Maleic acid levorotation amlodipine drug active pharmaceutical composition and preparation method thereof
A technology of levamlodipine and drug activity, applied in the direction of drug combination, pharmaceutical formula, organic active ingredients, etc., can solve the problems of not being able to know the product of levamlodipine maleate, not being able to fully reflect, not being able to know the stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
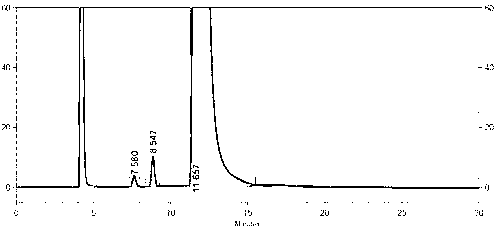
Image
Examples
preparation example 1
[0099] Preparation Example 1: Preparation of Racemic Amlodipine
[0100] (1) Preparation of phthaloyl amlodipine
[0101] Add 5Kg of ethyl 4-(2-phthalimidoethoxy)acetoacetate, 20L of anhydrous methanol, 2.3Kg of o-chlorobenzaldehyde, and 5.5Kg of methyl 3-aminobutenoate into the ring-closure kettle , heat preservation reaction for 22 hours, reclaim methanol under reduced pressure, after recovery, add 10kg of glacial acetic acid, stir at 15°C for about 12 hours, and filter in a centrifuge to obtain 5.1Kg of phthaloyl amlodipine crude wet product.
[0102] Add the crude amlodipine phthaloyl amlodipine into 7.0L of mixed solvent (toluene: glacial acetic acid = 1:1), heat to dissolve it completely, cool down to 10°C and keep it warm for 1 hour, shake off the filter, and dry under reduced pressure , to obtain 2.6Kg of phthaloyl amlodipine.
[0103] (2) Preparation of racemic amlodipine
[0104] Add 2.6Kg of phthaloyl amlodipine, 12.0Kg of toluene and 16.5Kg of 40wt% monomethyl...
preparation example 2
[0106] Preparation example 2: The preparation of levamlodipine, according to the preparation method disclosed in Chinese patent CN101528697
[0107] (1) Preparation of (S)-(-)-amlodipine solvate
[0108] 500 g of racemic amlodipine was dissolved in 2.5 L of N,N-methylacetamide (DMAC), then 190 g of L-tartaric acid was added thereto, the resulting mixture was cooled to 5° C. and stirred for 3 hours, and the precipitate was removed by filtration. Add 2.0L of dichloromethane and 14L of n-hexane to the filtrate, stir for 1.0 hour, filter, wash the filter cake with 800ml of n-hexane, and dry under reduced pressure at 50°C to obtain S-(-)-amlodipine-semi-L-tartaric acid Salt-DMAC solvate.
[0109] (2) Preparation of Levoamlodipine
[0110] The resulting S-(-)-amlodipine-hemi-L-tartrate-DMAC solvate was added to 5 L of methanol, heated for 7 hours, cooled to room temperature, filtered, and the obtained precipitate was dissolved in 2 L of dichloromethane, Add 1.5L of 2N NaOH solu...
preparation example 3
[0111] Preparation example 3: The preparation of levamlodipine, according to the preparation method disclosed in Chinese patent CN1927836
[0112] (1) Preparation of methyl ethyl sulfoxide:
[0113] Add 35L of acetone and 200mol of methyl ethyl sulfide to the reaction kettle, cool down to 0°C, add 200mol of hydrogen peroxide (concentration: 31%), control the temperature below 40°C during the addition, and keep warm at 25-35°C for 4 Hours, 65 ° C under reduced pressure to remove excess methyl ethyl sulfide, acetone and water, to obtain crude methyl ethyl sulfoxide 17.2Kg, water content of 4.8%, gas chromatography purity of 99.4%.
[0114] Take 17.2Kg of crude methyl ethyl sulfoxide (water content 4.8%), add 15.0Kg of calcium oxide, raise the temperature to 70°C, dry for 5 hours, filter after cooling down to room temperature, filter the filtrate, and distill under reduced pressure to obtain 14.8Kg of methyl ethyl sulfoxide, The water content is 0.35%, and the gas chromatograp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com