Preparation method for vortioxetine
A technology of vortioxetine and chlorobenzene, which is applied in the field of drug synthesis, can solve the problems of potential safety hazards, high equipment requirements, product adsorption, etc., and achieve the effects of reducing production costs, easy access to raw materials, and simple processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
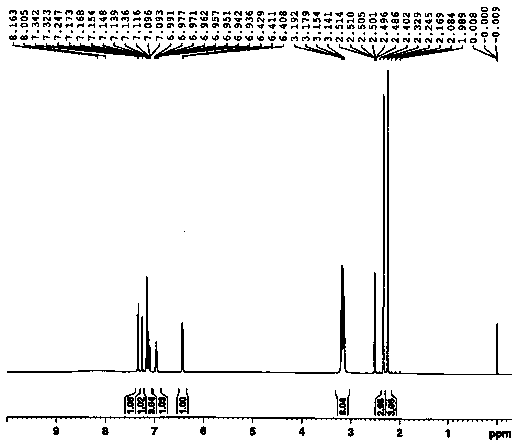
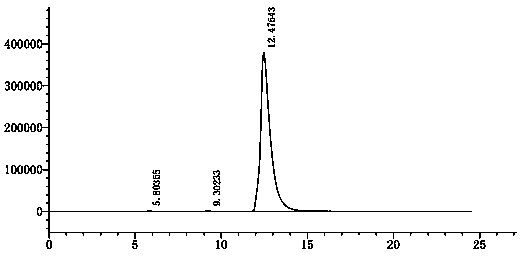
Image
Examples
Embodiment 1
[0041] ① Synthesis of 2-(2,4-dimethylphenylthio)chlorobenzene:
[0042]Add 14g of 2,6-dimethylthiophenol (101.4mmol), 29.8g of o-dichlorobenzene (202.8mmol), 965mg of CuI (5.1mmol) and 29.2g of NaOtBu (304.2mmol) into 210mL of In toluene, start stirring. The entire reaction system was replaced with nitrogen for 3 times (reaction needs to be carried out under the protection of nitrogen), the temperature of the external temperature oil bath was raised to 120°C, and the reaction system was kept in a reflux state, and the reaction was kept at this temperature for 16 hours. The reaction mixture was cooled, 50 mL of water was added, and stirred for 1 hour, then the resulting mixture was filtered through filter aid. The filtrate was washed with saturated brine (3*100 mL). Next, the combined aqueous phases were extracted with 200 ml of toluene. Then, the combined toluene phases were concentrated under reduced pressure to obtain 21.4 g of oil (86% yield).
[0043] ②Synthesis and pu...
Embodiment 2
[0047] ③ Synthesis of 2-(2,4-dimethylphenylthio)chlorobenzene:
[0048] Add 14g of 2,6-dimethylthiophenol (101.4mmol), 29.1g of 2-bromochlorobenzene (152.1mmol), 965mg of CuI (5.1mmol) and 29.2g of NaOtBu (304.2mmol) to 210mL In toluene, start stirring. The entire reaction system was replaced with nitrogen for 3 times (reaction needs to be carried out under the protection of nitrogen), the temperature of the external temperature oil bath was raised to 120°C, and the reaction system was kept in a reflux state, and the reaction was kept at this temperature for 16 hours. The reaction mixture was cooled, 50 mL of water was added, and stirred for 1 hour, then the resulting mixture was filtered through filter aid. The filtrate was washed with saturated brine (3*100 mL). Next, the combined aqueous phases were extracted with 200 ml of toluene. Then, the combined toluene phases were concentrated under reduced pressure to obtain 22.9 g of an oil (91% yield).
[0049] ④ Synthesis and...
Embodiment 3
[0053] ⑤ Synthesis of 2-(2,4-dimethylphenylthio)chlorobenzene:
[0054] Add 14 g of 2,6-dimethylthiophenol (101.4 mmol), 25.4 g of 2-iodochlorobenzene (106.5 mmol), 965 mg of CuI (5.1 mmol) and 29.2 g of NaOtBu (304.2 mmol) in sequence In 210 mL of toluene, start stirring. The entire reaction system was replaced with nitrogen for 3 times (reaction needs to be carried out under the protection of nitrogen), the temperature of the external temperature oil bath was raised to 120°C, and the reaction system was kept in a reflux state, and the reaction was kept at this temperature for 16 hours. The reaction mixture was cooled, 50 mL of water was added, and stirred for 1 hour, then the resulting mixture was filtered through filter aid. The filtrate was washed with saturated brine (3*100 mL). Next, the combined aqueous phases were extracted with 200 ml of toluene. Then, the combined toluene phases were concentrated under reduced pressure to obtain 22.7 g of oil (90% yield).
[0055...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com