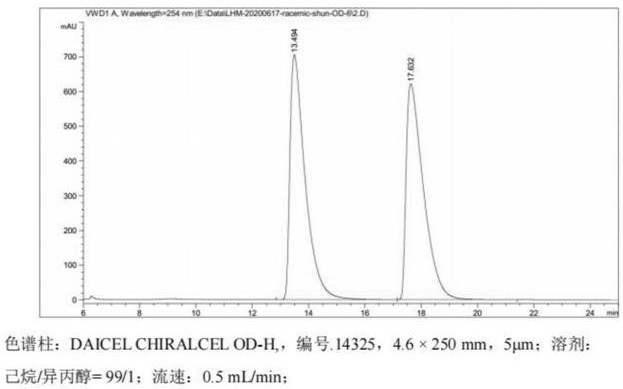
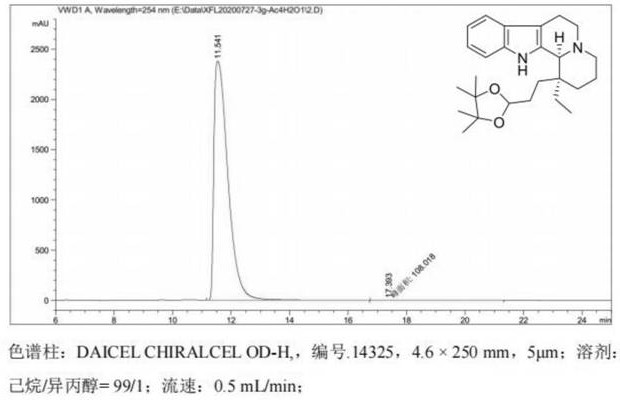
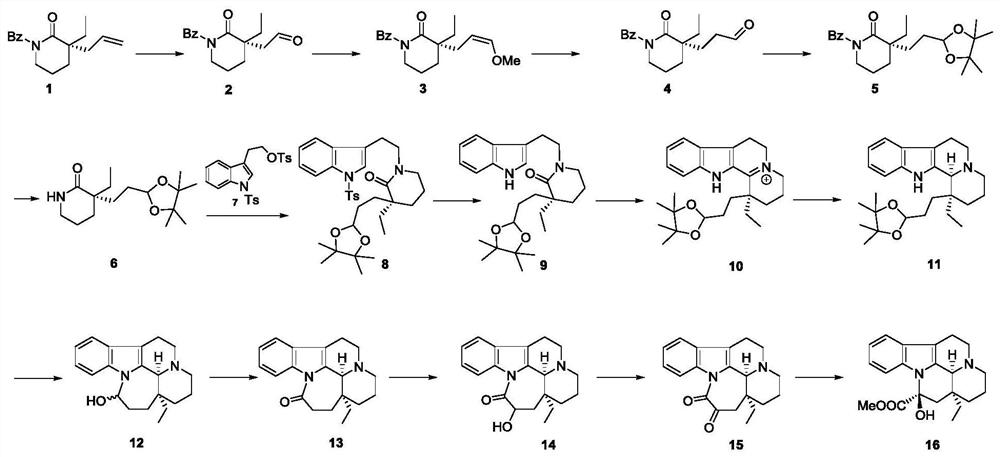
Intermediate, preparation method and application of intermediate in synthesis of vincamine
An intermediate, vincamine technology, applied in the field of chemical drug synthesis, can solve the problems of low total yield, long steps, and low industrial value, and achieve the effect of simple operation, simple reaction, and easy scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] The synthesis of embodiment 1 compound 2, reaction scheme is as follows:
[0098]
[0099] Include the following steps:
[0100]Compound 1 (98%ee) (1.73g, 6.4mmol) was dissolved in 100mL of dichloromethane, and ozone was passed through at -78°C for 10 minutes, and the reaction solution turned blue. Oxygen was introduced into the reaction solution for 10 minutes, and excess ozone was discharged, and triphenylphosphine (3.34 g, 12.8 mmol) was added, and the mixture was heated to room temperature and stirred. After TLC detection showed that the reaction was complete, the reaction solution was directly concentrated, and the obtained crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 5:1 to 4:1) to obtain yellow oil 2 (1.63 g, yield 97 %).
[0101] Compound 2: 1 H NMR (400MHz, CDCl 3 )δ9.67(s,1H),7.51(d,J=7.2Hz,2H),7.48–7.41(m,1H),7.41–7.31(m,2H),4.06–3.91(m,1H),3.88 –3.70(m,1H),2.97(d,J=17.6Hz,1H),2.47 (d,J=17.6Hz,1H),2....
Embodiment 2
[0102] The synthesis of embodiment 2 compound 4, reaction scheme is as follows:
[0103]
[0104] Include the following steps:
[0105] Disperse (methoxymethyl)triphenylphosphine chloride (3.01 g, 8.8 mmol) in dry tetrahydrofuran (50 mL) under the protection of argon, and after cooling to 0 °C, slowly add potassium tert-butoxide ( 1.0M in THF, 6.59mL, 6.6mmol), after the addition, the reaction solution was stirred at 0°C for 30 minutes. Then it was cooled to -78°C, and 10 mL of dry tetrahydrofuran solution of compound 2 (1.20 g, 4.4 mmol) was added thereto, reacted under the protection of argon for 2 hours, then opened for reaction, and gradually rose to room temperature for reaction. After TLC detection showed that the reaction was complete, saturated ammonium chloride solution was added to quench the reaction, the organic layer was separated, the aqueous layer was extracted with ethyl acetate (20mL×2), the organic layers were combined, and the organic layer was washed on...
Embodiment 3
[0107] The synthesis of embodiment 3 compound 5, reaction scheme is as follows:
[0108]
[0109] Include the following steps:
[0110] Compound 4 (1.16g, 4.0mmol) was dissolved in 23mL of dichloromethane, pinacol (716mg, 6.1mmol) and p-toluenesulfonic acid (76.8mg, 0.404mmol) were added to react at room temperature. After the complete disappearance of the raw material was detected by TLC, the reaction solution was directly concentrated, and the crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate = 8:1) to obtain yellow oil 5 (1.49 g, yield 97%).
[0111] Compound 5: 1 H NMR (400MHz, CDCl 3 )δ7.50(d,J=7.6Hz,2H),7.47–7.41(m,1H),7.40–7.31(m,2H),5.01(t,J=5.2Hz,1H),3.76(t,J =6.4Hz,2H),2.06–1.93(m,2H),1.88–1.81(m,2H),1.81–1.63(m,4H),1.62–1.51(m,2H),1.25–1.08(m,12H ),0.88(t,J=7.6Hz,3H). 13 C NMR (100MHz, CDCl 3 )δ178.1, 175.6, 136.8, 131.1, 128.0, 127.4, 100.8, 81.7, 81.7, 47.0, 46.9, 31.1, 30.9, 30.7, 29.8, 24.3, 24.2, 22.0, 22.0,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com