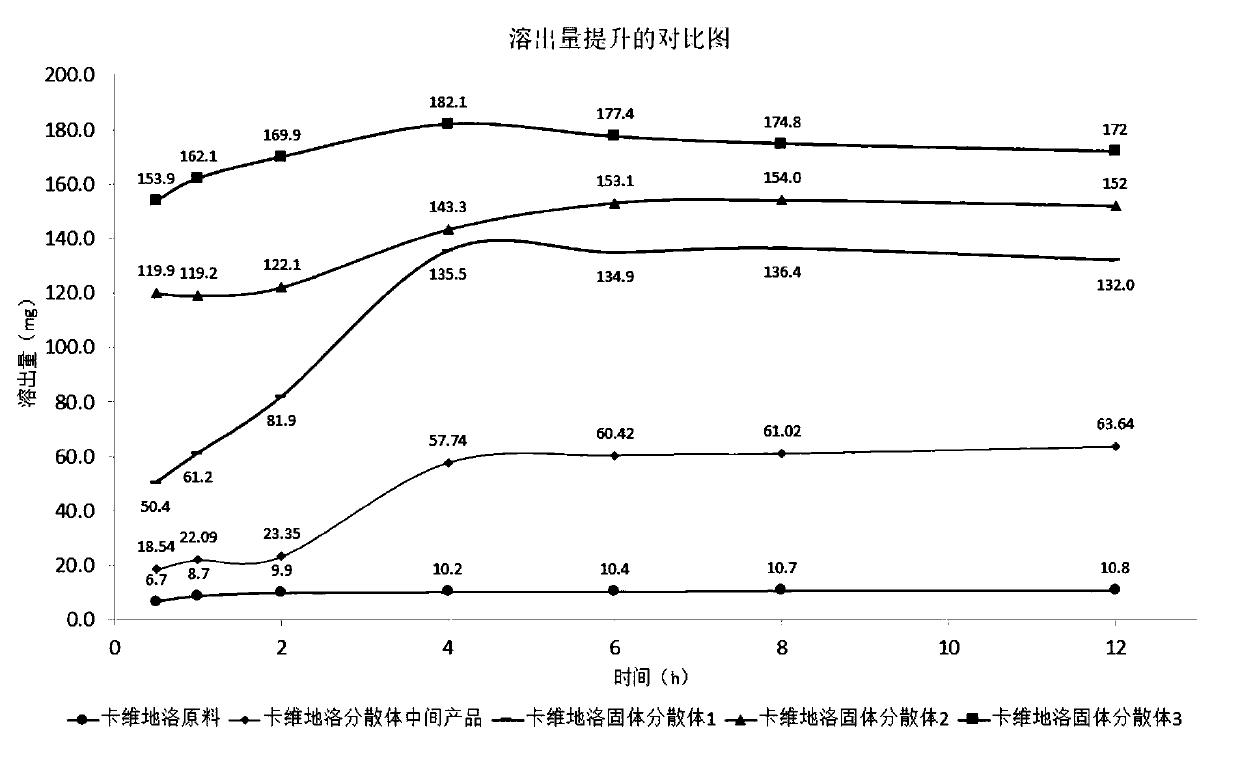
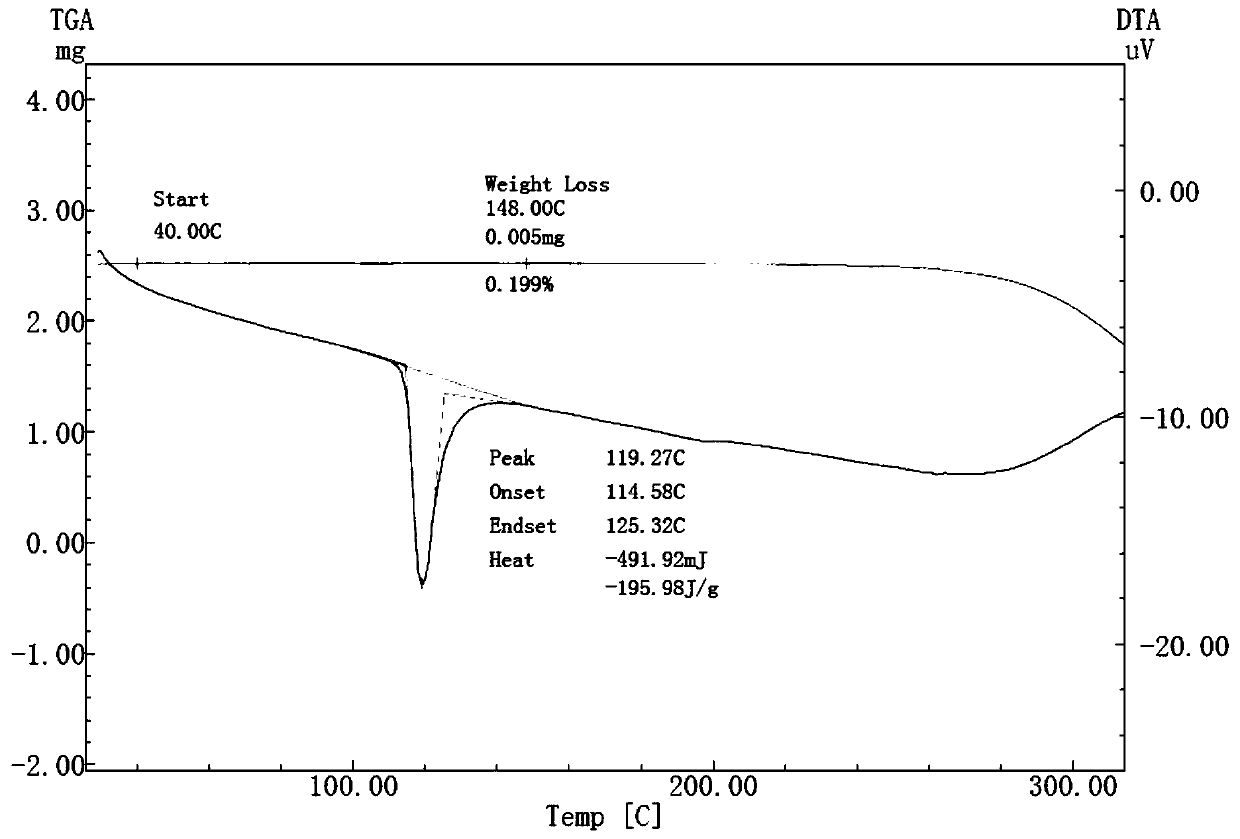
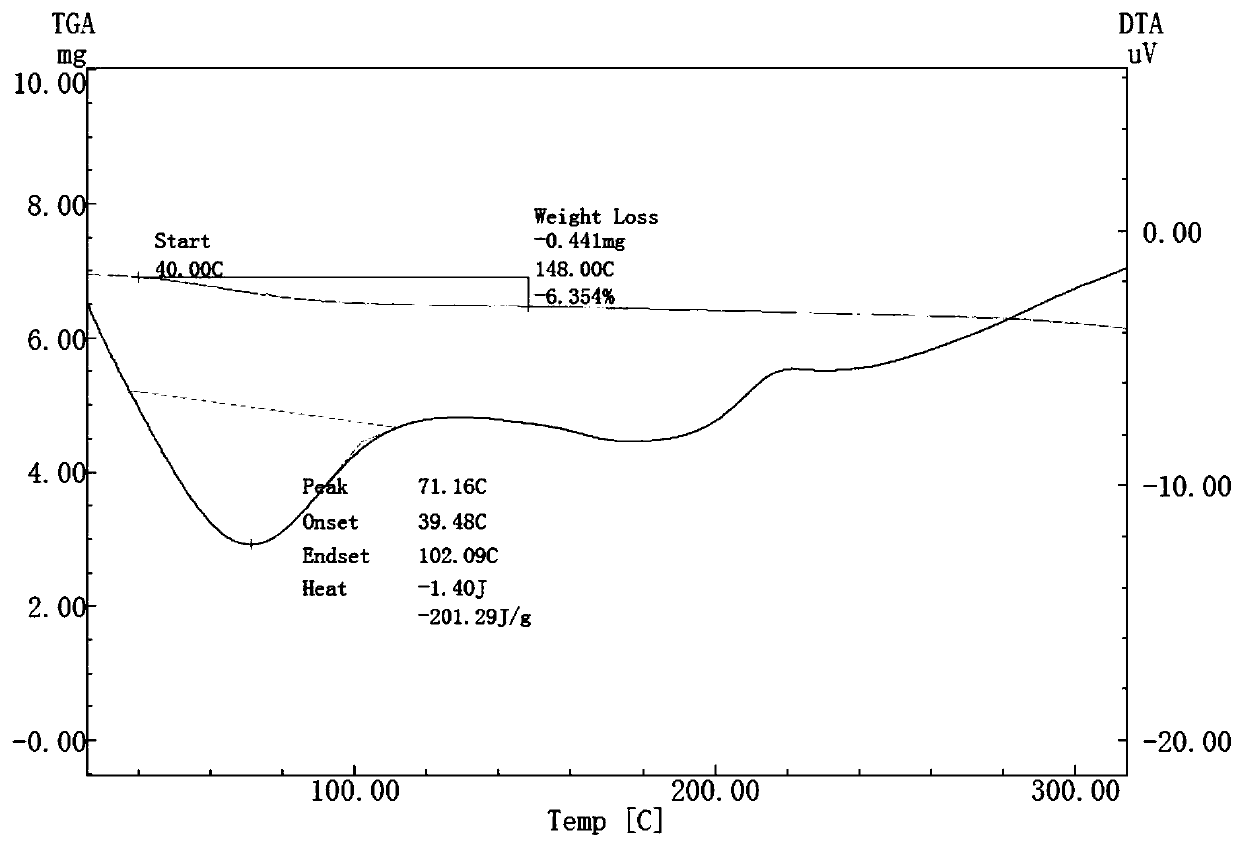
Solid dispersoid of poorly soluble drug CVD (carvedilol), preparation method and application
A technology for solid dispersion and insoluble drugs, applied in the field of medicine, can solve the problems of long grinding cycle, limited solubility and bioavailability of carvedilol, poor solubility, etc., and achieves improved solubility and dissolution rate, good long-term storage stability properties, improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1) Preparation of carvedilol solid dispersion intermediate product: add 10g of carvedilol and 2.78g of phosphoric acid to 50ml of 60% ethanol solution at 80°C, stir to obtain a clear liquid, then cool to room temperature, and use IKA T18 to homogenize Disperse with an instrument to control the particle size from 10 μm to 50 μm; vacuum dry to remove the solvent to obtain the co-precipitate, and sieve with 40 mesh to obtain the intermediate product of the dispersion (carvedilol + protonating reagent).
[0050] 2) Preparation of Carvedilol Solid Dispersion 1: Dissolve 20 g of Povidone K30 in 100 ml of 60% ethanol solution, stir to make the dissolution clear;
[0051] Add 10g of carvedilol and 2.78g of phosphoric acid to 50ml of 60% ethanol solution at 80°C, and stir to obtain a clear liquid; then add the above-mentioned povidone K30 solution, stir, then cool to room temperature, and use an IKA T18 homogenizer to carry out Disperse and control the particle size to 10 μm to ...
Embodiment 2
[0059] Dissolve 100g of povidone K30 in 500ml of 50% ethanol solution, stir to make the solution clear; take 2g of crospovidone and disperse it in 20ml of 50% ethanol solution, stir and suspend evenly;
[0060] Add 10g of carvedilol and 2.78g of phosphoric acid into 50ml of 50% ethanol solution at 70°C, stir to obtain a clear liquid, then add 2g of polyoxyethylene hydrogenated castor oil RH40, then add the above-mentioned povidone K30 solution, keep stirring Clarify, then cool to room temperature; then add crospovidone suspension to obtain a suspension. IKA T18 homogenizer was used for dispersion, and the particle size was controlled to be 10 μm to 50 μm; the solvent was removed by fluidized bed spray drying to obtain co-precipitates, and carvedilol solid dispersion was obtained by sieving with 80 mesh.
Embodiment 3
[0062] Dissolve 2.5g of povidone K30 in 15ml of 60% methanol solution, stir to make the solution clear; take 20g of crospovidone and disperse it in 180ml of 60% methanol solution, stir and suspend evenly;
[0063] Add 10g of carvedilol and 2.78g of phosphoric acid to 50ml of 60% methanol solution at 70°C, stir to obtain a clear solution, then add 40g of polyoxyethylene hydrogenated castor oil RH40, then add the above-mentioned povidone K30 solution, and keep stirring Clarify, then cool to room temperature; then add crospovidone suspension to obtain a suspension. The dispersion is carried out by mechanical stirring and shearing, and the particle size is controlled to be 10 μm to 50 μm; the solvent is removed by rotary evaporation to obtain a co-precipitate, and the carvedilol solid dispersion is obtained after jet milling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com