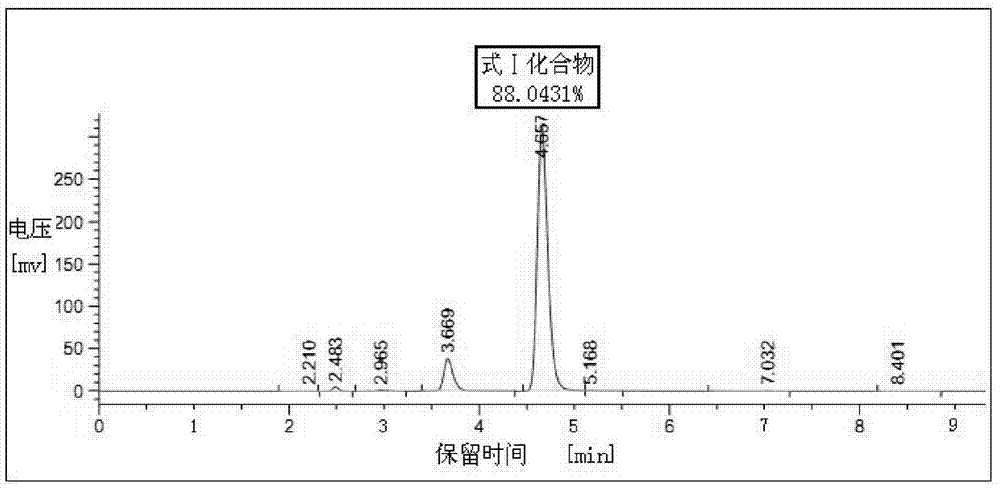
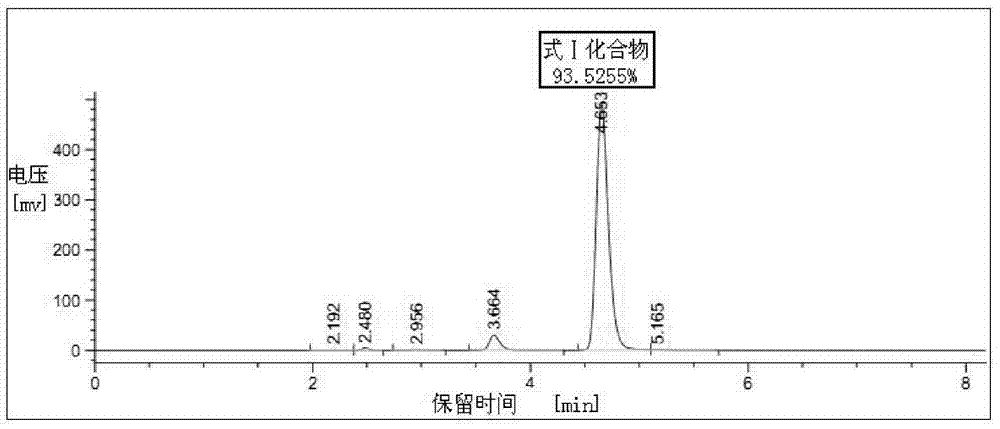
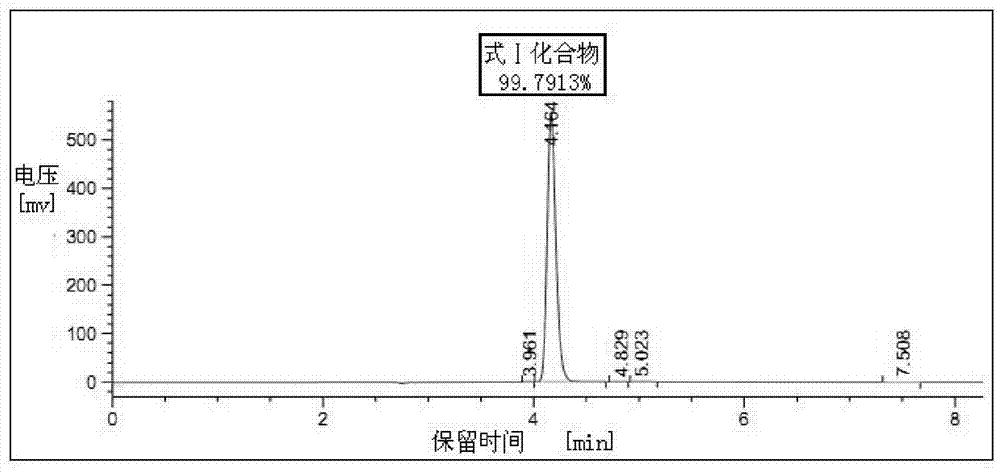
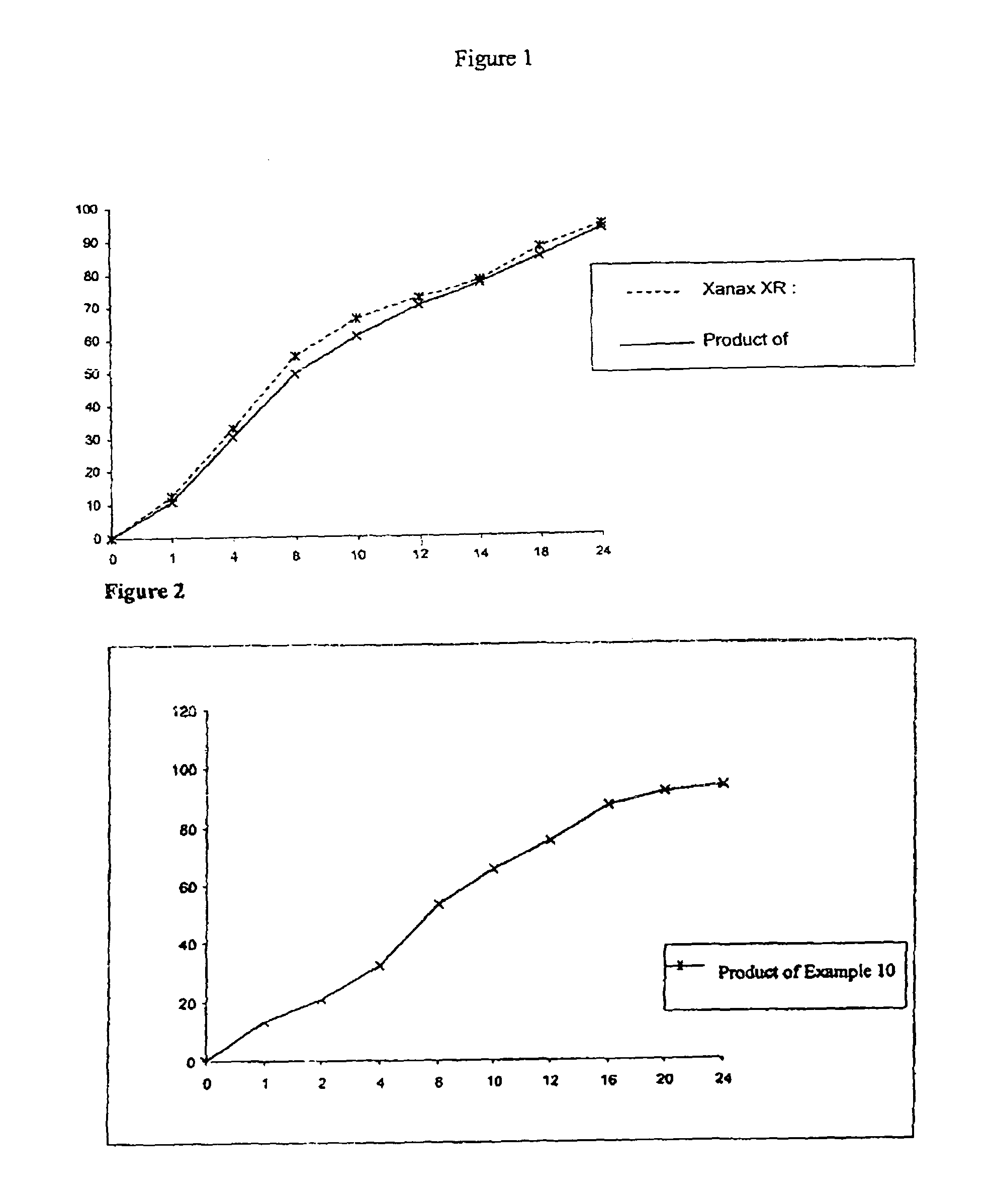
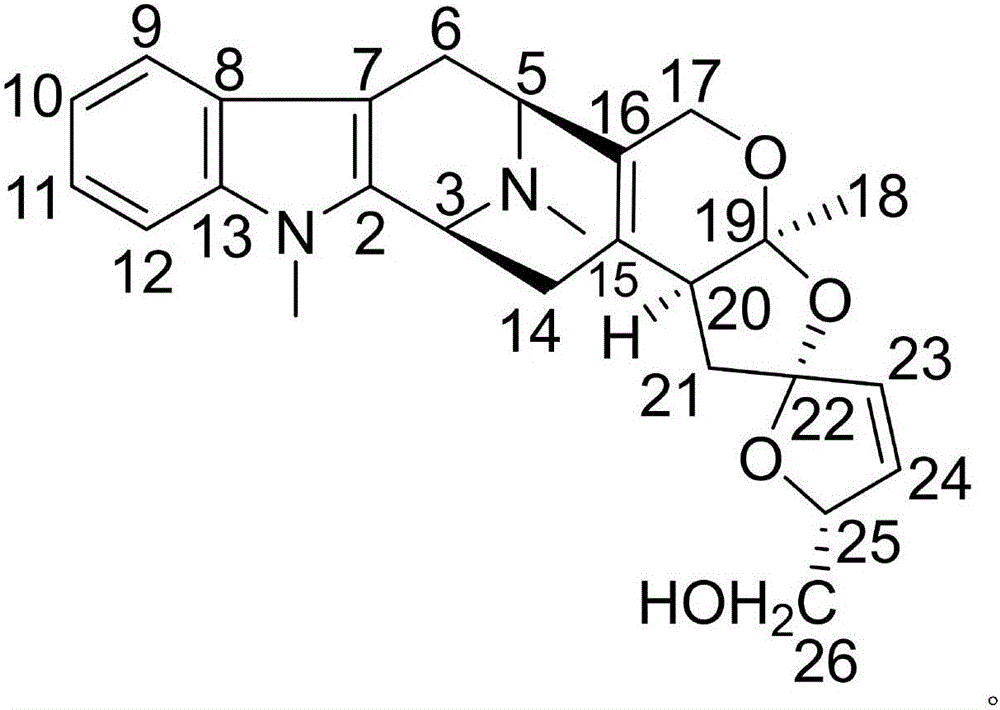
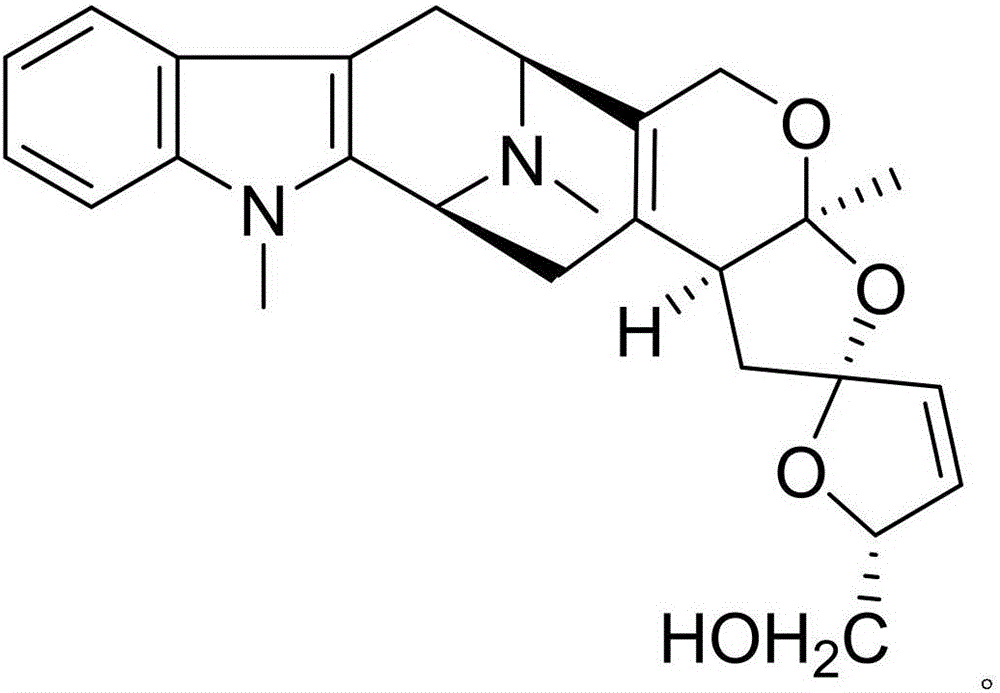
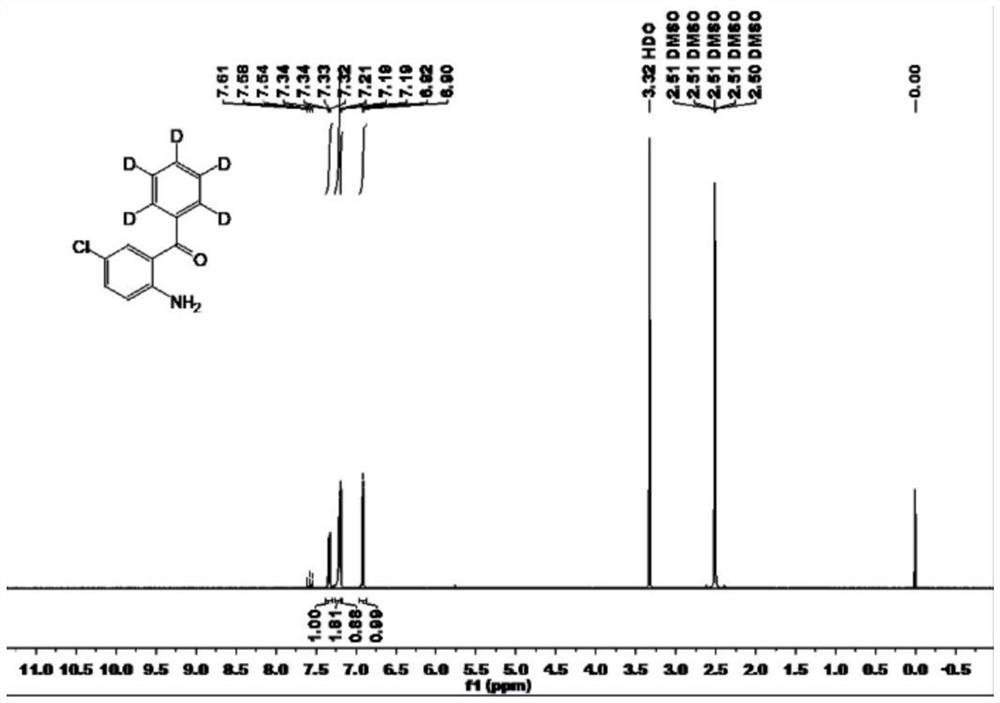
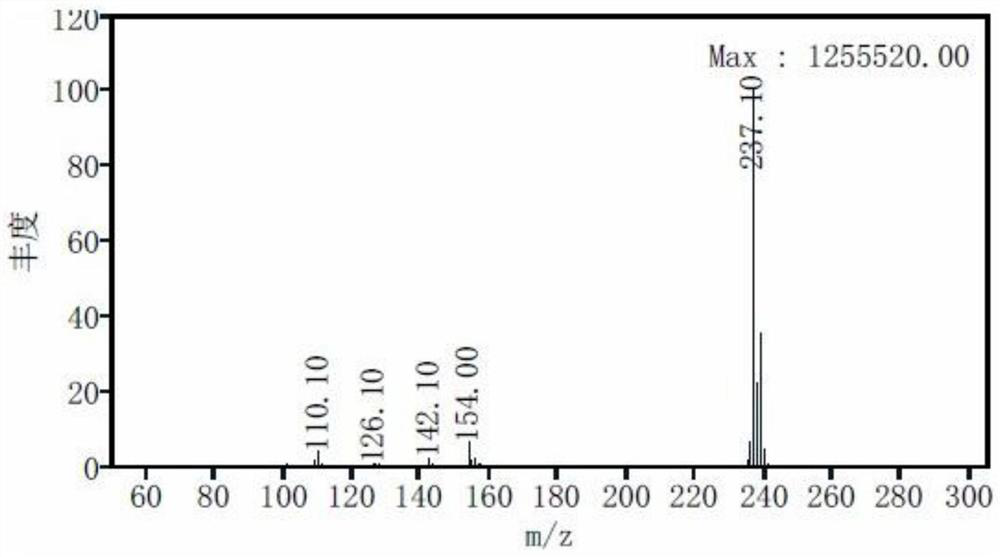
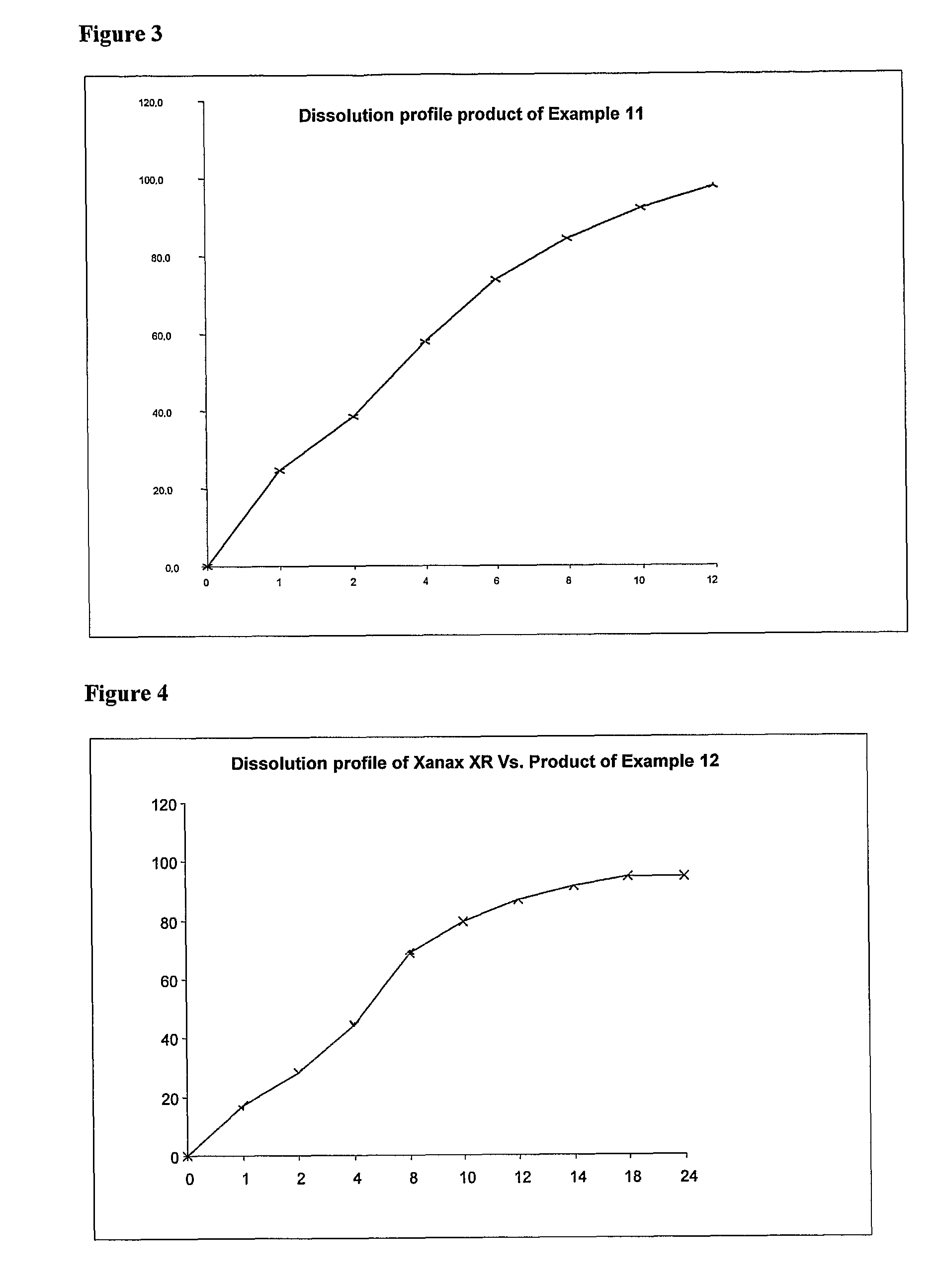Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
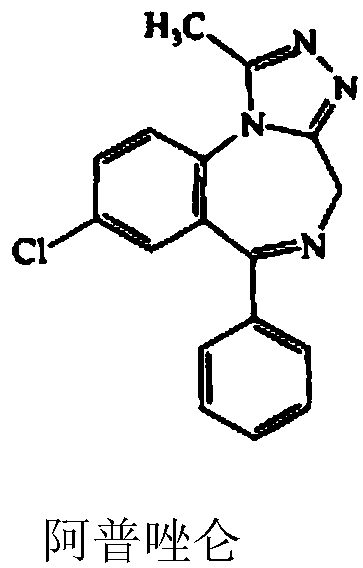
52 results about "Alprazolam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alprazolam is used to treat anxiety and panic disorders.
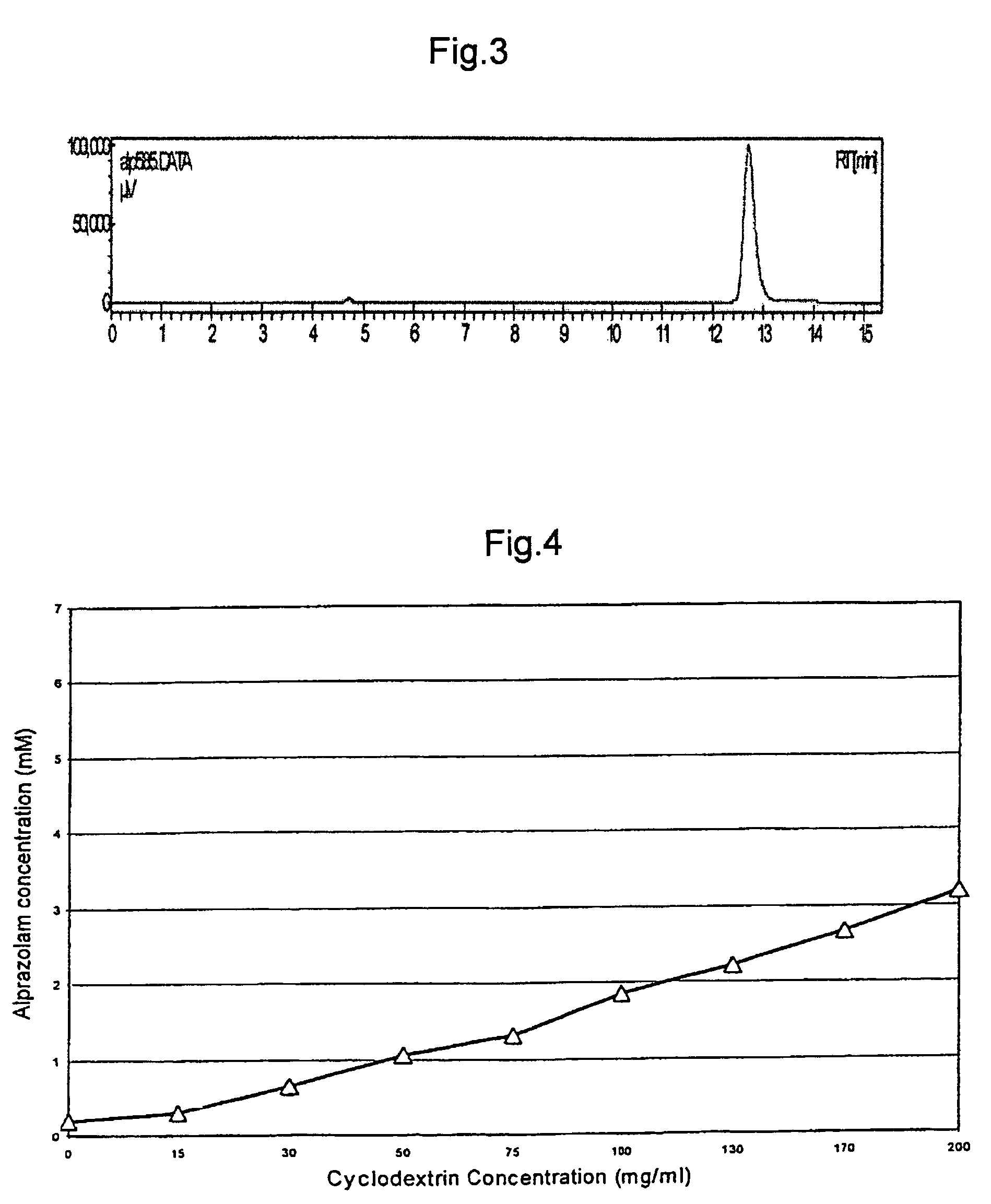
Alprazolam inclusion complexes and pharmaceutical compositions thereof
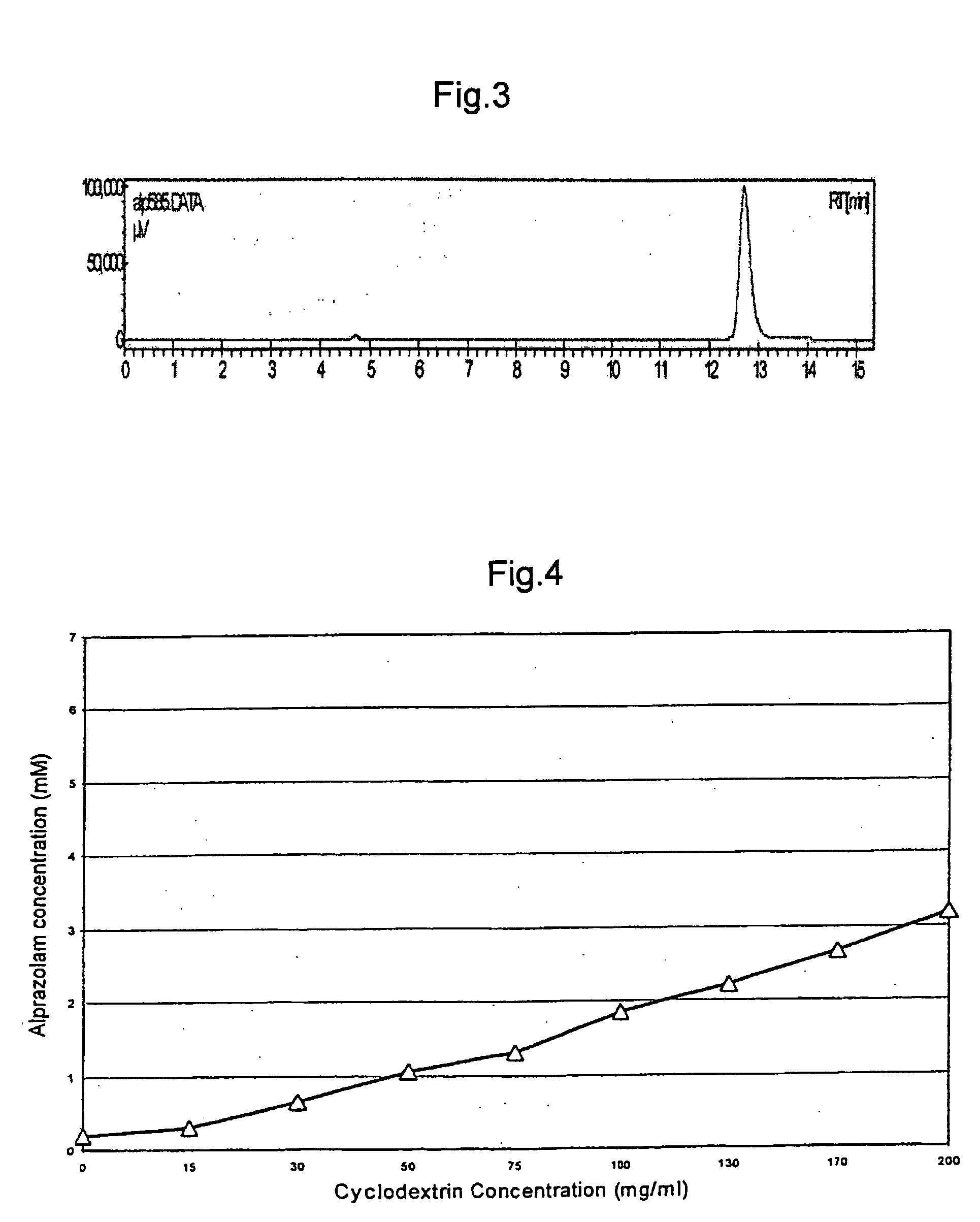
A pharmaceutical composition an inclusion complex and methods for treating patients and preparing said complex disclosed for transmucosal delivery comprising an inclusion complex of (a) alprazolam and (b) a water soluble 2-hydroxypropyl-beta-cyclodextrin, and a pharmaceutically acceptable carrier therefor, wherein all the alprazolam is present in ring-closed form.
Owner:FARMARC NEDERLAND
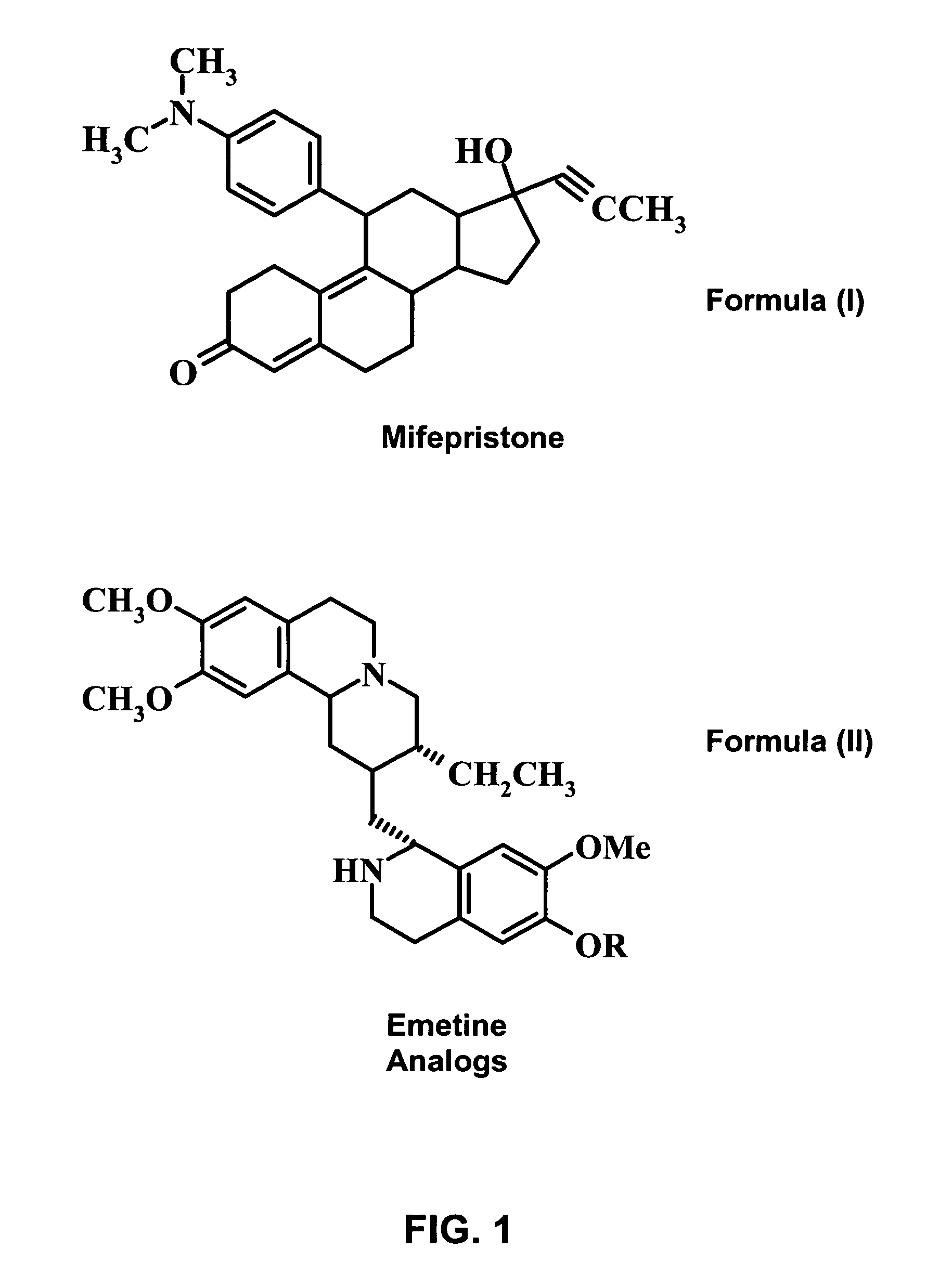
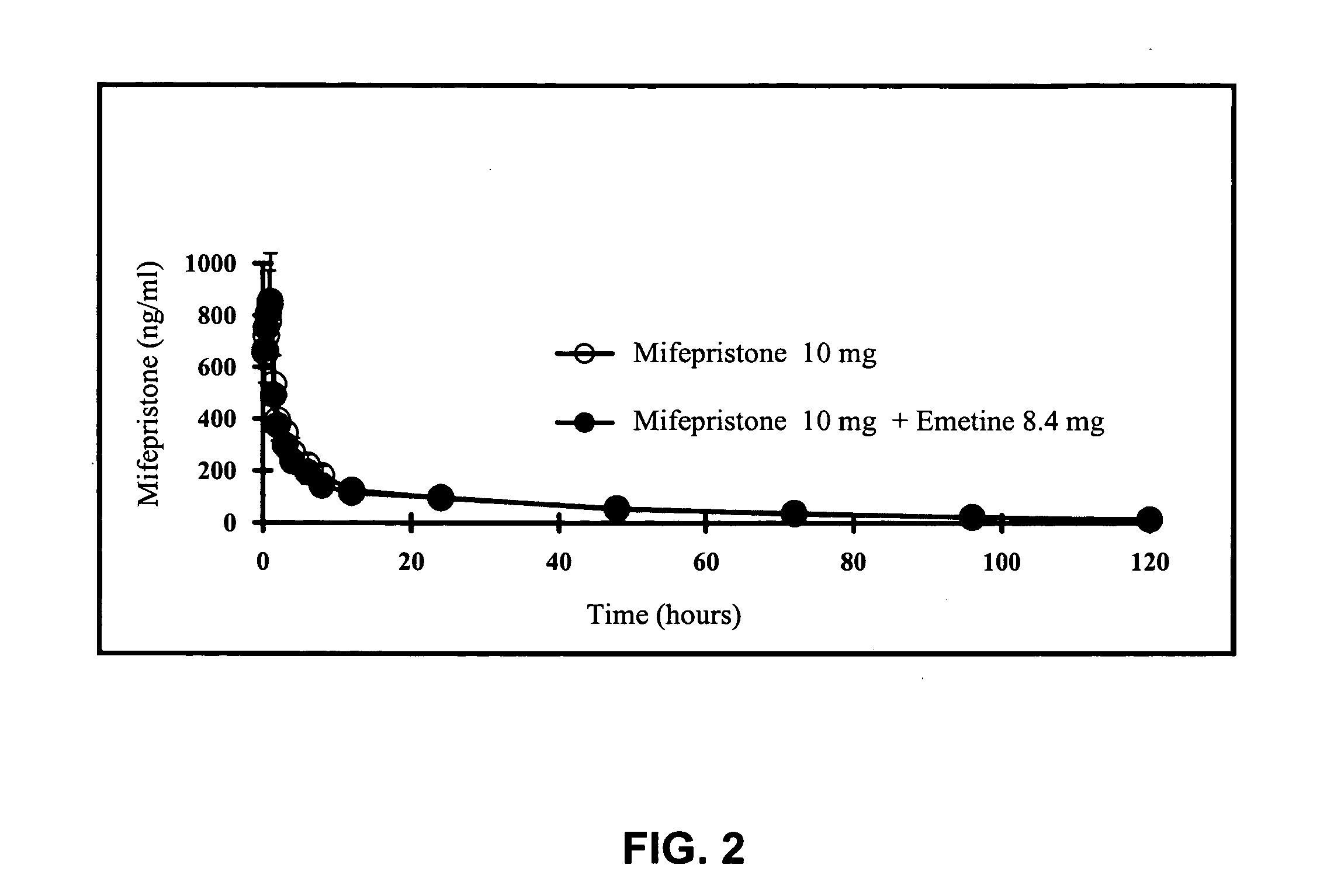
Methods and compositions for improving drug safety
A pharmaceutical composition with improved safety includes a selected amount of a vomit-inducing agent, wherein the selected amount is less than an amount needed to induce vomit in a user; and a therapeutic agent. The therapeutic agent may be selected from a sleeping pill, an anxiolytic, a hypnotic, a contraceptive agent. The therapeutic agent may also be selected from diazepam, flunitrazepam, alprazolam, triazolam, fludiazepam, midazolam, estazolam, zopiclone, and a combination thereof. The vomit-inducing agent may be selected from emetine, cephaeline, and a combination thereof.
Owner:LOTUS PHARMA CO LTD
Buccal, polar and non-polar spray containing alprazolam
Buccal aerosol sprays or capsules using polar and non-polar solvents have now been developed which provide alprazolam for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: aqueous polar solvent, alprazolam, and optional flavoring agent; formulation II: aqueous polar solvent, alprazolam, optionally flavoring agent, and propellant; formulation III: non-polar solvent, alprazolam, and optional flavoring agent; formulation IV: non-polar solvent, alprazolam, optional flavoring agent, and propellant; formulation V: a mixture of a polar solvent and a non-polar solvent, alprazolam, and optional flavoring agent; formulation VI: a mixture of a polar solvent and a non-polar solvent, alprazolam, optional flavoring agent, and propellant.
Owner:DUGGER HARRY A III +1
Pharmaceutical composition based on agonist of benzodiazepine
The present invention describes the use of pharmaceutical compounds in pharmaceutical compositions for sublingual administration, including as active ingredient thereof, an agonist of the central receptor of benzodiazepinics chosen among diazepam, lorazepam, bromazepam, triazolam, alprazolam, flunitrazepam, nitrazepam and midazolam maleate, in a mixture with a pharmaceutical excipient consisting of, at least, 70% of the weight of the final formulation containing 40-45% by weight of lactose, 15-27% by weight of sorbitol and 12-16% by weight of cellulose.
Owner:SIGMA PHARMA LTDA
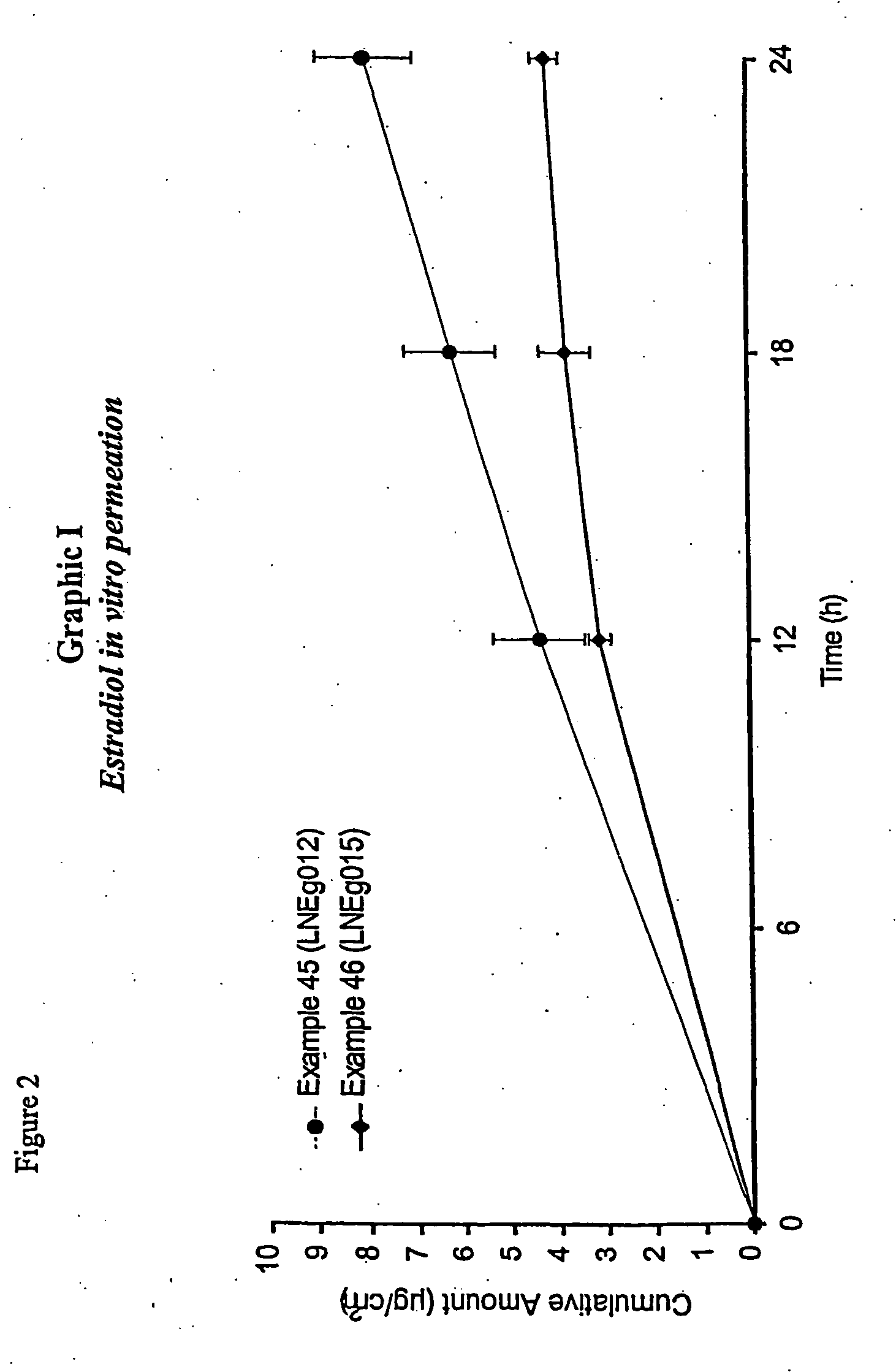
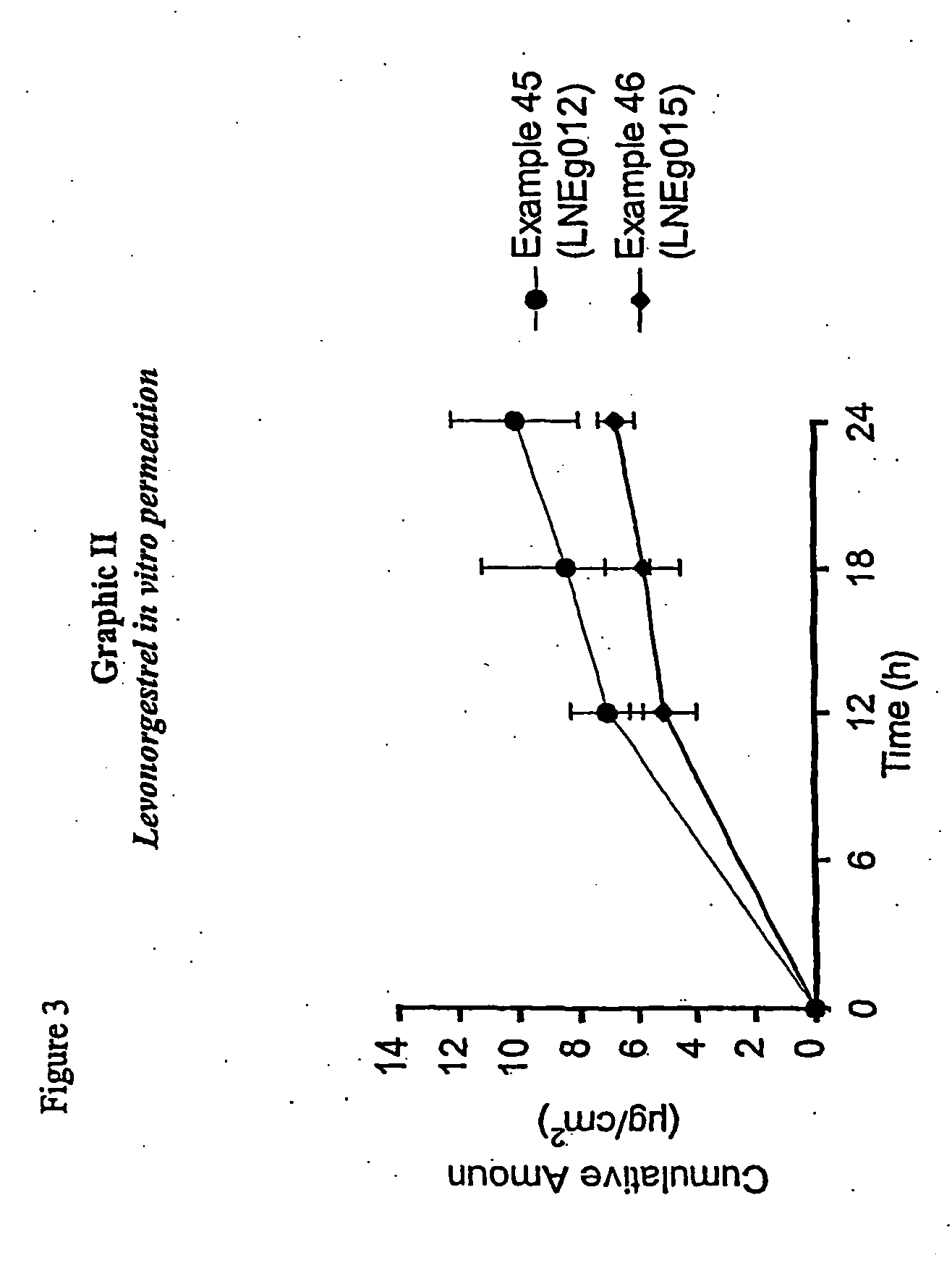
Novel composition for transdermal and/or transmucosal administration of active compounds that ensures adequate therapeutic levels
InactiveUS20070098775A1Easy to usePromote absorptionBiocideAerosol deliveryCompound (substance)Suppository
A pharmaceutical composition in the form of a solution, cream, lotion, spray, ointment, gel, aerosol, tablet, suppository or patch device for transdermal or transmucosal administration of alprazolam to a subject, which includes as a permeation enhancing mixture a fatty component in an amount of 0.1% to 20% by weight which is one of (a) a saturated fatty alcohol of formula CH3—(CH2)n—CH2OH, a saturated fatty acid of formula CH3—(CH2)n—CH2COOH, (b) an unsaturated fatty alcohol of formula CH3(CnH2(n−1))—OH, or (c) a fatty acid of formula CH3(CnH2(n−l))—COOH, wherein n is an integer of between 8 and 22; and a vehicle that includes a C1-C4 alkanol, a polyalcohol, and water.
Owner:ANTARES PHARMA IPL
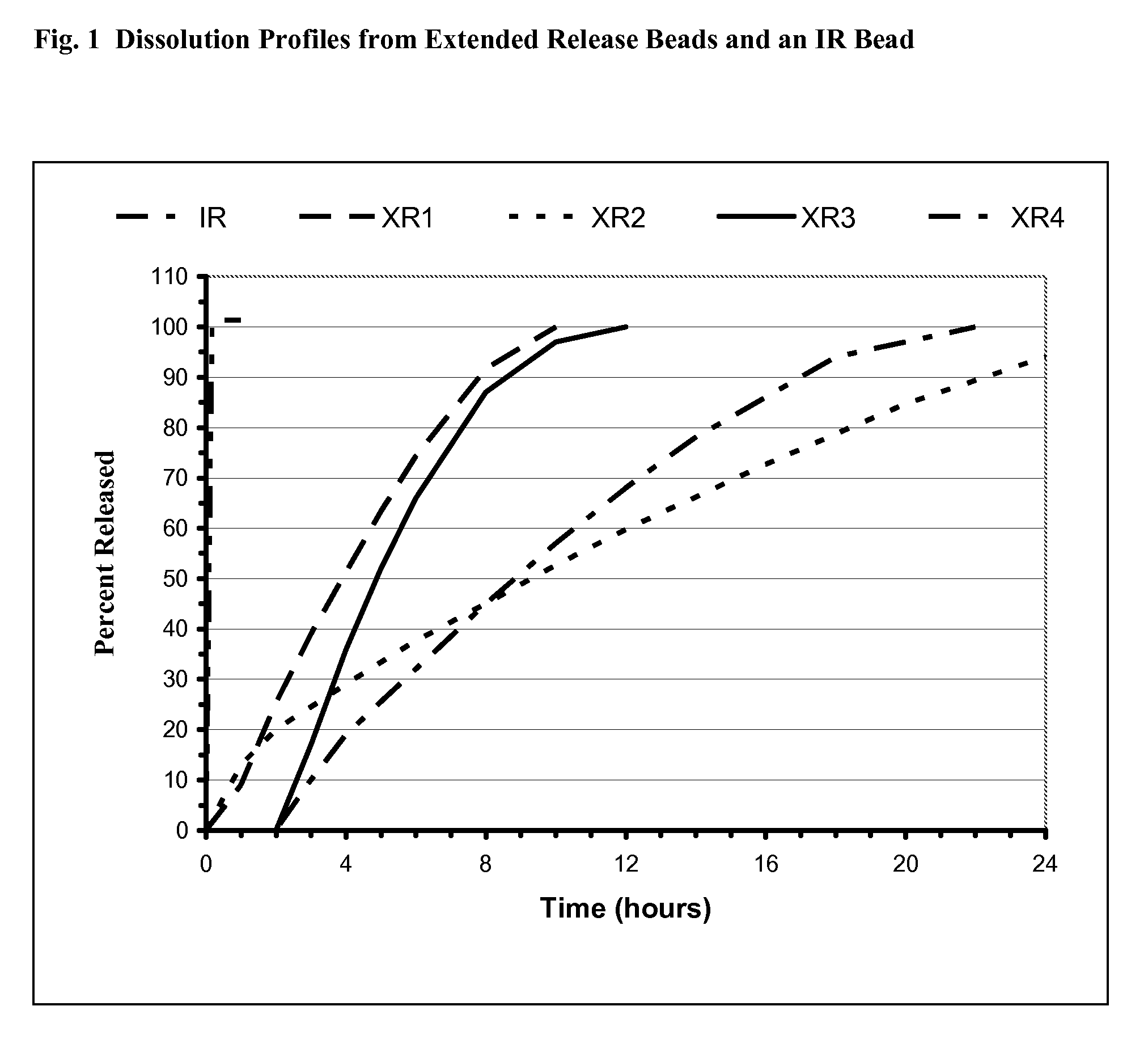
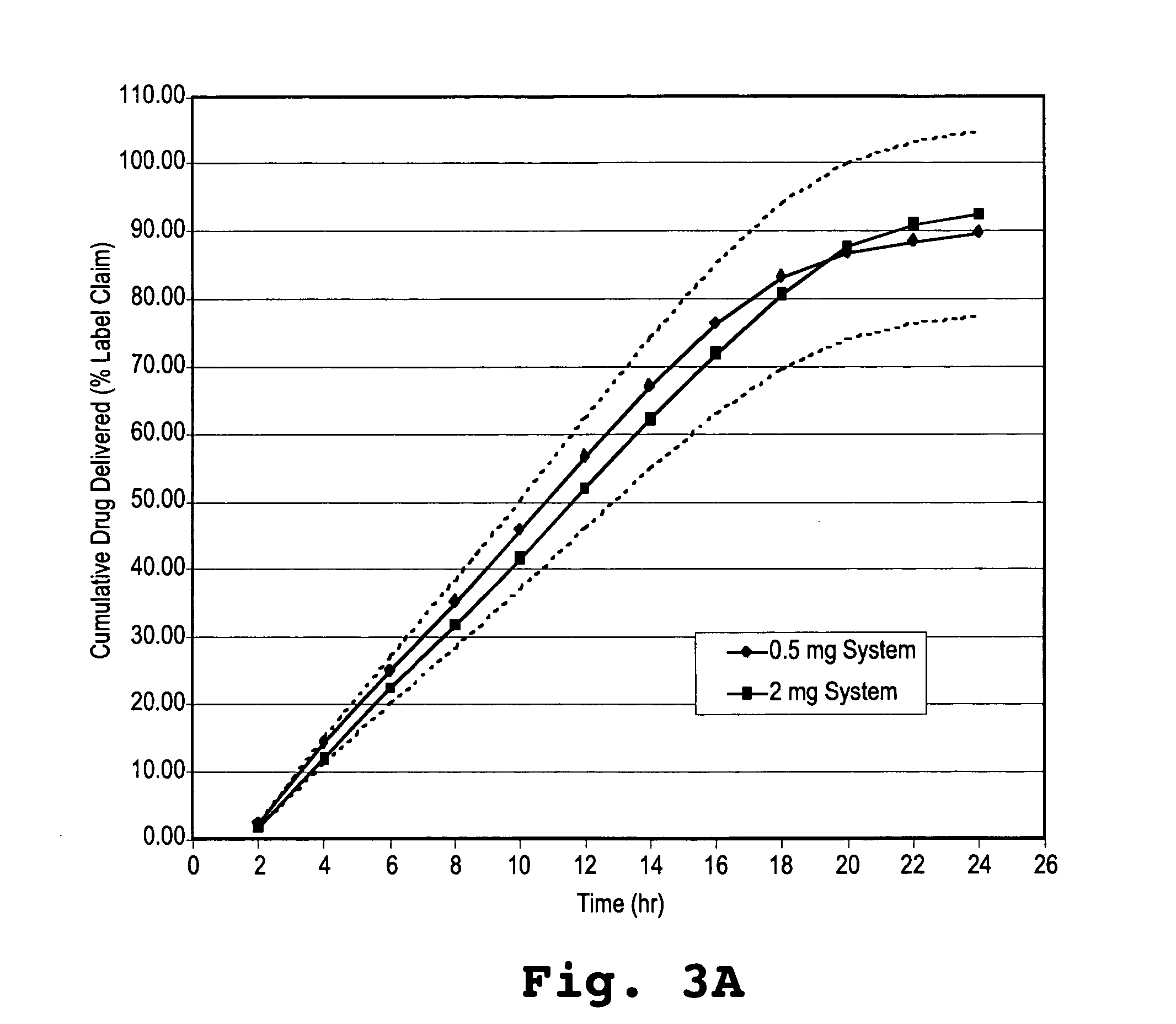
Controlled release formulations of alprazolam
A controlled release formulation of alprazolam for once a day administration to a mammalian subject, which formulation releases alprazolam along a pre-determined release profile, is provided.
Owner:SUPERNUS PHARM INC
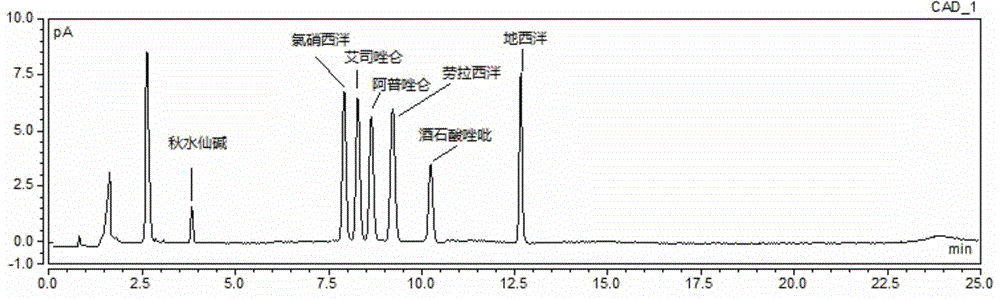
Method for simultaneous determination of contents of 6 kinds of sedative-hypnotic drugs in serum by UHPLC-CAD technology
ActiveCN104133030ASimultaneous measurementShort analysis timeComponent separationSide effectSedative/hypnotic
The invention relates to a method for simultaneous determination of the contents of 6 kinds of sedative-hypnotic drugs in serum by a UHPLC-CAD combined technology, wherein the 6 kinds of sedative-hypnotic drugs are diazepam, lorazepam, alprazolam, estazolam, clonazepam and zolpidem tartrate. The method is characterized by including the following steps: 1) reference substance preparation, 2) internal standard solution preparation, 3) sample solution preparation, 4) mobile phase solution preparation, 5) chromatographic condition setting, 6) electrospray detector condition optimization, 7) sample determination, 8) gradient-concentration reference substance solution preparation, 9) standard curve preparation, and 10) data calculation and analysis. The method is advanced, has good clinical application prospects and effects, can rationally use drugs for clinic, increases drug curative effects, lowers toxic and side effects and drug costs, and plays a positive role.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Medication and treatment for disease
Owner:ALTMAN ENTERPRISES
Kit for determining antianxiety/hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085265AReduce matrix effectThe test result is accurateComponent separationBromazepamTandem mass spectrometry
The invention provides a kit for determining antianxiety / hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry. The kit comprises the following constituents: drug standards comprising bromazepam, clonazepam, diazepam, lorazepam, midazolam, nitrazepam, oxazepam and Temazepam; drug internal standard compounds comprising alprazolam-d5, clonazepam-d4, diazepam-d5, lorazepam-d4, midazolam-d4, nitrazepam-d5, oxazepam-d5 and Temazepam-d5; drug extraction compositions comprising, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropanol solution and 10% of purified water; negative plasma; and diluent: 50% of carbinol water solution. The kit can be used for simultaneously determining antianxiety / hypnotic type drugs and active metabolites thereof, and has the advantages of short determination time and high flux.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Novel pharmaceutical compositions and methods for anxiety, depression and other psychiatric disorders
PendingUS20200323876A1Organic active ingredientsPharmaceutical delivery mechanismSeasonal Affective DisordersCompulsive disorders
Pharmaceutical compositions comprising azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients suffering from one or more psychiatric disorders such as major depressive disorder, generalized anxiety disorder, panic disorder, agitation, social anxiety disorder, mild chronic depression, obsessive-compulsive disorder, premenstrual dysphoric disorder, seasonal affective disorder, dysthymia, childhood enuresis, bipolar disorder, posttraumatic stress disorder, sleep disorder related to anxiety, are also disclosed.
Owner:LA PHARMATECH INC
Novel pharmaceutical compositions and methods for psychiatric symptoms of patients with alzheimer's disease
Pharmaceutical compositions containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients with Alzheimer's disease for symptoms of depression, anxiety, agitation, delusions, hallucination, irritability and sleeping disorder, are also disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for psychiatric symptoms of patients with Alzheimer's disease
Pharmaceutical compositions containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients with Alzheimer's disease for symptoms of depression, anxiety, agitation, delusions, hallucination, irritability and sleeping disorder, are also disclosed.
Owner:LA PHARMATECH INC
Medication and Treatment for Disease
ActiveUS20110217278A1Shorten the progressPromote formationOrganic active ingredientsNervous disorderDiseaseVitamin B12
A treatment is described for diseases with symptoms that can include fatigue, muscle aches and spasms, weakness, demylenation, and nerve pain. Diseases can include fibromyalgia, depression, and auto-immune and immuno-suppressive diseases, such as MS. The treatment comprises about 1-10 mg naltrexone, at least about 20 μg vitamin B12, at least about 5 mg vitamin B6, at least about 2 mg coenzyme Q, and preferably at least one ancillary medication selected from the group consisting of diazepam, cyclcobenzaprine, clonazepam, alprazolam, 9-tetrahydrocannibinol, fumarate, caffeine, and combinations thereof. The treatment can be administered orally, and can decrease mental and physical symptoms such as, for example, fatigue, gait problems, visual dysfunction, and pain while improving cognitive skills.
Owner:ALTMAN ENTERPRISES
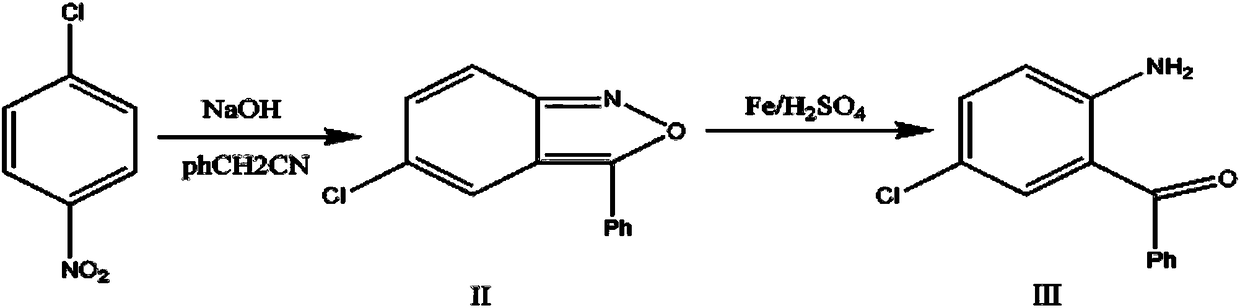
Preparation method of alprazolam intermediate
InactiveCN108250091AInhibition of dissolutionReduce pollutionOrganic chemistryOrganic compound preparationSodium hydroxideMethanol
The invention discloses a preparation method of an alprazolam intermediate. The method comprises the following steps: sequentially adding methanol and p-nitrochlorobenzene in a sodium hydroxide watersolution, dropwise adding phenylacetonitrile under stirring, reacting at 40 + / -5 DEG C after the dropwise addition is finished, and carrying out post-treatment to obtain an intermediate (II) ; and dissolving the intermediate (II) in ethanol, adding iron powder, refluxing, dropwise adding sulfuric acid, refluxing for 1 hour, and carrying out post-treatment to obtain an intermediate(III). Accordingto the method disclosed by the invention, the sodium hydroxide water solution is used in the synthesis of the intermediate (II) to replace a dangerous mode of refluxing and dissolving solid sodium hydroxide by using ethanol, a small amount of methanol is added into the reaction system, and the problem of two-phase reaction is successfully solved. The sulfuric acid is used for replacing volatile hydrochloric acid during the synthesis of the intermediate (III), the reaction time is effectively shortened, and the reaction efficiency is improved. The method disclosed by the invention is safer to operate, short in reaction time and more suitable for industrial production.
Owner:山东安信制药有限公司
Novel pharmaceutical compositions and methods for menopause related anxiety and depression
InactiveUS20210069209A1Lower estrogen levelsNervous disorderPharmaceutical delivery mechanismDuring menopauseAzelastine
Pharmaceutical compositions comprising azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating perimenopausal or menopausal patients, such as patients suffering from, experiencing, exhibiting and / or having one or more symptoms of anxiety or depression, are also disclosed.
Owner:LA PHARMATECH INC
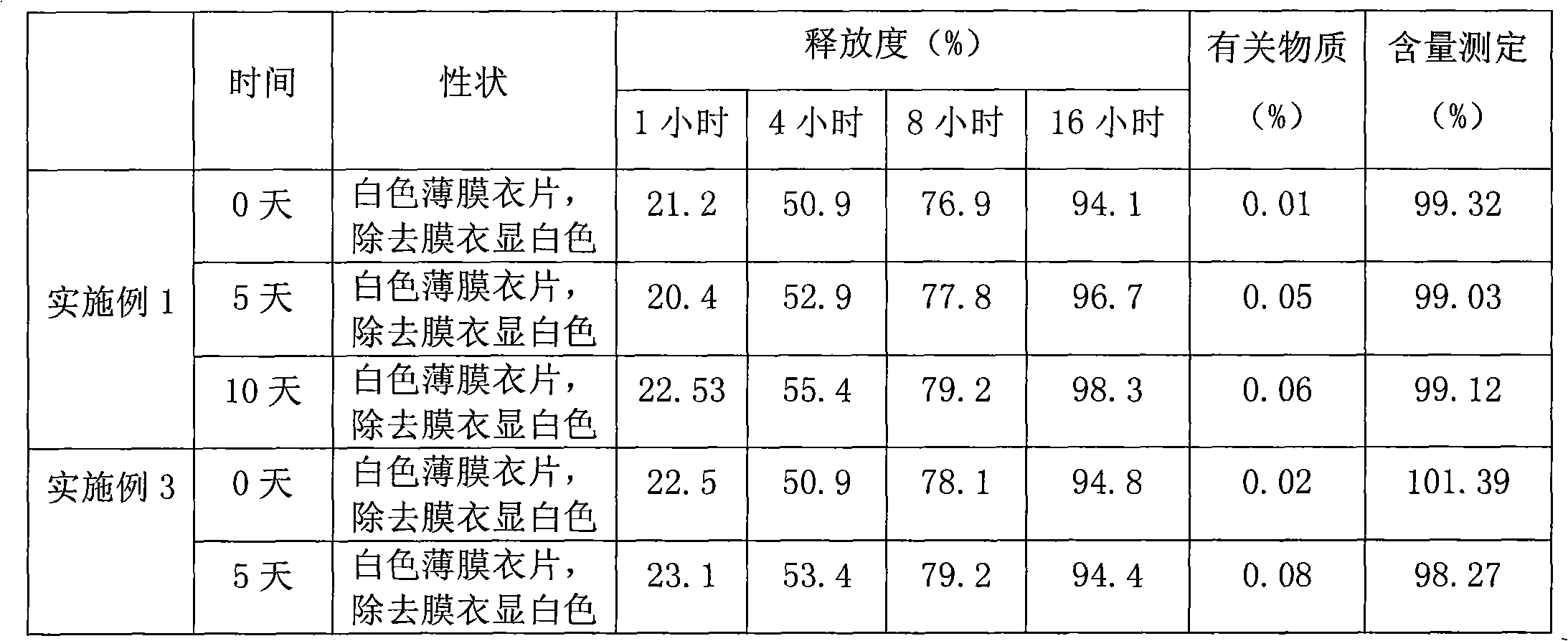
Alprazolam sustained-release preparation and preparation method thereof
InactiveCN102106839AHigh peak blood concentration fluctuationsStable drug releaseOrganic active ingredientsNervous disorderSide effectWhole body
The invention relates to alprazolam sustained-release preparation and the preparation method thereof. The alprazolam sustained-release preparation is characterized in that alprazolam, framework material, filling agent, lubricating agent and film-forming material are mainly included. The invention belongs to the technical field of medicinal preparations and aims at providing the alprazolam sustained-release preparation with favorable compliance, small side effects, durable drug effect and stable curative effect and the preparation method of the alprazolam sustained-release preparation. The alprazolam sustained-release preparation stably and stably releases the drug, avoids the phenomenon that the peak valley is easy to generate when the general preparation releases the drug, has more durable drug effect, stably exists in the blood for 24 hours and can be widely distributed on the whole body; and the alprazolam sustained-release preparation has the characteristics of high bioavailability, low toxic or side effects, stable curative effect, low administration frequency, long administration time, and the like and is convenient for administration; and the abuse is avoided and the alprazolam sustained-release preparation is easily stopped administrating.
Owner:COSCI MED TECH CO LTD
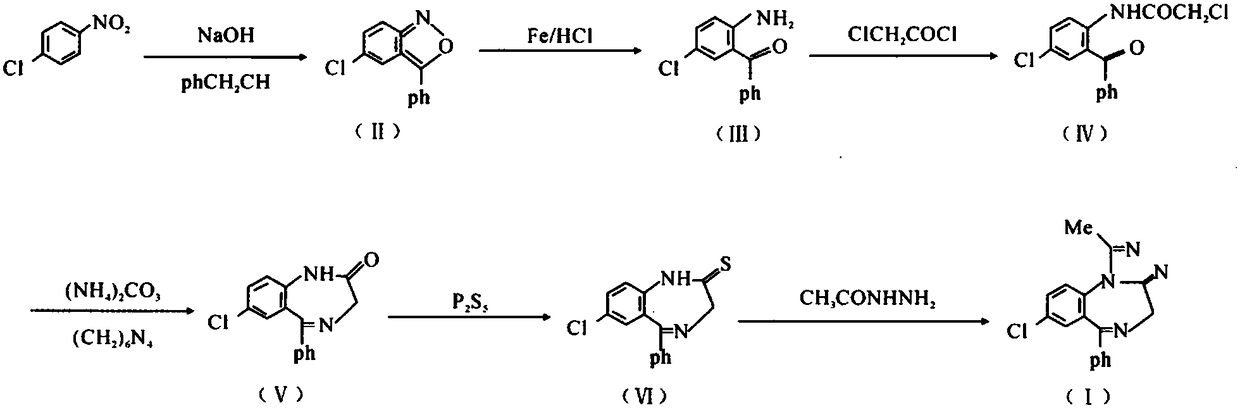
Method for preparing high-purity 7-chloro-5-phenyl-benzodiazepine-2-one
The invention relates to a method for preparing a high-purity important intermediate of alprazolam, namely 7-chloro-5-phenyl-benzodiazepine-2-one. The method comprises the following steps: after a ring-closure reaction taking 2-chloroacetylamino-5-chloro-diphenyl ketone and hexamethylenetetramine as main raw materials, performing vacuum concentration to dryness, and directly adding a refining solvent for clear dissolution; then, washing and layering, and dropwise adding dilute acid for salifying; adding alkaline water into the separated salt in purified water, and freeing to obtain 7-chloro-5-phenyl-benzodiazepine-2-one of which the HPLC (high performance liquid chromatography) purity is not lower than 99.0%. In the method, the end point of the concentration operation after complete reaction is easy to control, the HPLC purity of the product is remarkably increased, and the interference of unavoidable impurities on later preparation of alprazolam is avoided.
Owner:HUAZHONG PHARMA
Sustained release formulation of alprazolam
The present invention is directed towards the preparation of extended release Alprazolam formulation. The formulation thus obtained provides an efficient mode of delivery of Alprazolam in a continuous manner.
Owner:EMCURE PHARAMACEUTICALS LTD
Alprazolam inclusion complexes and pharmaceutical composition thereof
A pharmaceutical composition an inclusion complex and methods for treating patients and preparing said complex disclosed for transmucosal delivery comprising an inclusion complex of (a) alprazolam and (b) a water soluble 2-hydroxypropyl-beta-cyclodextrin, and a pharmaceutically acceptable carrier therefor, wherein all the alprazolam is present in ring-closed form.
Owner:FARMARC NEDERLAND
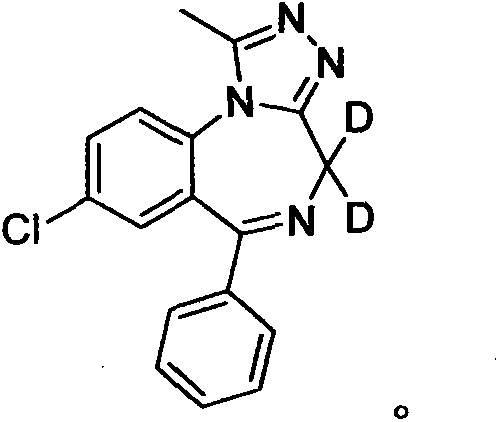
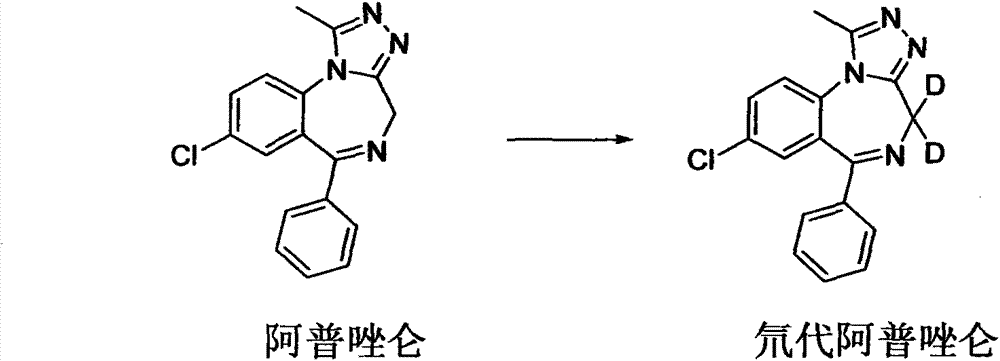
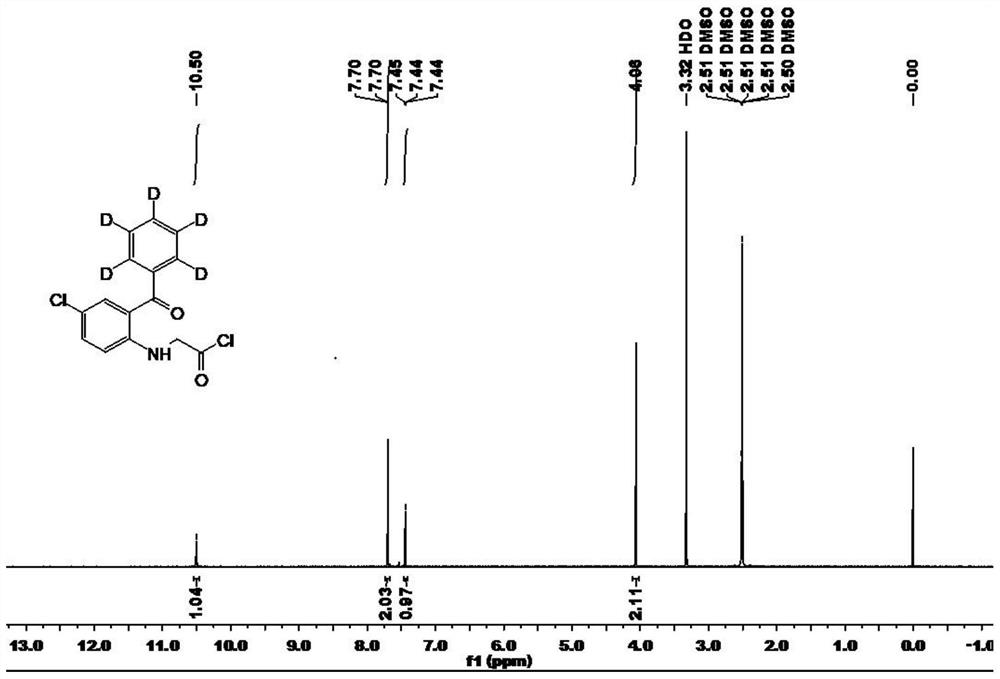
Deuterated alprazolam and preparation method thereof
InactiveCN103204856ASimple reaction conditionsEasy to purifyOrganic chemistryDeuterated chloroformPotassium carbonate
The invention discloses deuterated alprazolam and a preparation method thereof. The preparation method of deuterated alprazolam comprises the following steps of: (1) mixing commercially available non-deuterated alprazolam and N,N-dimethyl formamide, and stirring; (2) adding potassium carbonate and deuterated chloroform, heating the mixture to more than 40 DEG C and stirring; and (3) carrying out separation to obtain the deuterated alprazolam. The preparation method provided by the invention is short, simple and convenient to operate, low in cost and easy to purify. The commercially available non-deuterated alprazolam uses a small amount of deuterated reagents as deuterium sources in a non-deuterated solvent atmosphere, so that deuterated alprazolam is obtained in a relatively short period of time, and through simple column chromatography purification, a purified product is purified. The deuterated alprazolam standard product prepared according to the invention is high in purity and stable in chemical property; and the preparation of the standard product for analysis is convenient. The preparation method provided by the invention can be used for producing deuterated internal standard substance used for analyzing and detecting alprazolam.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Taste masking system for alprazolam
InactiveUS20060147516A1Eliminate needDisintegrates quicklyPowder deliveryOrganic active ingredientsAlprazolamSolvent
The present invention relates to taste masking system, taste masked formulations, dosage forms made from those formulations and methods of making those formulations that involve dissolving or dispersing a pH dependant polymer and alprazolam in a solvent, granulating using that material or forming layers over a solid support therewith. This can be followed with the use of an overcoating layer.
Owner:CIMA LABS
Method of treating epilepsy
Alprazolam formulated as an inhaled condensation aerosol and method for treating epilepsy and / or seizures.
Owner:ALEXZA PHARMA INC
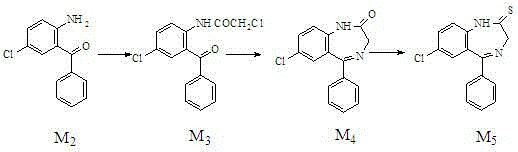
Preparation method of benzodiazothioketone serving as intermediate of alprazolam
ActiveCN104130201AReduce pollutionFew synthetic stepsOrganic chemistryBiochemical engineeringThioketone
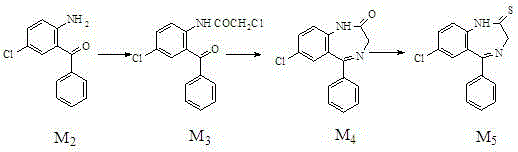
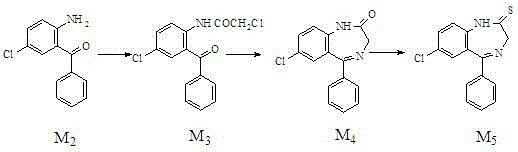
The invention relates to a preparation method of benzodiazothioketone serving as an intermediate of alprazolam. The preparation method comprises the following steps: performing a typical acylation reaction, a cyclization reaction and a vulcanization reaction on 2-amino-5-chloro-benzophenone (M2) serving as a raw material to obtain a benzodiazothioketone crude product (the HPLC (High Performance Liquid Chromatography) content is about 94 percent); and recrystallizing the crude product in a mixed solvent at the normal temperature to obtain a fine product of which the HPLC content is over 98.5 percent, wherein the melting point of the fine product is 238-242 DEG C (the melting range is below 3 DEG C). The preparation method has the advantages of small number of synthesis steps, mild process condition, easiness in controlling a refining method, high yield, high fine product content, controllable low production cost, reduction in environmental pollution, and contribution to industrialization.
Owner:河南豫辰药业股份有限公司
Preparation method of compound lamotrigine subcutaneous implantable controlled-release glue rod
InactiveCN103989685AEasy to useImprove quality controlNervous disorderPharmaceutical delivery mechanismControlled releaseCurative effect
The invention relates to a compound lamotrigine subcutaneous implantable controlled-release glue rod, which contains lamotrigine, alprazolam and a controlled-release agent. The compound lamotrigine subcutaneous implantable controlled-release glue rod can be implanted into a loose part of the lower abdominal wall subcutaneous tissues of a patient by using small incision and trocar introduction, after the patient obtains satisfactory curative effect from acute treatment; the controlled-release glue rod is lasting in curative effect, economical and practical, and the curative effect can be controlled at about 1 year for each implantation; and the drug is guided by the controlled-release agent and releases slowly at a uniform speed through a medical silica gel film wall, so as to guarantee stable and effective blood drug level and achieve the purpose of therapy maintenance. The controlled-release glue rod is applicable to patients with all types of epilepsy (induced mental disorders), mood disorders and other indications for lamotrigine treatment.
Owner:郑玉华 +2
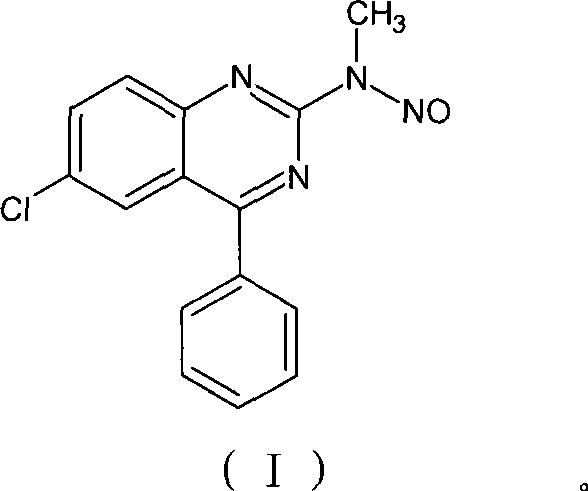
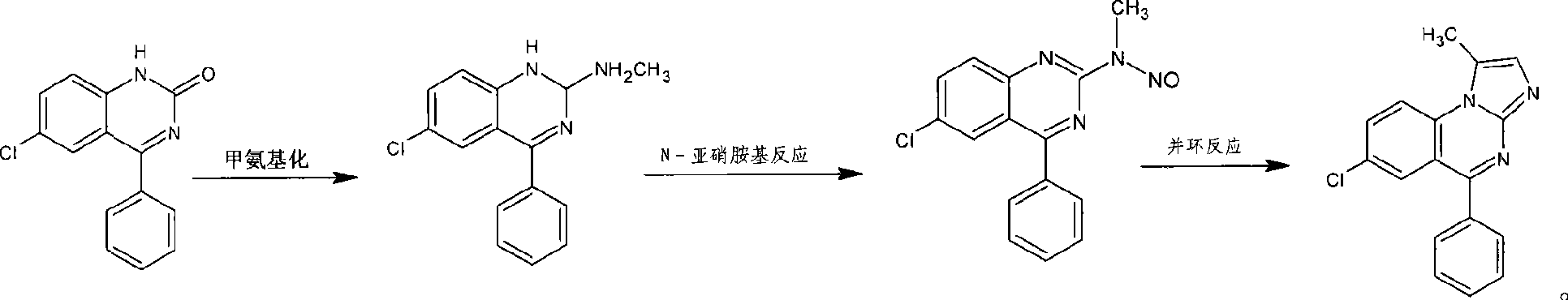
Benzodiazepinic amine nitrite compound, preparation and use thereof
InactiveCN101440066AImprove product qualityProcess conditions are mild and easy to controlOrganic chemistryBenzodiazepineBenzene
The invention relates to a 1, 4-nitrosation-N-nitrosamine intermediate (I) compound in the synthesis of alprazolam, a preparation method and application thereof to the preparation of alprazolam. 7-chloro-5-phenylbenzodiazepinone is taken as an initial raw material, and the alprazolam is obtained by the synthesis through certain processes such as condensation ring-closure after a methylamination reaction and an N-nitrosation reaction. Other benzodiazepine derivatives can be synthesized through a 1, 4-nitrosation-N-nitrosamine intermediate obtained by the synthesis. The method has the advantages of unique process, steady product quality, mild process conditions, easy control, and high yield, and can control lower production cost and reduce environmental pollution.
Owner:QILU TIANHE PHARMA
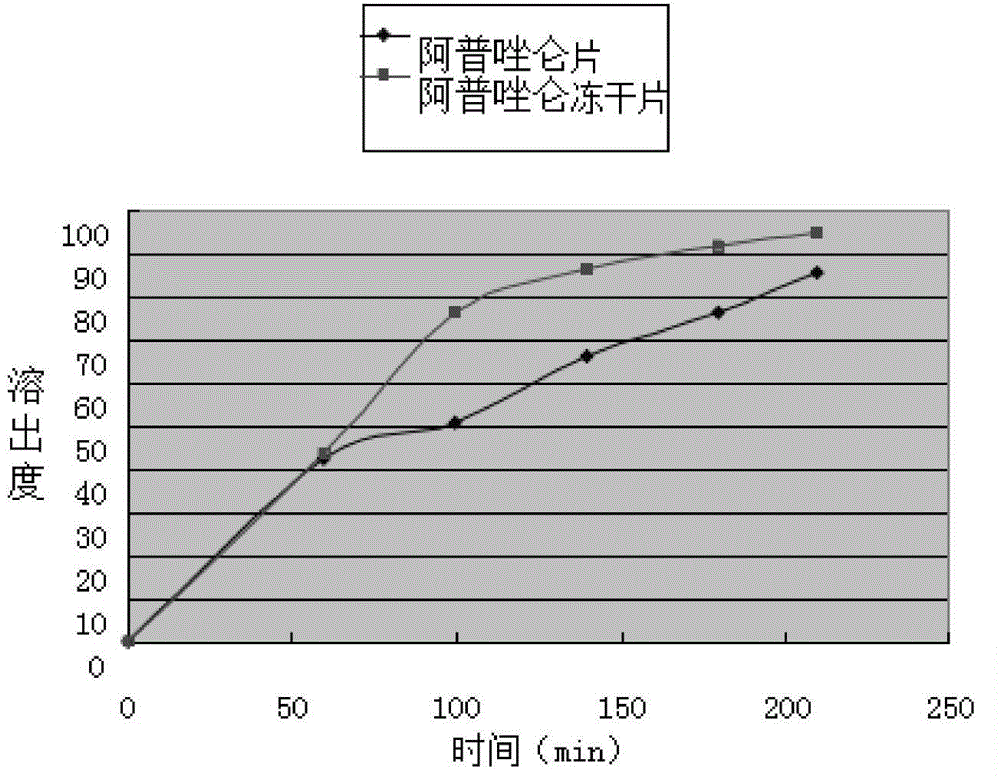
Alprazolam composition lyophilized tablet and preparation method thereof
InactiveCN104622823AGood molding effectHigh dissolution rateOrganic active ingredientsNervous disorderSolubilityCurative effect
The invention provides an alprazolam composition lyophilized tablet and a preparation method thereof, and relates to the technical field of medicines and medicine production. The tablet is prepared from alprazolam, starch and cane sugar, wherein starch and cane sugar are used as auxiliaries, common corn starch is subjected to heating treatment to improve the adhering and disintegrating effects of the starch in the tablet, so that the moldability of the tablet can be improved. The alprazolam composition lyophilized tablet only needs two auxiliaries of starch and cane sugar. According to the preparation method, a twice-cooling twice-heating lyophilizing process is adopted for the alprazolam composition lyophilized tablet, the moldability of the tablet can be better by means of twice cooling and twice heating, the solubility of the tablet can be improved, and the bioavailability of the tablet can be improved; the tablet is capable of overcoming the defect of conventional alprazolam tablets and reducing the types and usage of auxiliaries in the alprazolam tablets, is high in solubility and high in bioavailability, and can be used for guaranteeing the curative effect and safety of clinical administration.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
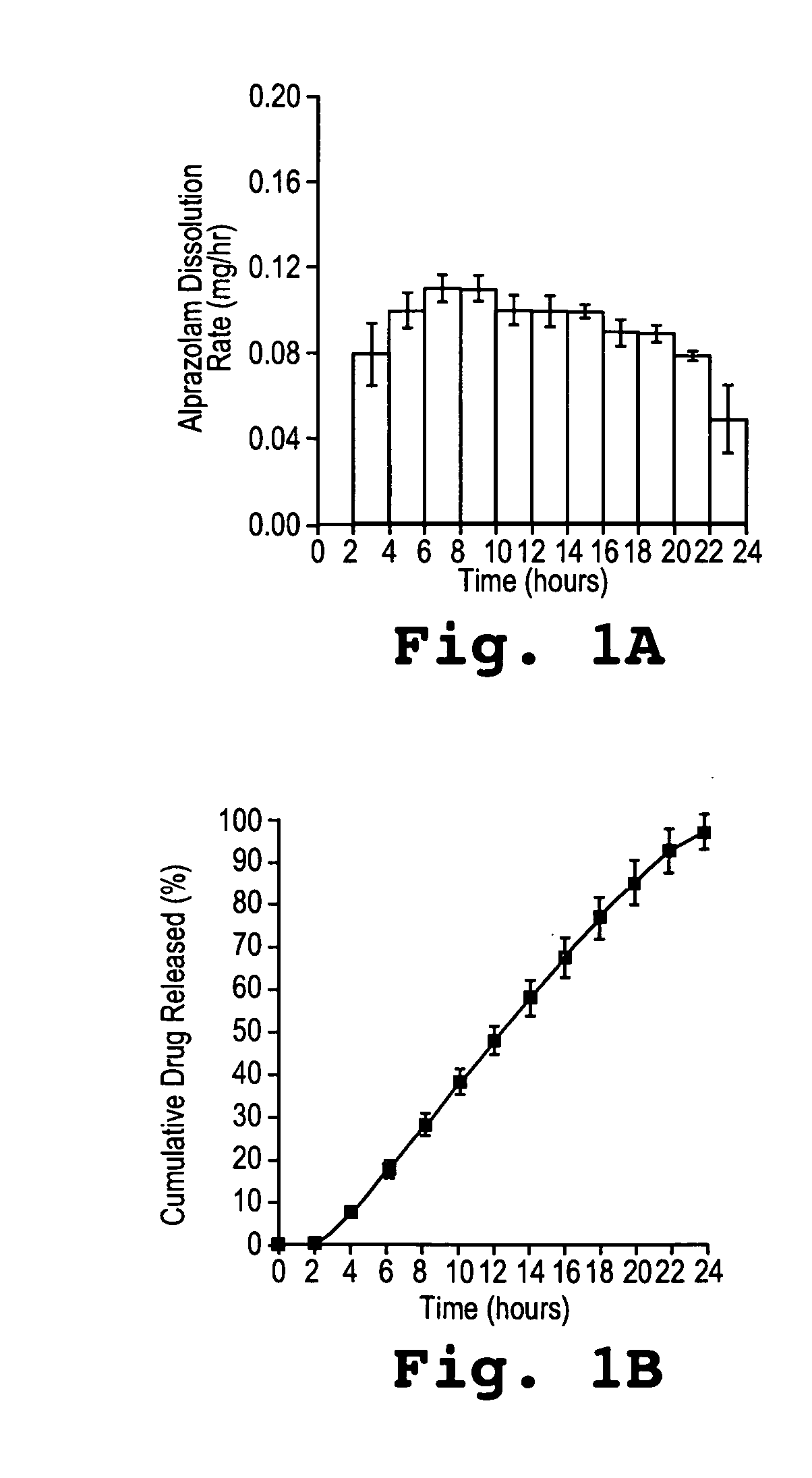
Methods and dosage forms for controlled delivery of alprazolam
InactiveUS20050260268A1Eliminate side effectsOrganic active ingredientsNervous disorderSide effectSustained release drug
A dosage form for delivery of alprazolam is described. The sustained release dosage form provides via once-a-day dosing a therapeutically effective average steady-state plasma alprazolam concentration, where the maximum attained plasma concentration is achieved more than about 14 hours after administration. The slow, sustained release reduces side effects such as sedation and abuse potential.
Owner:ALZA CORP
Pharmaceutical composition based on agonist of benzodiazepine
Owner:SIGMA PHARMA LTDA
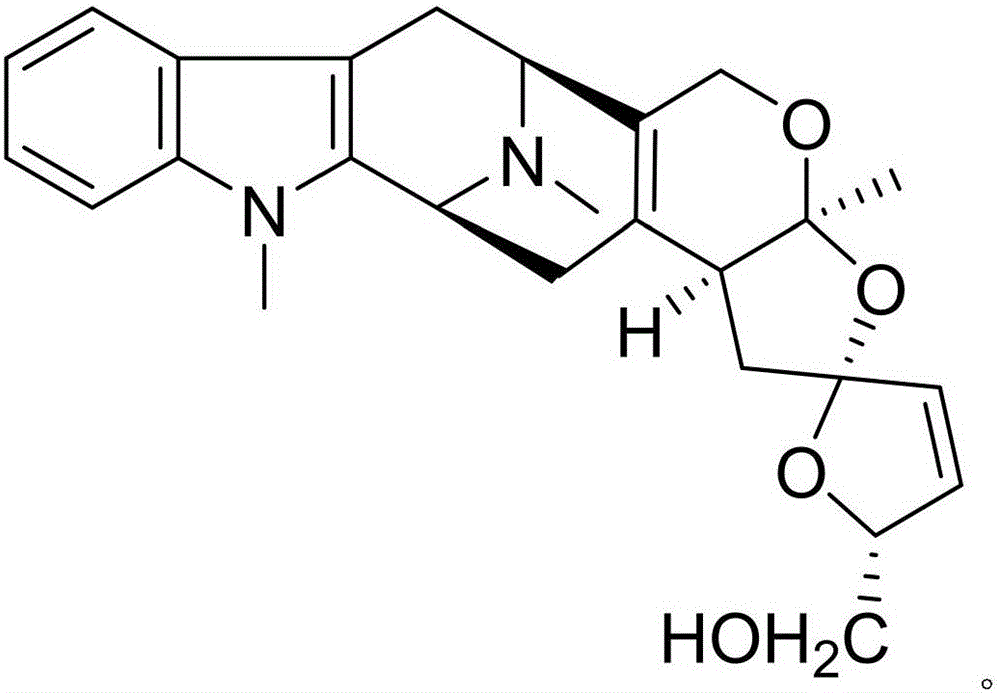
Alprazolam pharmaceutical composition and anti-inflammatory and analgesic effects thereof
InactiveCN105801590AIncreased anti-inflammatory and analgesic effectsHighlight substantive featuresOrganic active ingredientsOrganic chemistryCyathula officinalisNatural product
The invention discloses an alprazolam pharmaceutical composition and anti-inflammatory and analgesic effects thereof. The alprazolam pharmaceutical composition contains alprazolam and a natural product compound (I) which is separated and obtained from dry rhizomes of cyathula officinalis kuan and is novel in structure; when alprazolam and the natural product act separately, the anti-inflammatory and analgesic effects are common; when alprazolam and the natural product act jointly, the anti-inflammatory and analgesic effects are significantly improved, and alprazolam and the natural product can be developed into a drug capable of achieving the anti-inflammatory and analgesic effects. Compared with the prior art, the alprazolam pharmaceutical composition has the prominent substantive advantage and significant progress.
Owner:李晨露
Preparation method of stable isotope labeled alprazolam and estazolam internal standard reagent
InactiveCN113549078AHigh chemical purityRaw materials are easy to obtainIsotope introduction to heterocyclic compoundsStable Isotope LabelingIsotopic labeling
The invention relates to a preparation method of a stable isotope labeled alprazolam and estazolam internal standard reagent for monitoring the blood concentration of a clinical treatment drug, and belongs to a standard substance for monitoring the clinical treatment drug. The stable isotope labeled alprazolam or stable isotope labeled estazolam is obtained by taking stable isotope labeled phenylboronic acid as a raw material, performing catalytic addition, acylation, cyclization, sulfur-oxygen exchange and cyclization and finally conducting separating and purifying. The process has the advantages that raw materials needed for synthesis are simple and easy to obtain, reaction conditions are mild, the chemical purity of the target product stable isotope labeled alprazolam and stable isotope labeled estazolam can reach 98% or above, and the isotope abundance can reach 98% or above. The preparation method can meet the technical requirements of a stable isotope labeling internal standard reagent required by a liquid chromatography-tandem mass spectrometry method for monitoring the blood concentration of a clinical treatment drug.
Owner:谱同生物医药科技常州有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com