Preparation method and application of nano nickel phosphide
A technology of nickel phosphide and nanotechnology, applied in chemical instruments and methods, phosphine, nanotechnology, etc., can solve the problems of complex process, harsh conditions, high reaction temperature, etc., and achieve good reusability, large specific surface area, catalytic The effect of more active points
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 0.1 g of sodium dodecylbenzenesulfonate (SDBS), 3 mmol of nickel chloride and 10 mmol of sodium hypophosphite in 30 ml of water, stir vigorously, and add 0.2 g of white phosphorus. After mixing evenly, the resulting mixed solution was poured into a stainless steel autoclave lined with polytetrafluoroethylene, and heated to 170° C. for 15 hours to react. After the reaction, cool the autoclave to room temperature, wash the obtained black product several times with deionized water, and dry it in a vacuum oven at 50°C. The obtained product is porous nickel phosphide nanospheres with catalytic hydrogenation activity of nitrobenzene
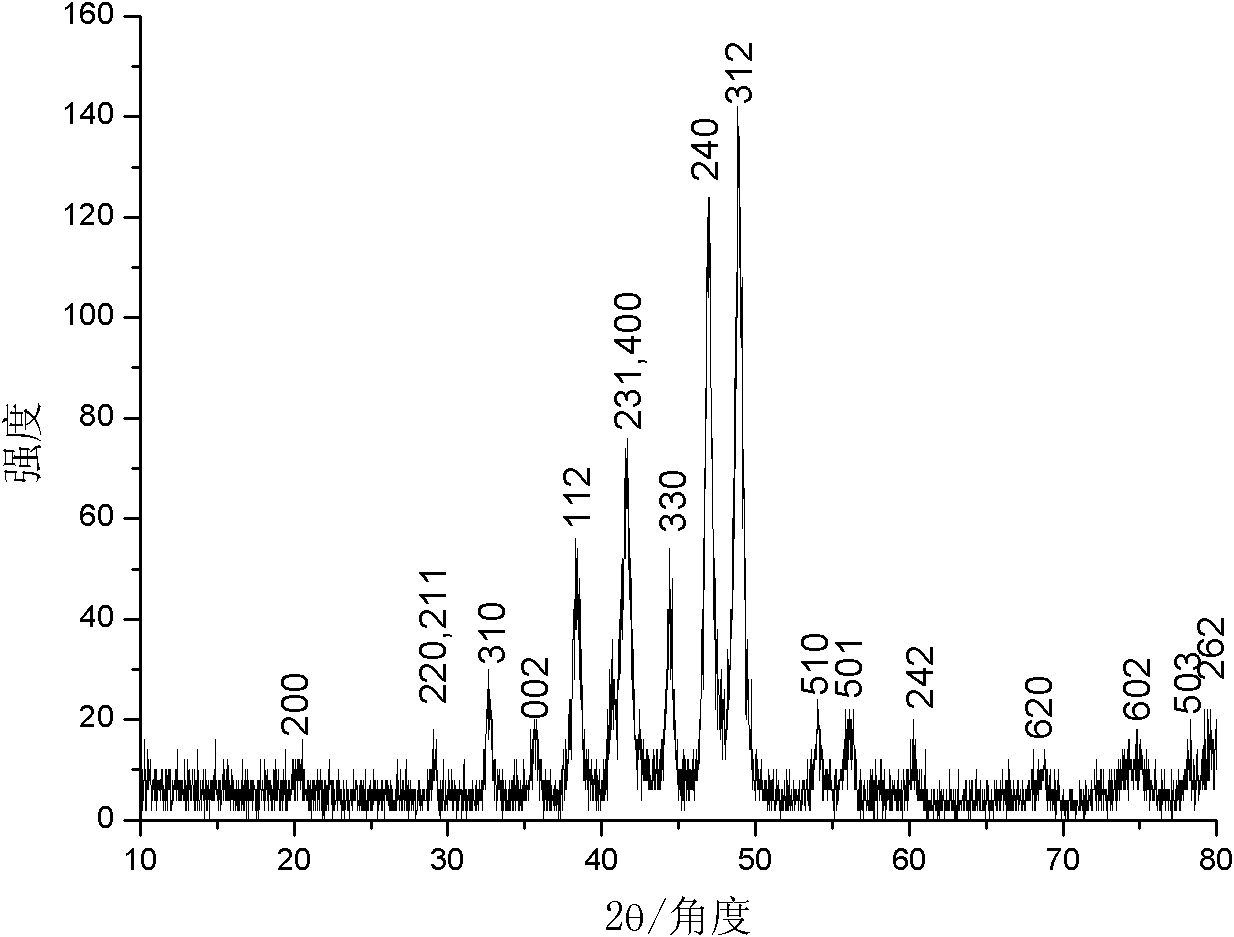
[0022] The product (Cu Kα ray, λ=0.154060nm, scanning speed 0.02° / s) of implementation experiment 1 is carried out phase identification with Japan Shimao Feng XRD-6000 type X-ray powder diffractometer, as figure 1 shown in . Compared with the JCPDS standard card (74-1381), all diffraction peaks are related to Ni 12 P 5 It fits perfec...
Embodiment 2
[0024] Dissolve 0.08 g of sodium dodecylbenzenesulfonate (SDS), 3 mmol of nickel acetate and 10 mmol of sodium potassium hypophosphite in 25 ml of water, stir vigorously, and add 0.25 g of white phosphorus. After mixing evenly, the resulting mixed solution was poured into a stainless steel autoclave lined with polytetrafluoroethylene, and heated to 160° C. for 15 hours. After the reaction, cool the autoclave to room temperature, wash the obtained black product several times with deionized water, and dry it in a vacuum oven at 50°C. The obtained product is porous nickel phosphide nanospheres with catalytic hydrogenation activity of nitrobenzene
Embodiment 3
[0026] Dissolve 0.15 g of sodium dodecylbenzenesulfonate (SDBS), 3 mmol of nickel nitrate and 12 mmol of sodium hypophosphite in 35 ml of water, stir vigorously, and add 0.3 g of white phosphorus. After mixing evenly, the resulting mixed solution was poured into a stainless steel autoclave lined with polytetrafluoroethylene, and heated to 160° C. for 12 hours. After the reaction, cool the autoclave to room temperature, wash the obtained black product several times with deionized water, and dry it in a vacuum oven at 50°C. The obtained product is porous nickel phosphide nanospheres with catalytic hydrogenation activity of nitrobenzene
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com