Composition of amorphous vortioxetine or amorphous vortioxetine salt and pharmaceutical adjuvants, and preparation method thereof
A technology of pharmaceutical excipients and vortioxetine, which is applied in the direction of drug combination and nervous system diseases, can solve the problems of difficulty in the development of pharmaceutical formulations and the limitation of the total amount of excipients, and achieve low price, increased stability, and good solubility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
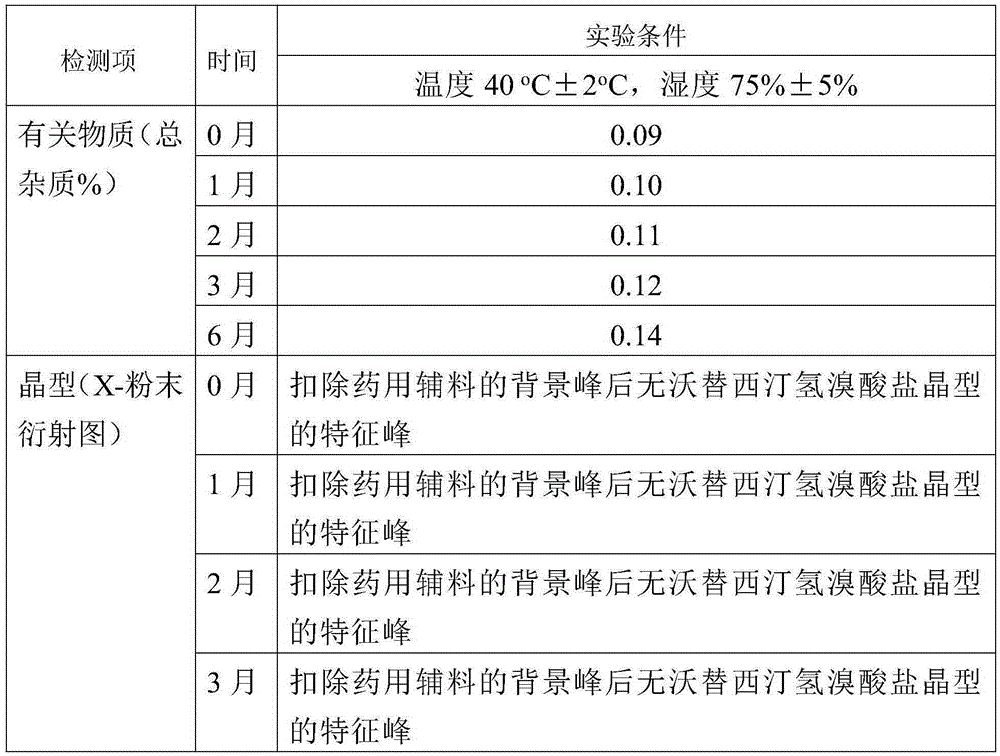
[0046] Dissolve vortioxetine hydrobromide (50 mg), D-mannitol (50 mg) and povidone K30 (50 mg) in methanol (800 microliters), heat to 60°C and stir to dissolve. The above solution was rapidly cooled to -10°C, a white solid was precipitated, filtered, and dried to obtain a composition of amorphous vortioxetine hydrobromide, D-mannitol and povidone K30, the X-ray of the composition Powder diffraction pattern as figure 1 As shown, there is no characteristic peak of the vortioxetine hydrobromide crystal form in the X-ray powder diffraction pattern after deducting the background peak of the pharmaceutical excipient.
Embodiment 2
[0048] Vortioxetine (50 mg), polyacrylic acid resin Eudragit L100 (50 mg) and polyethylene glycol 4000 (200 mg) were dissolved in ethanol (600 μl) and water (600 μl) at -40 °C Stir down to mix well. The above solution is slowly concentrated to dryness in a rotary evaporator to obtain a white solid, which is a composition of amorphous vortioxetine, polyacrylic acid resin Eudragit L100 and polyethylene glycol 4000, and the X-ray powder diffraction pattern of the composition Among them, there were no characteristic peaks of vortioxetine crystal form after deducting the background peaks of pharmaceutical excipients.
Embodiment 3
[0050] Add vortioxetine hydrochloride (2 g), lactose (2 g) and polyethylene glycol 8000 (10 g) into water (300 ml), heat to 60°C and stir to dissolve. The above solution was dried with JISL micro-spray dryer LSD-48, and the inlet temperature was maintained at 60°C and the outlet temperature was 50°C. The composition of ethylene glycol 8000, in the X-ray powder diffraction diagram of the composition, there is no characteristic peak of vortioxetine hydrochloride crystal form after deducting the background peak of pharmaceutical excipients.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com