Pharmaceutical composition containing flurbiprofen and patch
A technology of flurbiprofen and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of poor compatibility between tackifying resins and drugs, damage to the cohesion of adhesive matrix, and poor drug-loading ability of patches, etc., to prolong storage time and effective action time, excellent transdermal absorption performance, and good sticking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
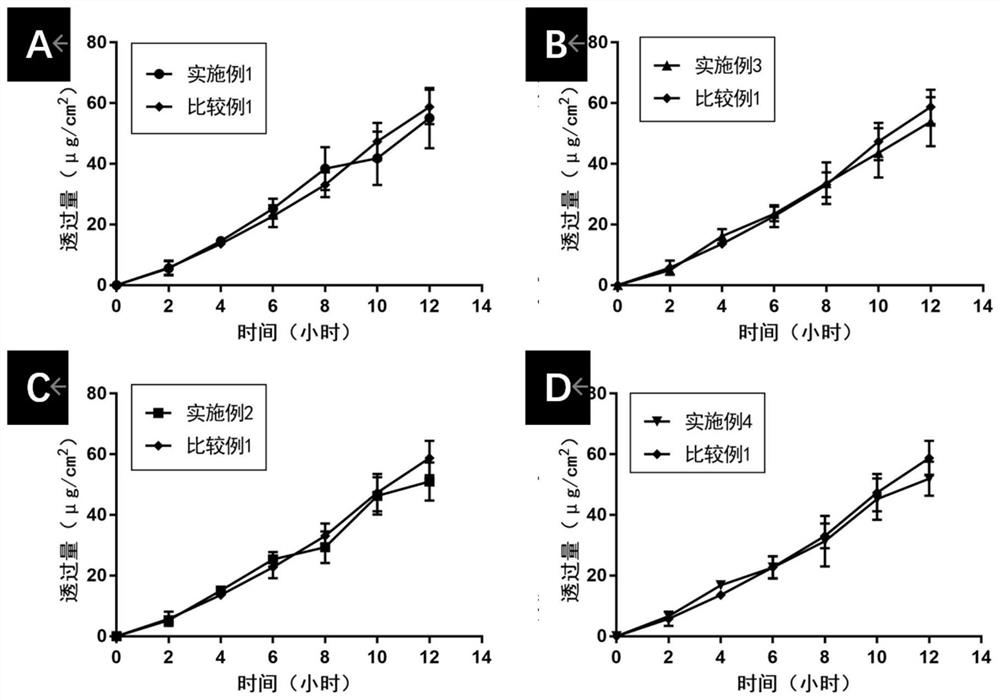
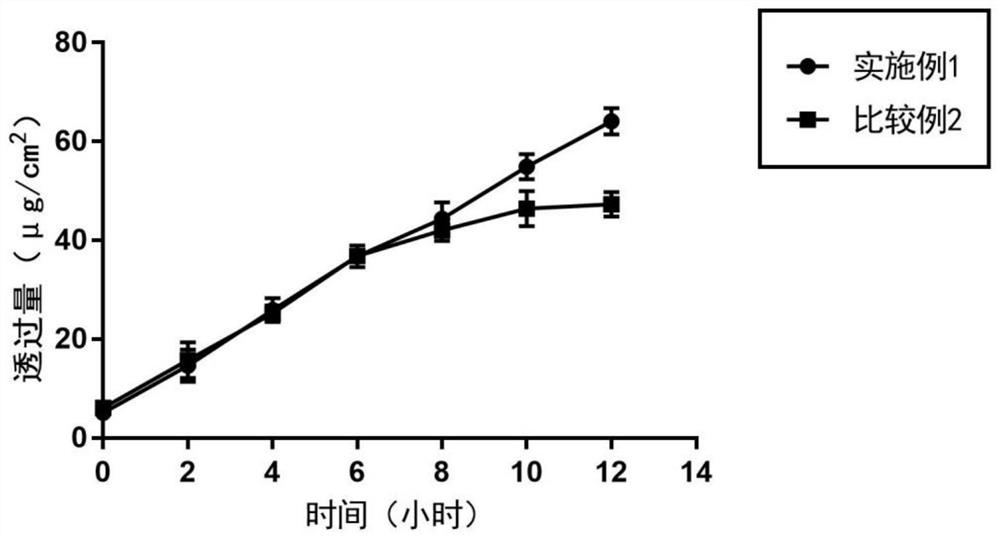
Embodiment 1
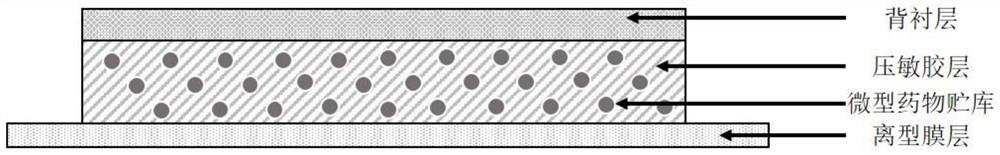
[0089] The flurbiprofen pressure-sensitive adhesive patch that present embodiment provides is made up of backing (9100 type, Japanese vilene Co., Ltd.), paste and anti-adhesive layer (KL12 type, Japan DYNIC Co., Ltd.), and the component of paste is as follows: Table 1 shows.
[0090] Table 1
[0091]
[0092]
[0093] Preparation Process:
[0094] (1) Add SIS, polyisobutylene and liquid paraffin into the reaction vessel, mix and swell at 150-160°C for more than 30 minutes, this step is carried out under nitrogen or vacuum conditions;
[0095] (2) Put terpene resin, L-menthol, calcium hydroxide, BHT and hydrogenated rosin glyceride into the mixture obtained in step (1) successively, and stir until melting at 150°C. Carry out under vacuum condition;
[0096] (3) In another reaction vessel, add flurbiprofen and poloxamer F68, heat up to 150°C, mix and stir to form a solid mixture of flurbiprofen and poloxamer;
[0097] (4) Add the solid mixture obtained in step (3) into...
Embodiment 2
[0100] The flurbiprofen pressure-sensitive adhesive patch provided in this example consists of a backing, a paste and an anti-adhesive film, and the components of the paste are shown in Table 2.
[0101] Table 2
[0102] Components and their sources Dosage Flurbiprofen (above 99.5% purity, British AESICA) 13.28g SIS (Vector 4111, DEXCO, USA) 20.29g Polyisobutylene (Oppanol B-10, BASF, Germany) 6.01g Terpene resin (A135, American PINOVA) 9.02g Hydrogenated Rosin Glycerides (HYDROGRAL-G5, French DRT) 3.01g Poloxamer F68 (BASF, Germany) 13.28g L-menthol (US SIGMA) 1.50g Liquid paraffin (Shanghai aladdin) 33.21g Calcium hydroxide (Shanghai aladdin) 0.04g BHT (MERCK, Germany) 0.38g total 100.02g
[0103] Preparation Process:
[0104] (1) Add SIS, polyisobutylene and liquid paraffin into the reaction vessel, mix and swell at 150-160°C for more than 30 minutes, this step is carried out under nitrogen or...
Embodiment 3
[0110] The flurbiprofen pressure-sensitive adhesive patch provided in this example consists of a backing, a paste and an anti-adhesive film. The components of the paste are shown in Table 3.
[0111] table 3
[0112] Components and their sources Dosage Flurbiprofen (above 99.5% purity, British AESICA) 13.28g SIS (Vector 4111, DEXCO, USA) 22.12g Polyisobutylene (Oppanol B-10, BASF, Germany) 6.55g Terpene resin (A135, American PINOVA) 9.83g Hydrogenated Rosin Glycerides (HYDROGRAL-G5, French DRT) 3.28g Polyvinylpyrrolidone K-90 (MERCK, Germany) 6.64g L-menthol (US SIGMA) 1.64g Liquid paraffin (Shanghai aladdin) 36.21g Calcium hydroxide (Shanghai aladdin) 0.04g BHT (MERCK, Germany) 0.41g total 100.00g
[0113] Preparation Process:
[0114] (1) Add SIS, polyisobutylene and liquid paraffin into the reaction vessel, mix and swell at 150-160°C for more than 30 minutes, this step is carried out under nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com