Preparation method of bazedoxifene oxide
A bazedoxifene oxide technology, applied in the field of preparation of bazedoxifene oxide, can solve the problems affecting the purity of bazedoxifene acetate, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
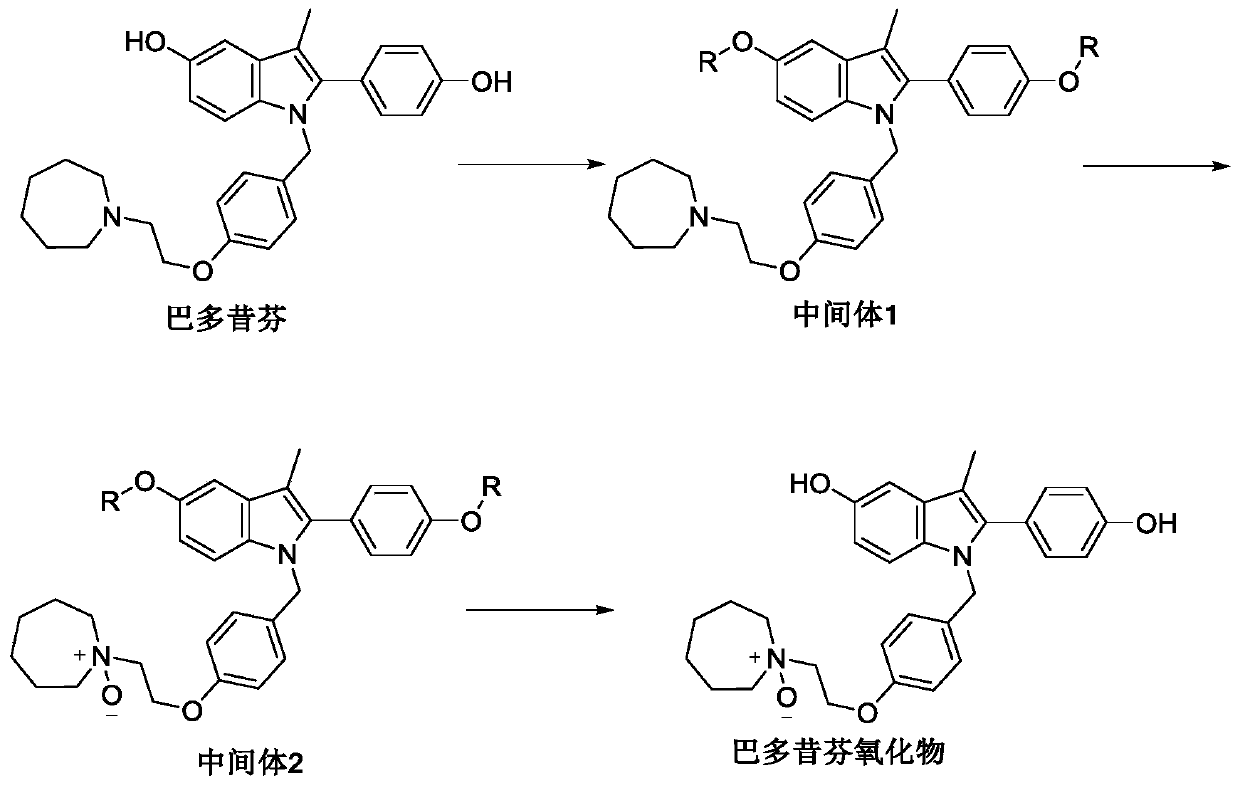
[0034] The embodiment of the present invention provides a preparation method of bazedoxifene oxide, comprising:
[0035] Step S11, using bazedoxifene free base as a raw material, dissolving it in the first benign solvent, adding an organic base, and then adding a silane protecting group to protect two phenolic hydroxyl groups to obtain intermediate 1;
[0036] Step S12, dissolving the intermediate 1 in a second benign solvent, adding an oxidizing agent to obtain intermediate 2 after oxidation;
[0037] Step S13, dissolving the intermediate 2 in a third benign solvent, adding a protecting group removing reagent to remove two molecules of silane protecting groups to obtain bazedoxifene oxide.
[0038] Further, in step S11, the first benign solvent includes at least one of dichloromethane, chloroform, tetrahydrofuran, dioxane and ethyl acetate; the silane protecting group includes trimethylchlorosilane , triethylchlorosilane, tert-butyldimethylchlorosilane, triisopropylchlorosil...
Embodiment 1
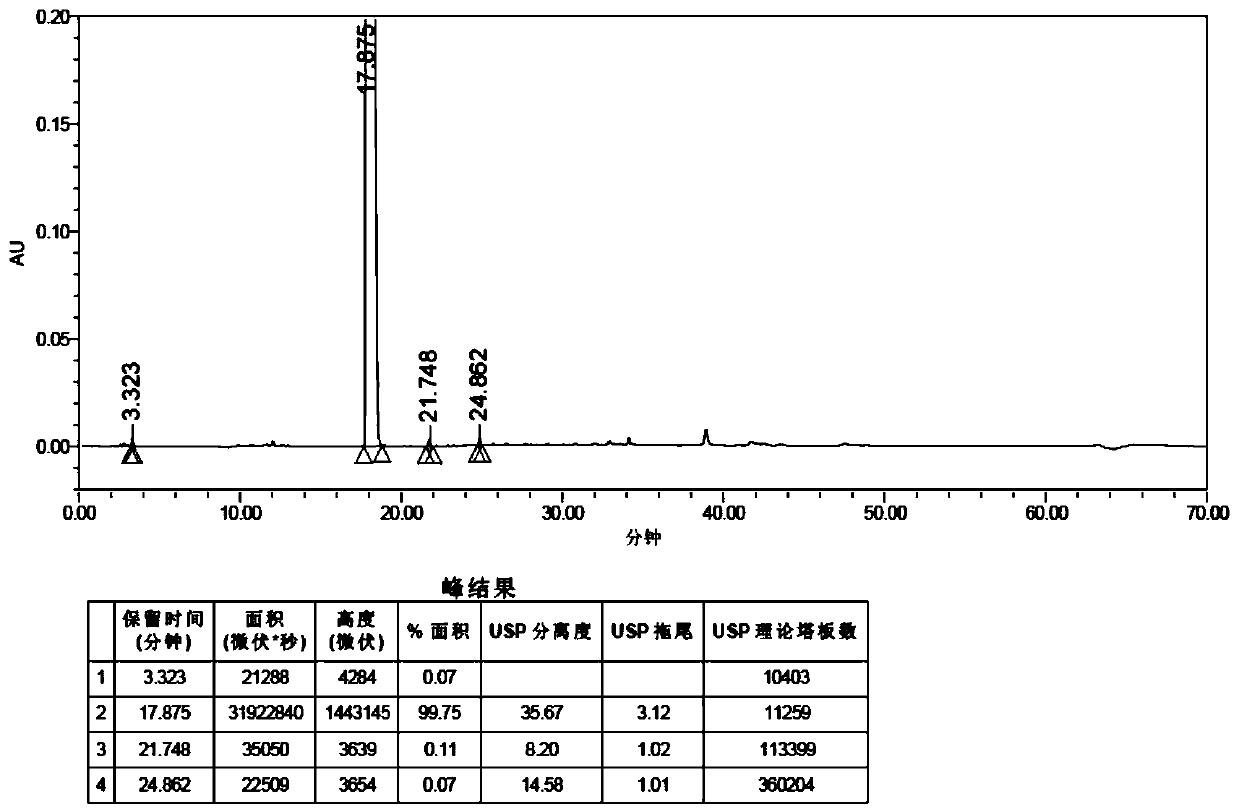
[0049] Step S201: Add bazedoxifene free base (3.1g, 6.6mmol) and dichloromethane (15mL) to the reaction flask, then add triethylamine (6.7g, 65.9mmol), and cool to 0°C, slowly add tert-butyldimethylsilyl chloride (5.0g, 32.9mmol) dissolved in dichloromethane (15mL) dropwise, after the dropwise addition, rise to room temperature and stir for 4 hours; after the reaction is complete, the reaction The solution was poured into 50 mL of purified water, extracted with dichloromethane (30 mL×3), the organic phases were combined, washed with saturated brine (50 mL×2), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and the concentrate After purification by column chromatography (eluent: dichloromethane:methanol=5:95), 4.3 g of intermediate 1 was obtained as a yellow oil with a yield of 93.48%.
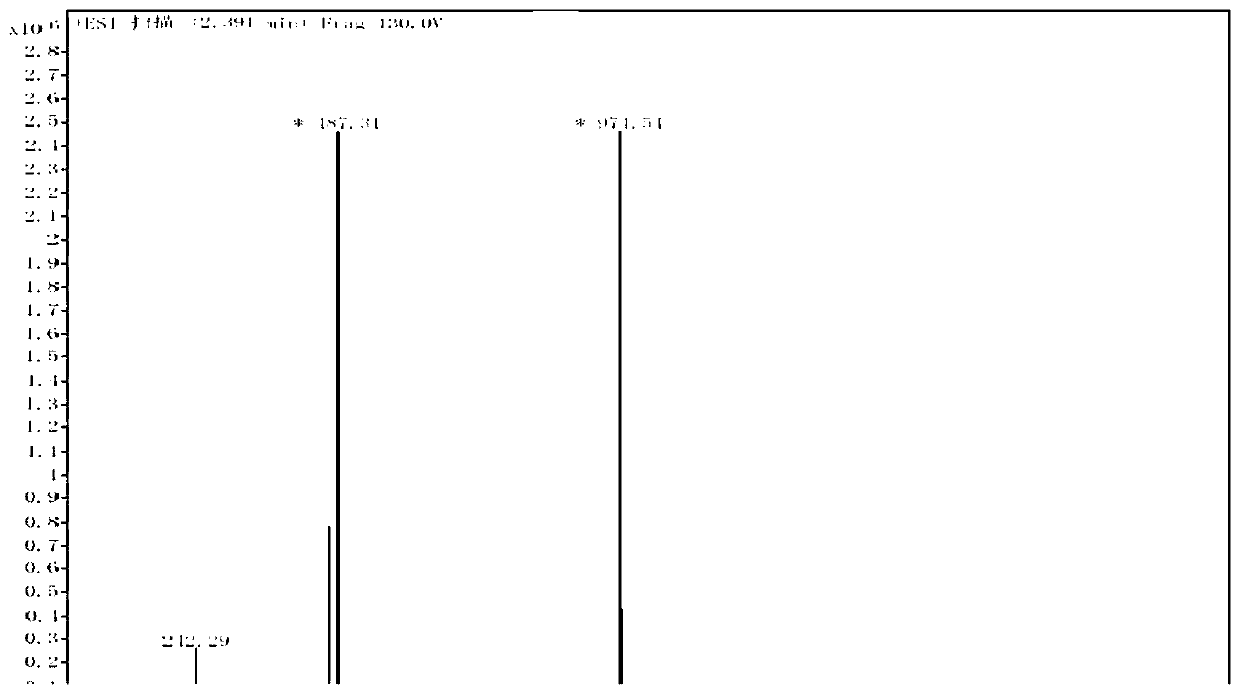
[0050] Step S202: Dissolve the intermediate 1 (4.1g, 5.86mmol) obtained in S201 in dichloromethane (60mL), and slowly add hydrogen peroxide (30%, 60mL) dropw...
Embodiment 2
[0055] Step S301: Add bazedoxifene free base (5.0g, 10.6mmol) and dichloromethane (30mL) to the reaction flask, then add triethylamine (6.5g, 63.7mmol), and cool to 0°C, slowly add a solution of trimethylchlorosilane (5.8g, 53.1mmol) in dichloromethane (30mL) dropwise, after the dropwise addition, rise to room temperature and stir for 4 hours; after the reaction is complete, pour the reaction solution into 100mL for purification In water, extracted with dichloromethane (50mL×3), combined the organic phases, washed the organic phase with saturated brine (100mL×2), dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and the concentrate was purified by column chromatography (The eluent is dichloromethane:methanol=5:95), 5.9 g of yellow oily intermediate 1 was obtained, and the yield was 90.30%.
[0056] Step S302: Dissolve the intermediate 1 (5.0g, 8.1mmol) obtained in S301 in dichloromethane (60mL), slowly add hydrogen peroxide (30%, 60mL) dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com