Butylphthalide drug activity composition and preparation method thereof
A technology of medicinal activity and butylphthalide, applied in the field of medicine, can solve the problems of poor product stability, inability to be used as a medicine, uncontrollable, etc., and achieve the effects of stable quality, ensuring clinical efficacy and drug safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
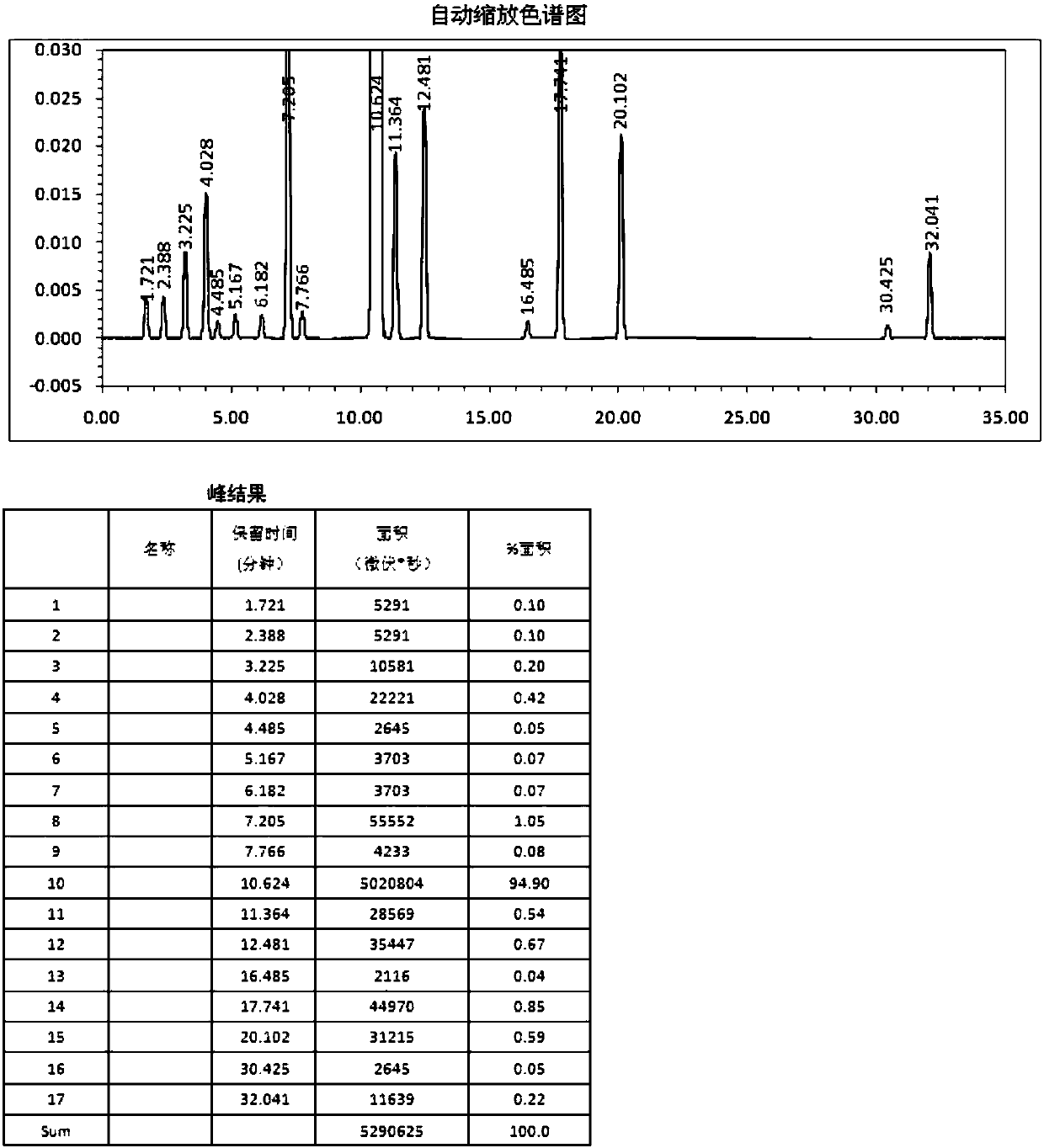
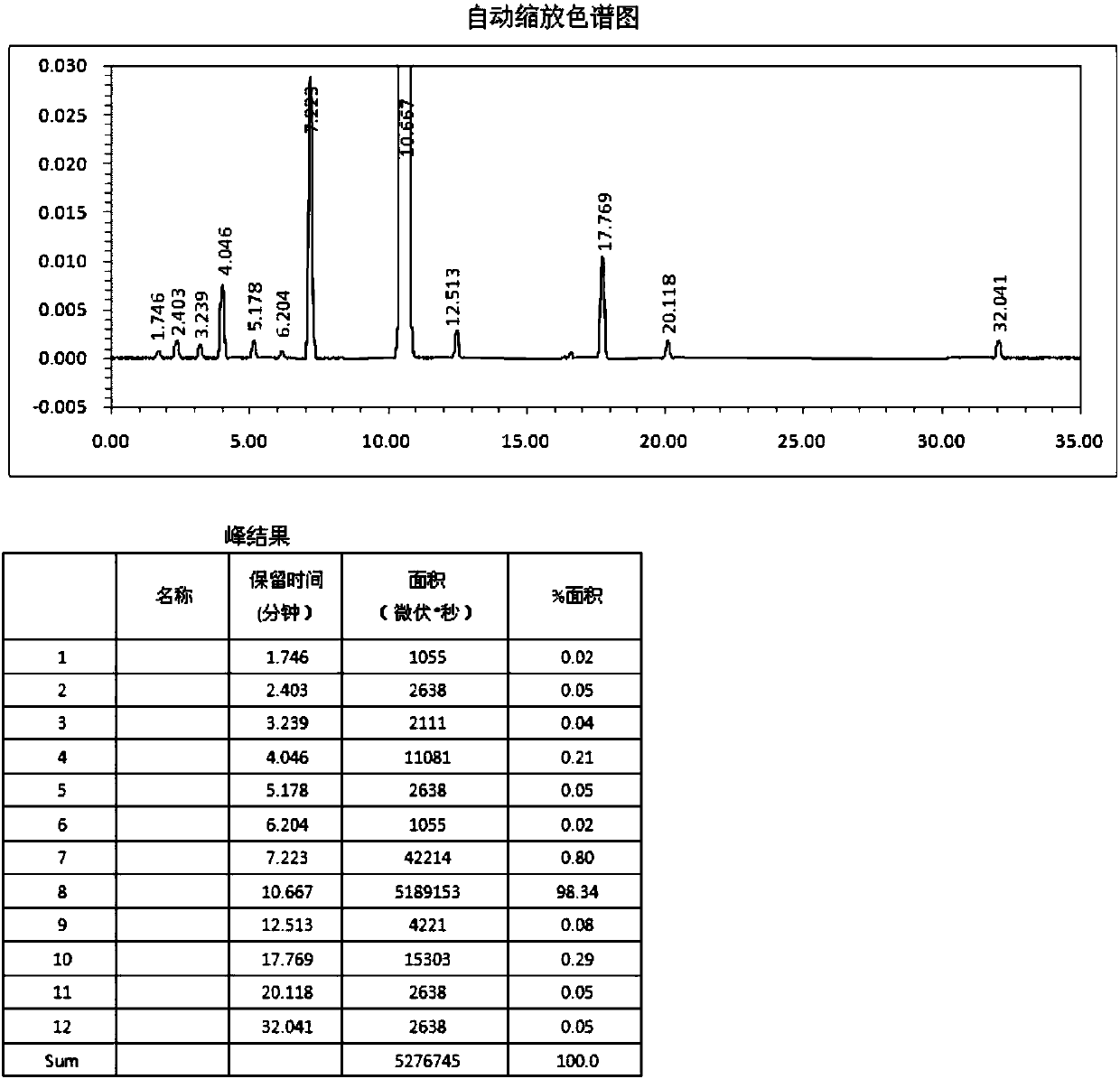
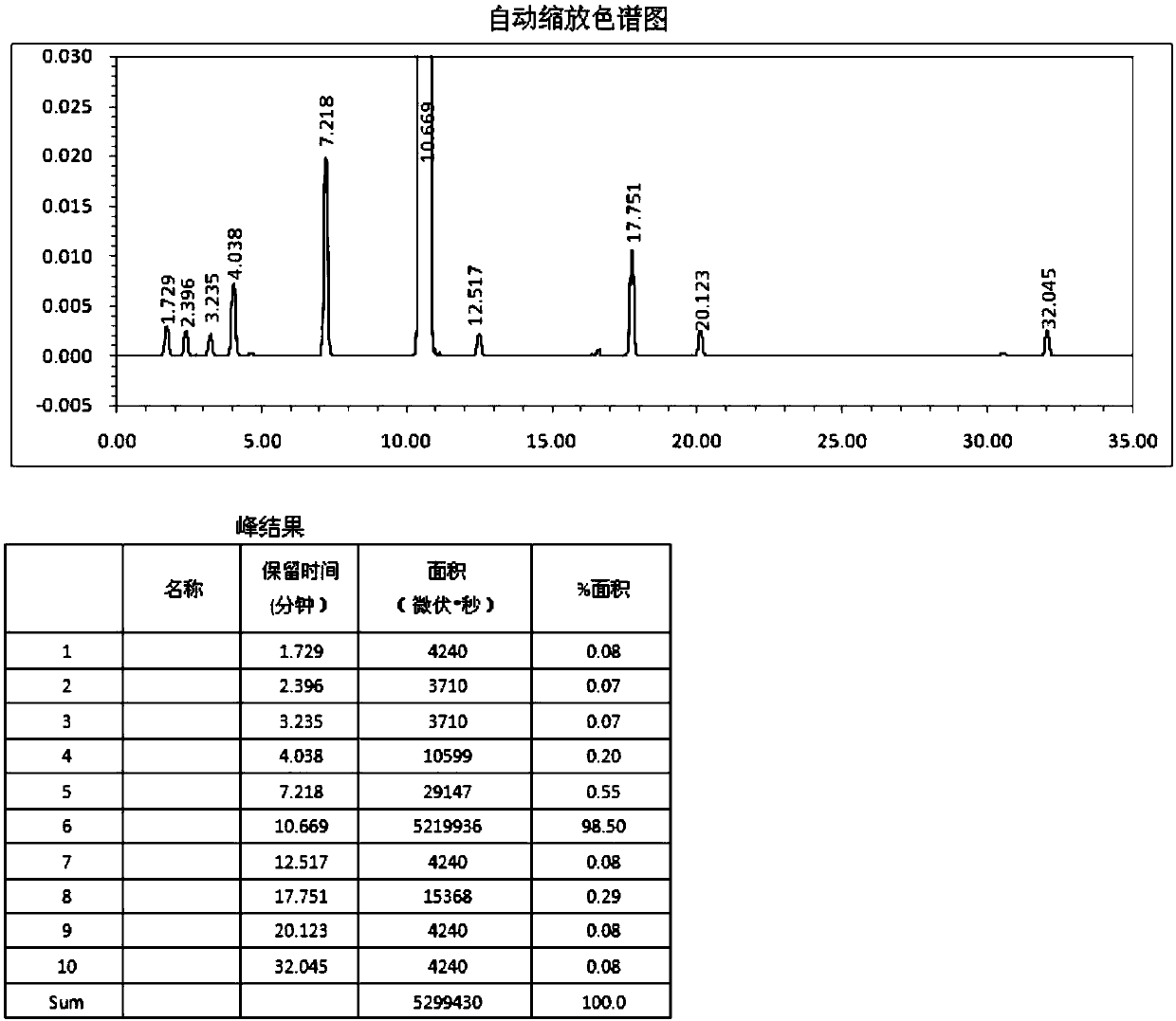
Image
Examples
preparation example 1
[0068] Preparation Example 1: Butylphthalide, a prior art product, prepared according to the preparation method disclosed by Li Shaobai et al. in "(±) Synthesis of Apigenin A"
[0069] (1) Preparation of butylene phthalide
[0070] 148.0Kg of phthalic anhydride, 82.0Kg of anhydrous sodium acetate and 300.0L of n-valeric anhydride were heated and refluxed at 300°C for 4 hours, and the low-boiling point fraction was evaporated (controlled below 150°C), the residue was dissolved in hot water, and then NaHCO 3 Neutralize to pH=6~7, extract with 7×500L ether, combine organic layers, anhydrous Na 2 SO 4 After drying, the desiccant was filtered off, diethyl ether was distilled off, and silica gel column chromatography was eluted with chloroform-petroleum ether to obtain 45.0 Kg of butylenephthalide.
[0071] (2) Preparation of butylphthalide
[0072] Dissolve 45.0Kg of 3-butenephthalide in ether, add 4.5Kg of 10% Pd / C, and use H 2 Gas replacement 6 times, filled with H 2 , stirr...
preparation example 2
[0073] Preparation example 2: Butylphthalide, a prior art product, according to the preparation method disclosed in Chinese patent CN101962374
[0074] (1) Preparation of Bromobutane Grignard Reagent
[0075] Under the protection of nitrogen, add 200L of tetrahydrofuran, 6.0Kg of magnesium flakes and 0.1Kg of iodine in a reaction tank equipped with stirring, thermometer and reflux condensing device, raise the temperature to 50°C, and add 31.50Kg of bromobutyl dissolved in 40L of tetrahydrofuran dropwise Alkanes, the temperature is controlled not to exceed 70 ° C, after the dropwise addition, continue to stir for 1 h to obtain the Grignard reagent of bromobutane.
[0076] (2) Preparation of o-valerylbenzoic acid
[0077] Under the protection of nitrogen, add 300L tetrahydrofuran, 30Kg phthalic anhydride and 2.5Kg copper iodide, cool to -10°C, add the Grignard reagent of bromobutane obtained in step (1) dropwise, control the dropwise addition for about 1h Complete; after the d...
Embodiment 1
[0081] Embodiment 1: Butylphthalide pharmaceutically active composition
[0082] Crude butylphthalide: butylphthalide prepared in Preparation Example 1
[0083] A. butylphthalide crude product 3.0Kg is joined in the rectifying tower;
[0084] B. Control the vacuum degree to 5mmHg, heat up to about 130°C, collect the distillate at this temperature, and discard it; continue
[0085] Raise the temperature to 154°C, control the reflux ratio to 3:1, and collect fractions at this temperature;
[0086] C. Re-add the collected fractions in the rectification tower, repeat step B 2 times to obtain 2.7Kg of butylphthalide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com