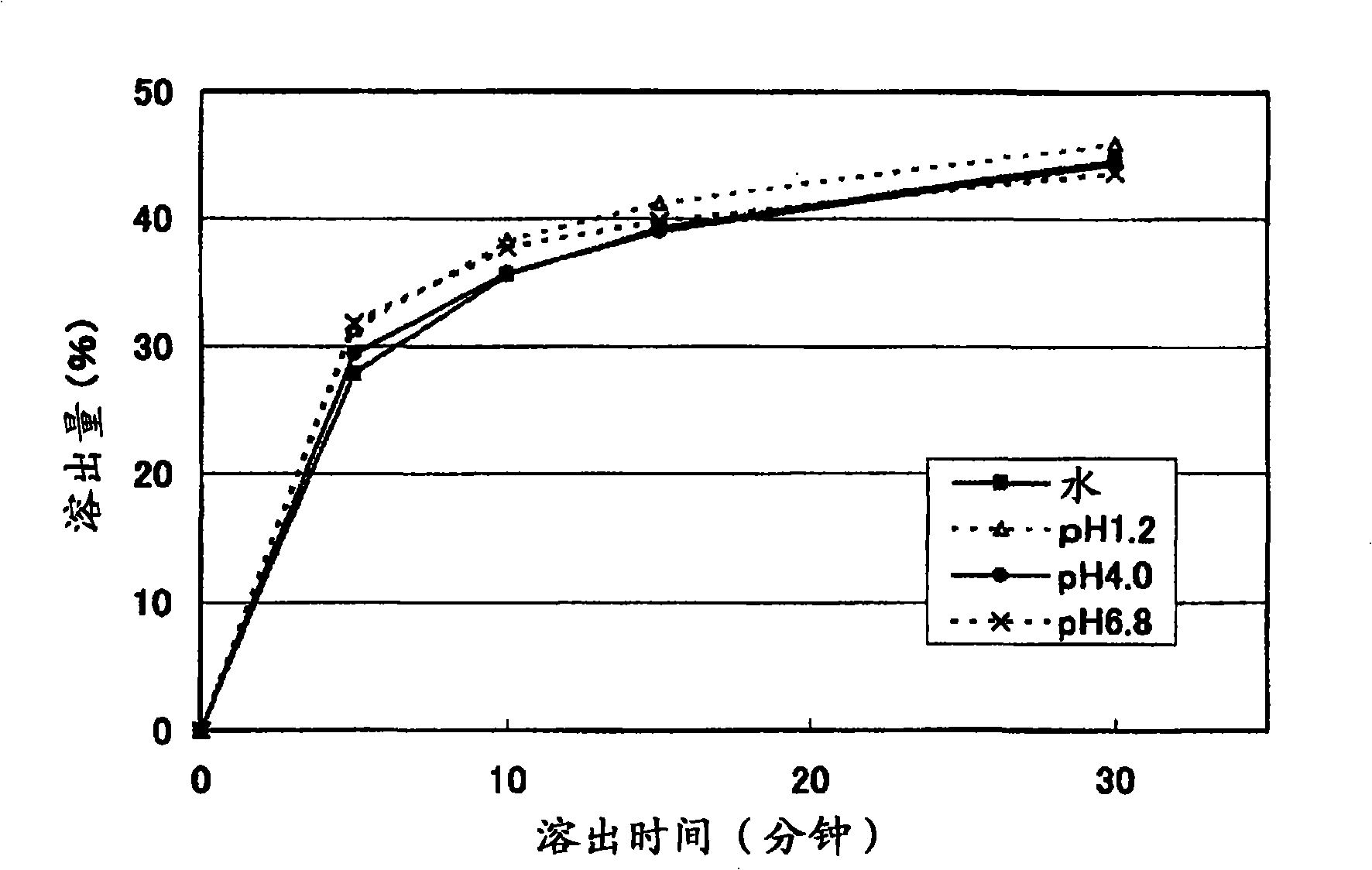
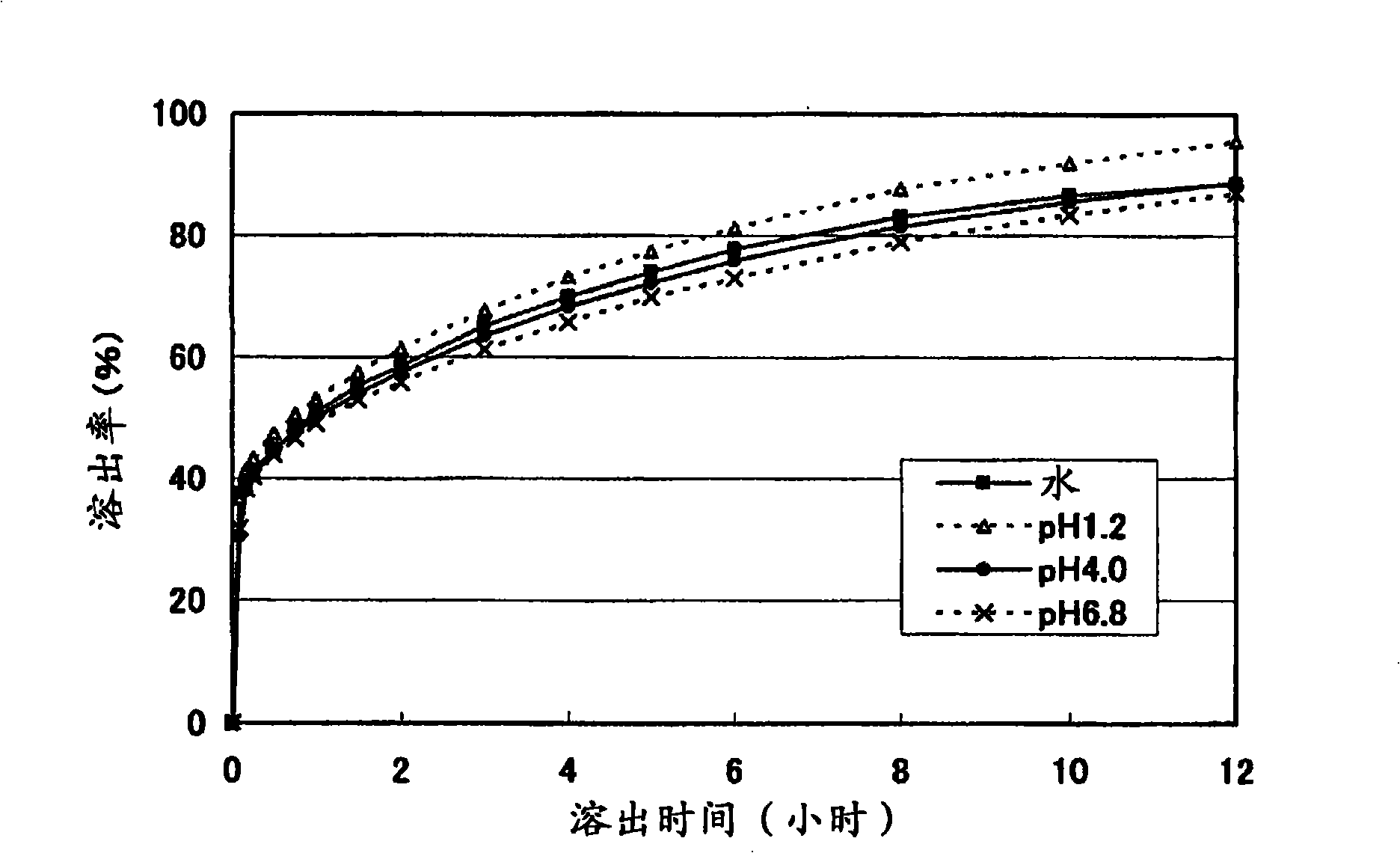
Solid pharmaceutical preparation
A solid pharmaceutical preparation and preparation technology, which can be used in drug combination, drug delivery, antipyretic and other directions, and can solve problems such as low pH value dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 2450 g of tramadol hydrochloride, 630 g of lactose, 273 g of crystalline cellulose, and 1470 g of partially gelatinized starch were mixed and pulverized, and granulated with a liquid in which 49 g of hydroxypropyl cellulose was dissolved in purified water. 28 g of magnesium stearate was added to the granulated granules and mixed to prepare immediate-release granules. On the other hand, 1625 g of tramadol hydrochloride, 3000 g of hydroxypropyl cellulose, and 150 g of carboxymethylcellulose sodium were mixed and pulverized, and granulated with purified water. 675 g of carboxyvinyl polymer was added to the granulated granules and mixed, and then 50 g of magnesium stearate was added and mixed to prepare sustained-release granules. The immediate-release granules and the sustained-release granules thus obtained were compressed into tablets to obtain tramadol bilayer tablets containing 100 mg of tramadol hydrochloride per tablet. In addition, for the formulations of 75 mg and...
Embodiment 2
[0072] [Example 2] Research on excipients
[0073] Immediate-release portions for 100 mg tablets of formulation A using lactose and crystalline cellulose as excipients and formulation B using only crystalline cellulose as excipients were prepared. The compounding quantity of each containing component per 1 tablet is shown in Table 4. Tablets having a tramadol hydrochloride content of 100 mg / tablet having the composition shown in Table 4 were produced according to the following preparation method.
[0074] [Table 4]
[0075]
[0076] 350g of tramadol hydrochloride, 133g of excipients (prescription A: 93g of lactose and 40g of crystalline cellulose, prescription B: 133g of crystalline cellulose), 210g of partially α-gelatinized starch were mixed, pulverized, and granulated with purified water . 7 g of magnesium stearate was added to the granulated granules and mixed to prepare immediate-release granules.
[0077] In the same manner as in Test Example (1), immediate-releas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com