Synthesizing and refining methods of rivaroxaban
A synthetic method, the technology of rivaroxaban, applied in the field of synthesis and refinement of rivaroxaban, can solve the problems of unfriendly environment, increased temperature control cost, cumbersome operation, etc., to be beneficial to industrial production and reduce temperature control cost , good response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
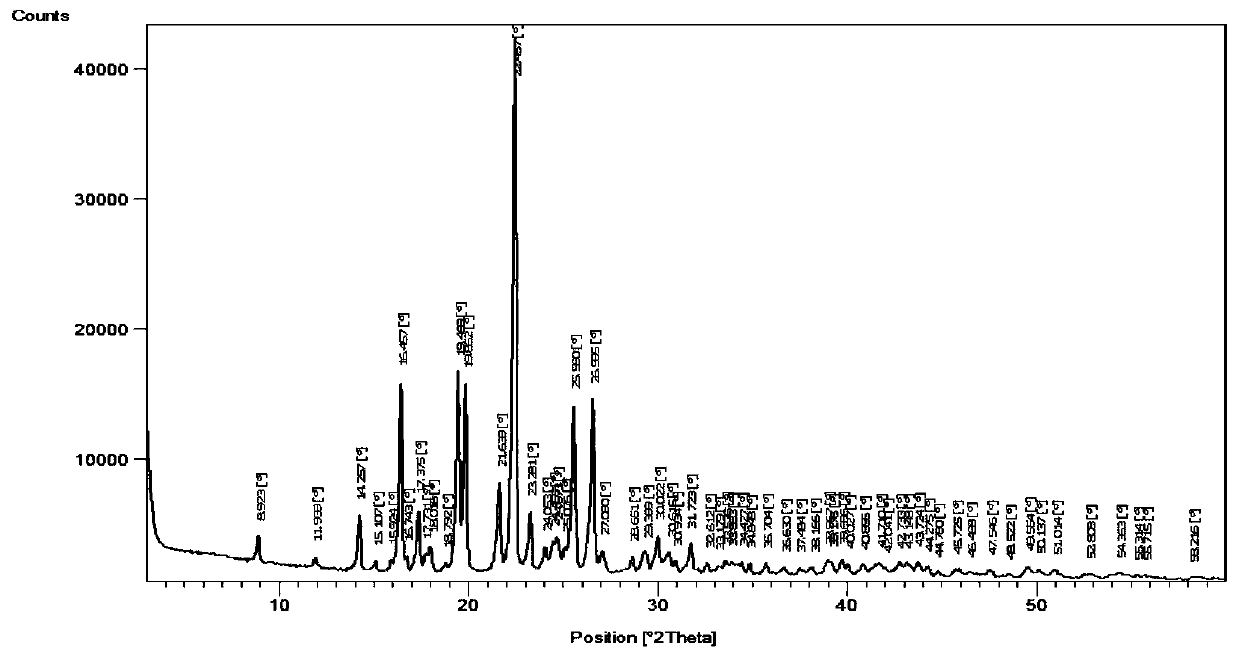
Image
Examples
Embodiment 1
[0045] (1) Preparation of crude product of rivaroxaban
[0046] At room temperature, add 650g of N,N-dimethylformamide (DMF) into the reaction flask, start stirring, cool down to 0°C, then add 40g of compound (LF-II), 73g of compound (SMI), 39.7g of CDI , the reaction solution is in a suspended state; temperature control is 0-5°C, and 67.6g of triethylamine is added dropwise to the reaction solution, and the internal temperature of the reaction solution is controlled at 0-5°C during the dropwise addition. to room temperature (20-25° C.), insulated and stirred for 2 hours. Suction filtration, the filter cake was washed with 80 g of DMF, the filtrate was added to 1200 g of water, stirred and crystallized at room temperature for 2 hours. Centrifuge the slurry mixture with a centrifuge until the flow is stopped, wash the reaction bottle with 500 g of purified water, and centrifuge the combined washings until the flow is stopped to obtain crude rivaroxaban.
[0047] (2) Refining ...
Embodiment 2
[0050] (1) Preparation of crude rivaroxaban At room temperature, add 600g of N,N-dimethylformamide (DMF) to the reaction flask, start stirring, cool down to 0°C, and then add 36g of compound (LF-II) in sequence , 65.5g compound (SMI), 39g CDI, the reaction solution is in a suspension state, and the temperature is controlled at 0-5°C. Add 60g of triethylamine dropwise to the reaction liquid, and control the internal temperature of the reaction liquid at 0-5°C during the dropwise addition. After the drop is complete, keep stirring for 30 minutes, then raise the temperature to room temperature (20-25°C), and keep stirring for 1.5 hours. Suction filtration, the filter cake was washed with 50g DMF, the filtrate was added to 1000g water, stirred and crystallized at room temperature for 1.5h. Centrifuge the slurry mixture with a centrifuge until the flow is stopped, wash the reaction bottle with 300 g of purified water, and centrifuge the combined washings until the flow is stopped t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com