Preparation method for pharmaceutical crystal form of febuxostat
A febuxostat, crystal form technology, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of unsuitable terminal solvent, cumbersome operation process, poor process stability, etc., and achieves strong operability and low process cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 (by the method of the present invention, the volume-to-weight ratio of aqueous ethanol and febuxostat is 7L / kg)
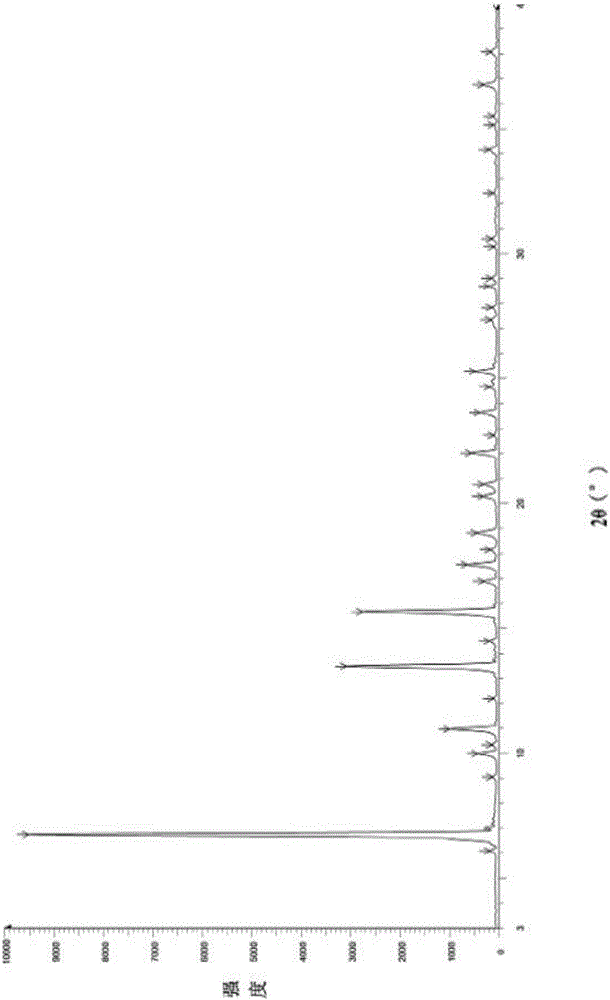
[0034] Add 50.0g of febuxostat to a 500ml three-necked bottle, add 350ml of ethanol with a weight percentage of 90% ethanol aqueous solution, stir, heat and reflux until completely dissolved and then stir for 30min. When the temperature of the reaction system was slowly lowered to 63° C., 0.15 g of febuxostat medicinal crystal form seed (form A) was added, and solids were precipitated in the reaction system. Continue to stir and cool down to 35°C, keep stirring for 1h, filter with suction until no liquid flows out, collect the filter cake in a hot air drying oven at a controlled temperature of 55-60°C and dry for 6h to obtain 41.0g of a white solid with a yield of 82% and a chromatographic purity of 99.96%. Sampling detection, powder X-ray diffraction spectrum and infrared spectrum are consistent with the spectrum disclosed in the patent applica...
Embodiment 2
[0035] Embodiment 2 (by the method of the present invention, the volume-to-weight ratio of aqueous ethanol and febuxostat is 8L / kg)
[0036] Add 10.0g of febuxostat to a 250ml three-necked bottle, add 80ml of ethanol with a weight percentage of 90% ethanol aqueous solution, stir, heat to reflux until completely dissolved and then stir for 30min. When the temperature of the reaction system was slowly lowered to 63° C., 30 mg of febuxostat medicinal crystal form seed crystal (form A) was added, and a solid precipitated out of the reaction system. Continue stirring to cool down to 35°C, keep stirring for 1 hour, filter with suction until no liquid flows out, collect the filter cake in a hot-air drying oven at a controlled temperature of 55-60°C and dry for 6 hours to obtain 6.7 g of white solid, with a yield of 67%. Sampling and detection showed that the powder X-ray diffraction spectrum and infrared spectrum were consistent with the spectrum disclosed in the patent application C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com