Enhanced CH2 structure domain mutant combined with newborn Fc receptor as well as preparation method and application thereof
A receptor binding and structural domain technology, applied in the biological field, can solve the problems of weak CH2 stability and achieve the effect of pH-dependent binding enhancement, good stability, and good anti-aggregation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Construction of yeast display library
[0027] Using the base sequence of the m01s backbone as a template (its gene sequence is Seq ID No.2 and its amino acid sequence is Seq ID No.1), through the combination of site-directed mutation and random mutation, according to the existing literature (Chao G, et al ., NatProtoc., 2006), constructing a yeast display library.
Embodiment 2
[0028] Embodiment 2: flow cytometry sorting
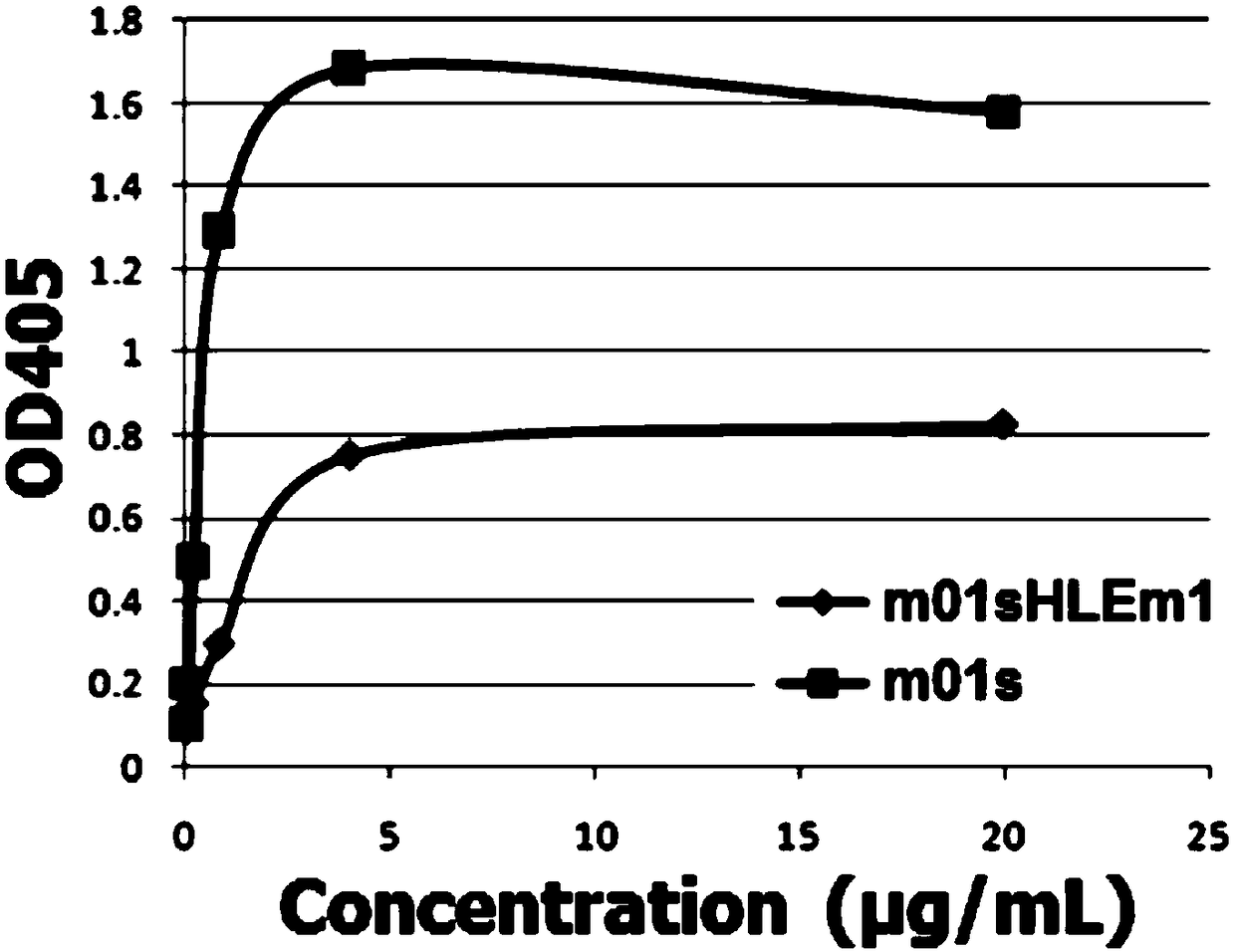
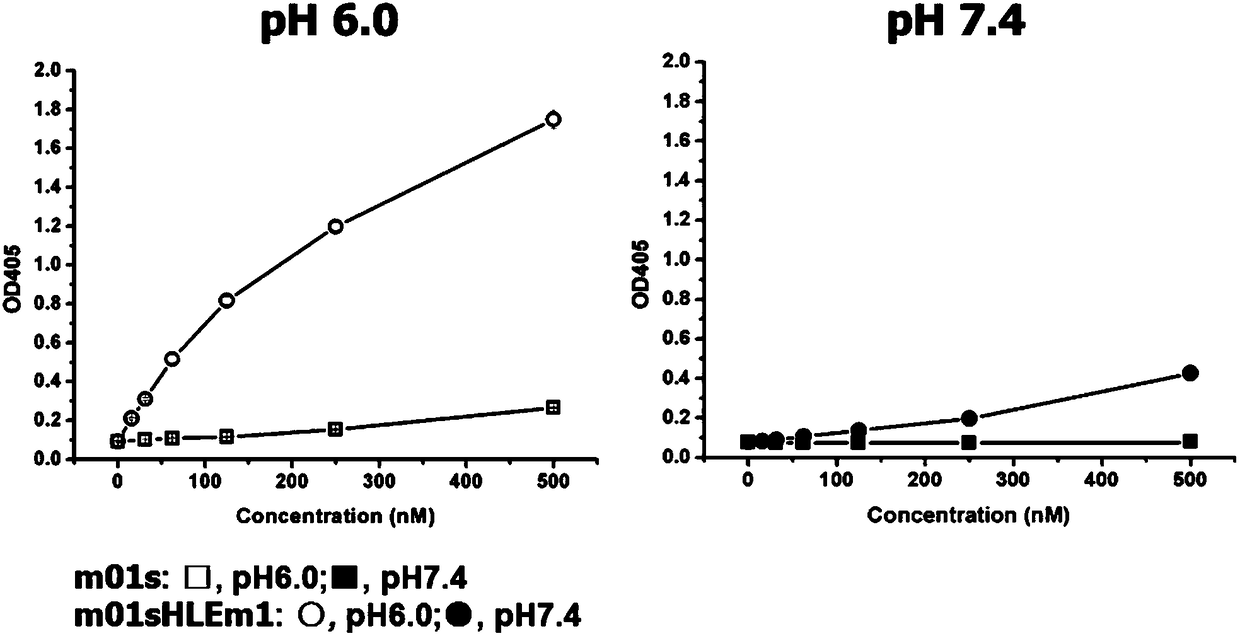
[0029] With the soluble expressed recombinant human FcRn protein as the target, the yeast cells that can bind to FcRn are sorted out by flow cytometry, and the next round of flow sorting is carried out after expanding culture, and the final four rounds of screening Finally, the polyclonal yeast was analyzed by flow cytometry, the enriched clones were further identified, and a mutant was identified, which was named m01sHLEm1.
[0030] The amino acid sequence of m01sHLEm1 is Seq ID No.3, and the gene sequence is Seq ID No.4.
Embodiment 3
[0031] Example 3: Prokaryotic expression and purification of m01sHLEm1 skeleton protein
[0032] The enriched clones were amplified by PCR to obtain the target fragment, that is, the base sequence of m01sHLEm1, and then the target fragment and the pCom empty load were digested by the restriction endonuclease sfi I, and the digested target fragment and the empty load were recovered. , under the action of T4 ligase, ligate the target fragment with empty load overnight at 16°C.
[0033] After the clone to be expressed was constructed, the recombinant plasmid was transferred to Escherichia coli HB2151 competent cells, and SB medium was used for protein expression.
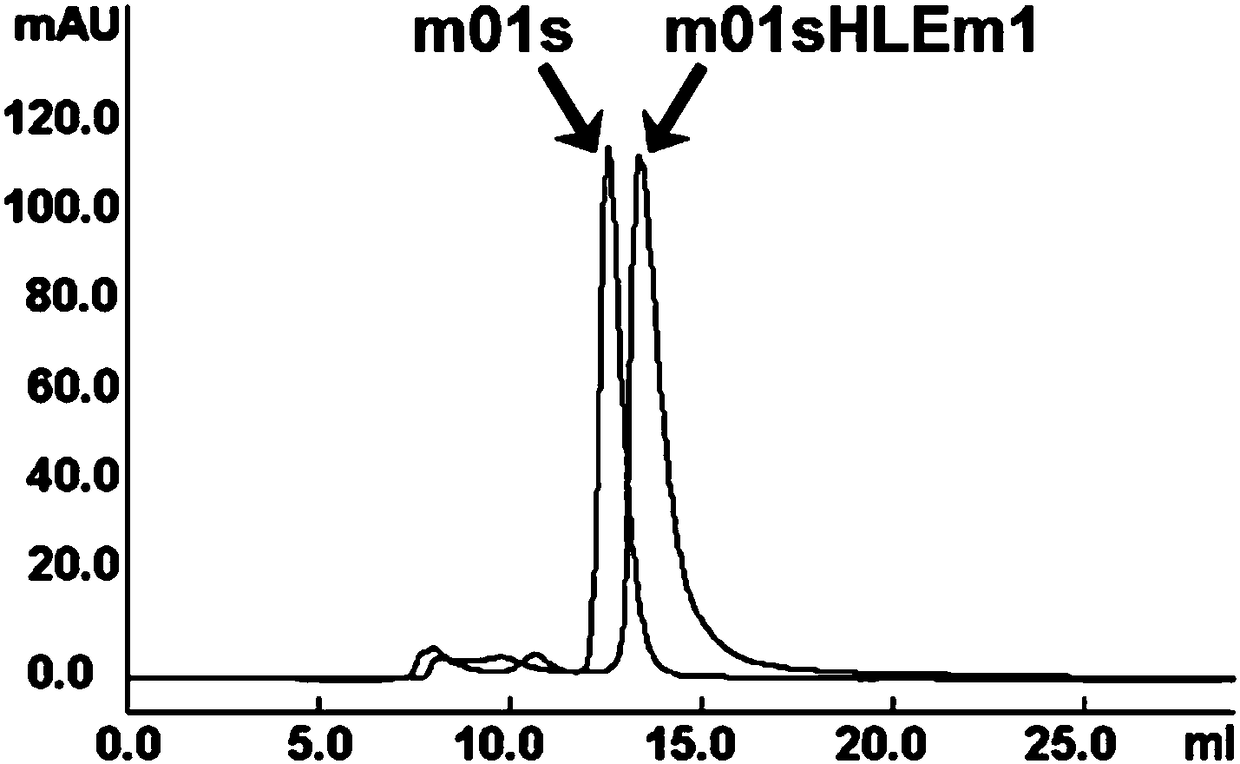
[0034] The prokaryotic expressed m01sHLEm1 protein was purified by nickel column, the eluate of different imidazoles was detected by SDS-PAGE, and the m01sHLEm1 protein eluate was concentrated by ultrafiltration and exchanged according to the results of Coomassie brilliant blue staining.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com