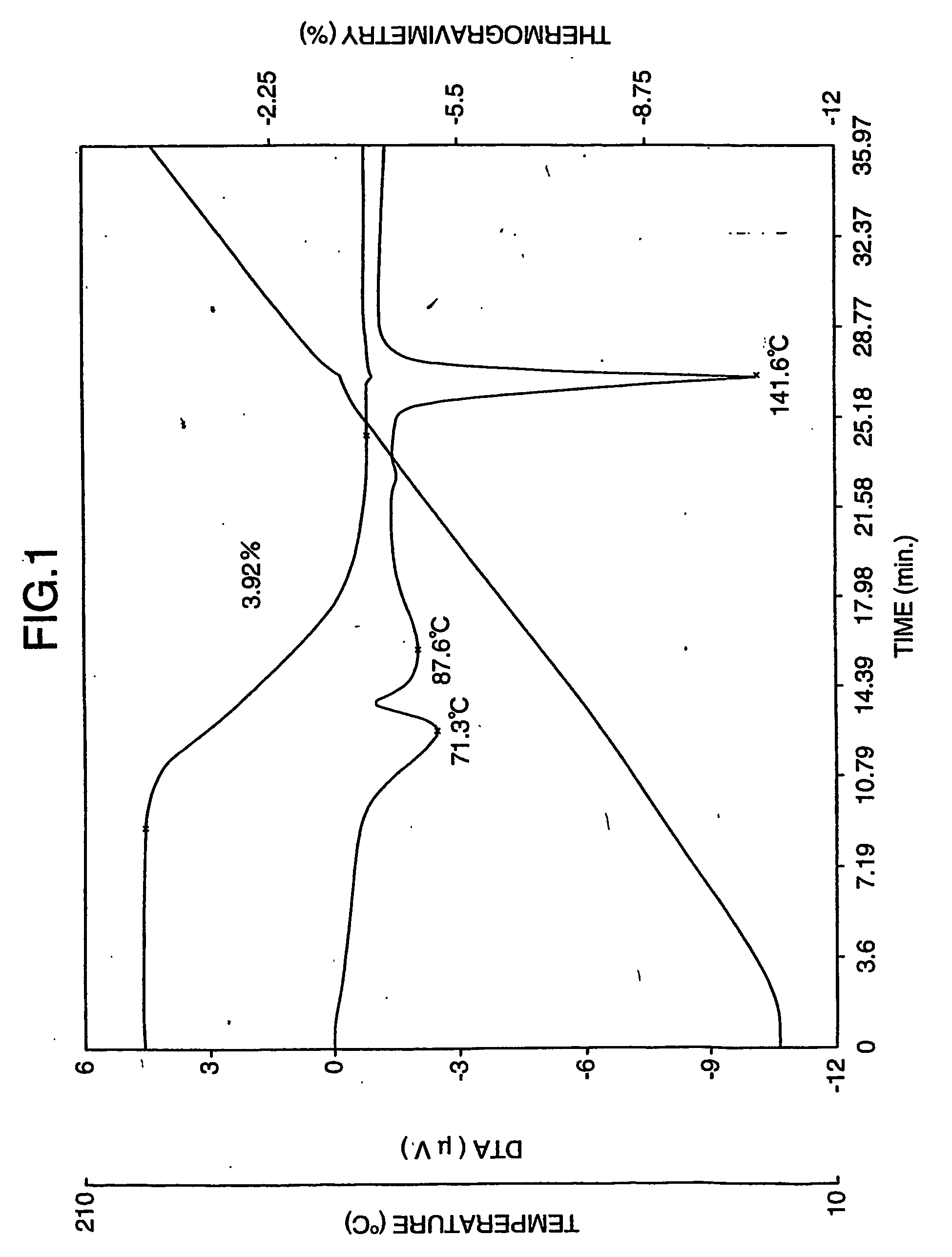
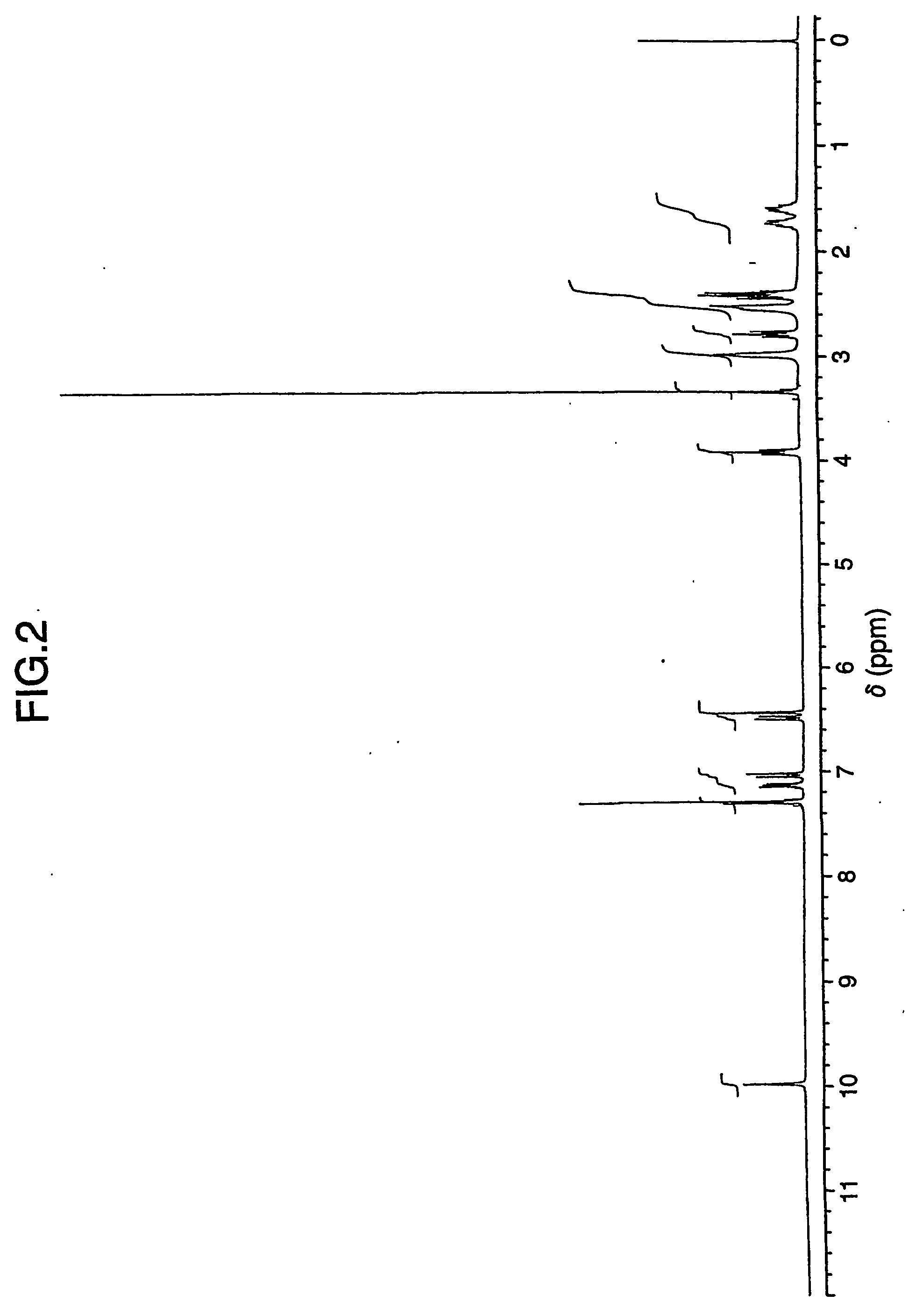
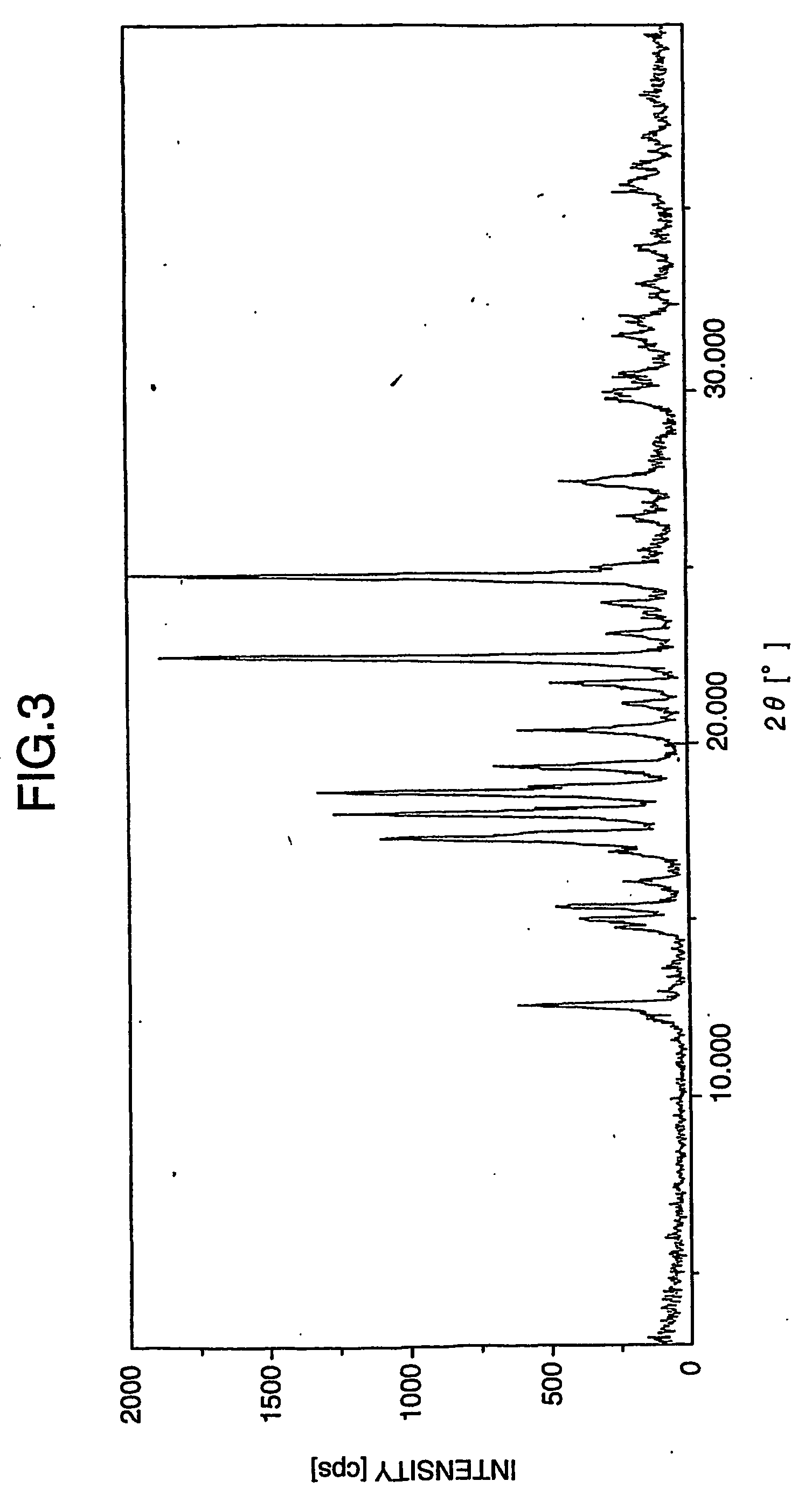
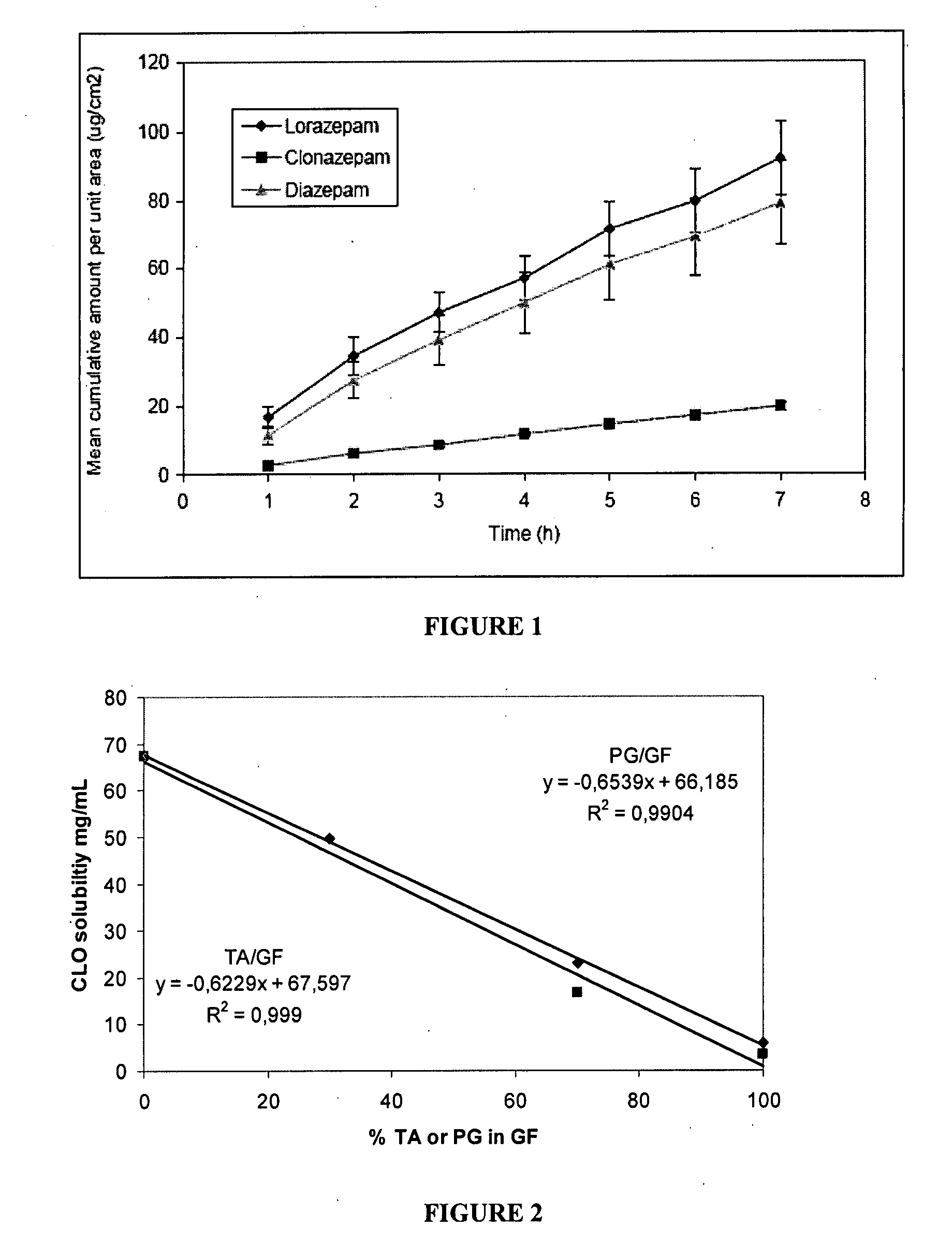
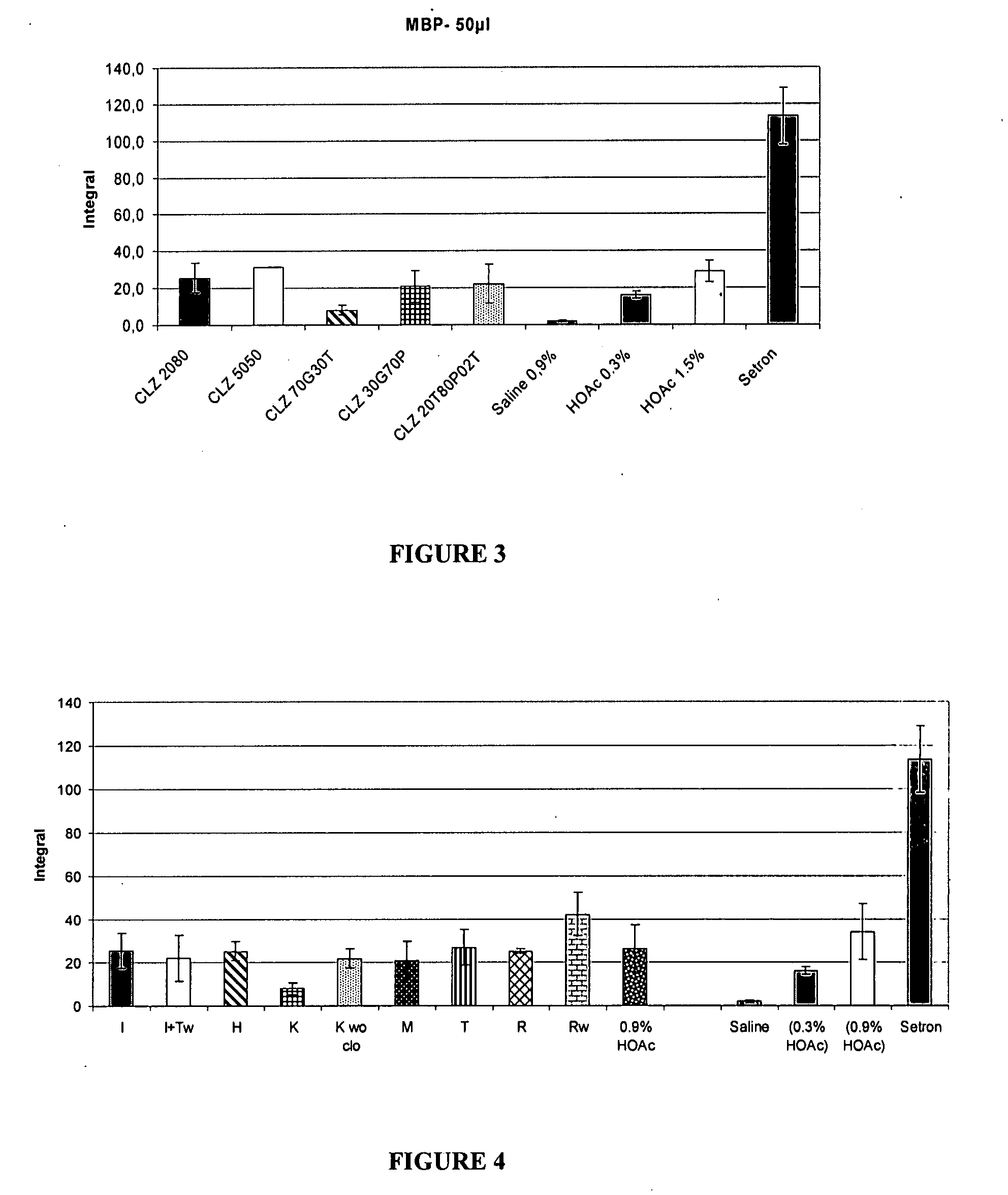
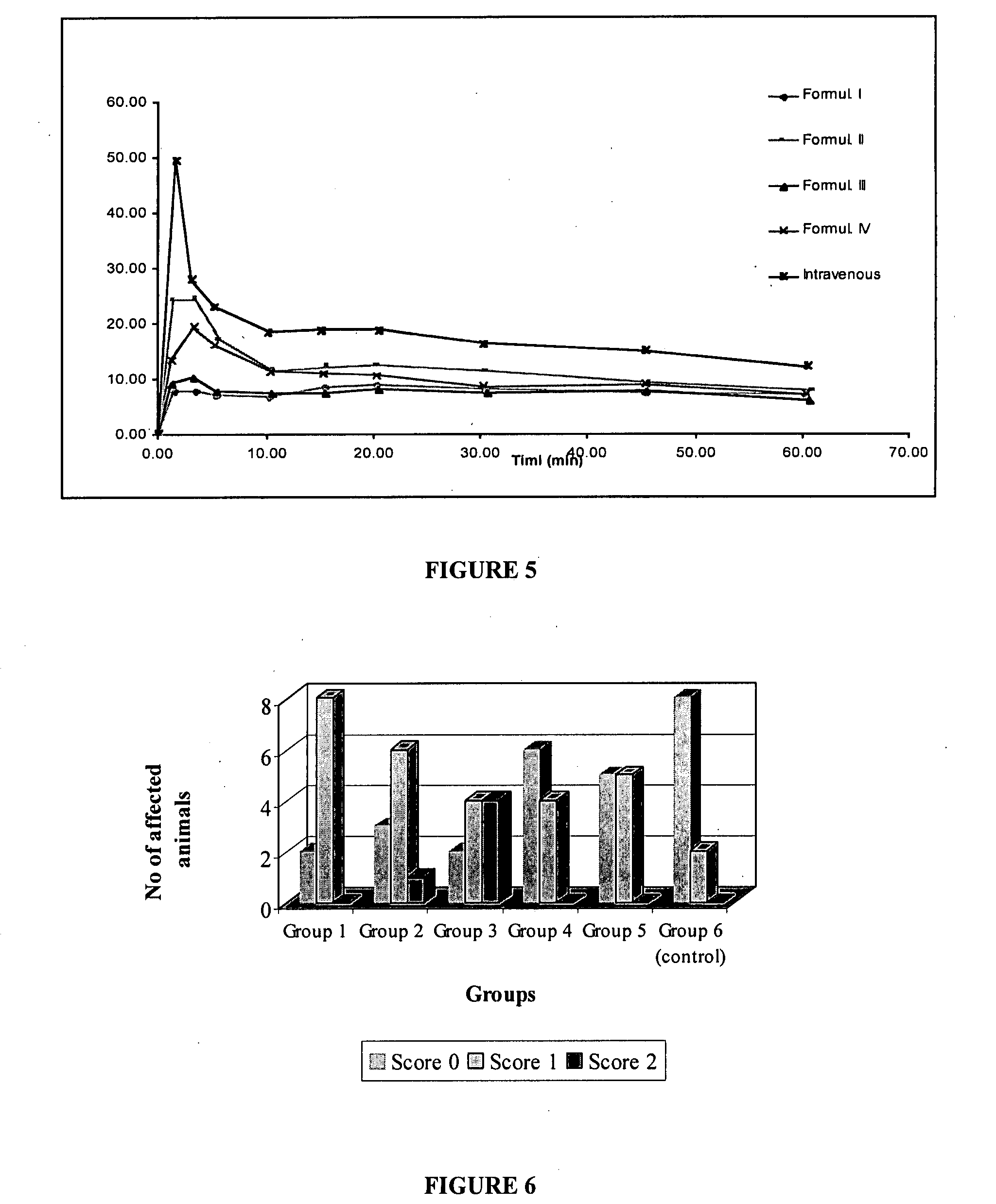
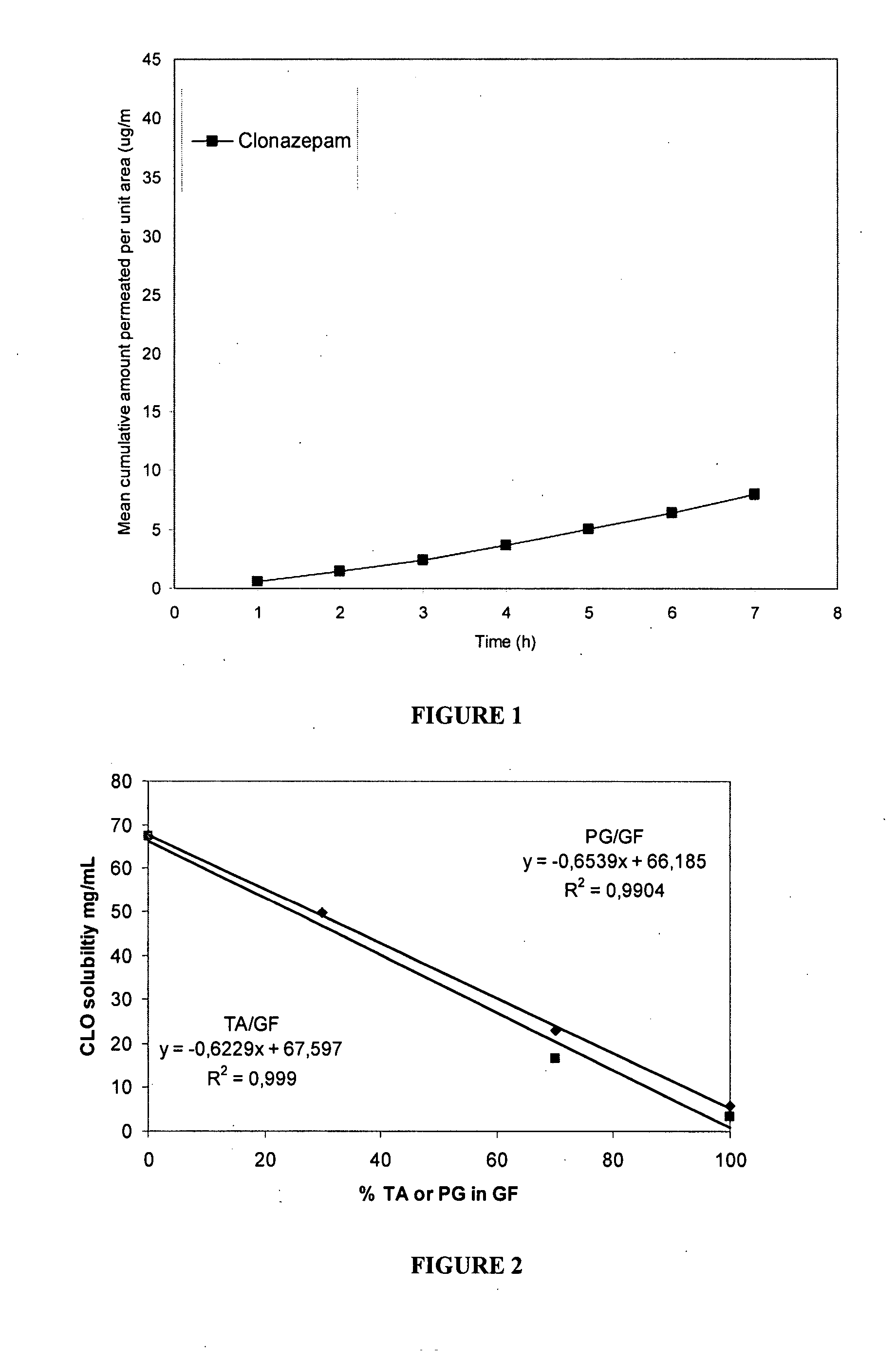
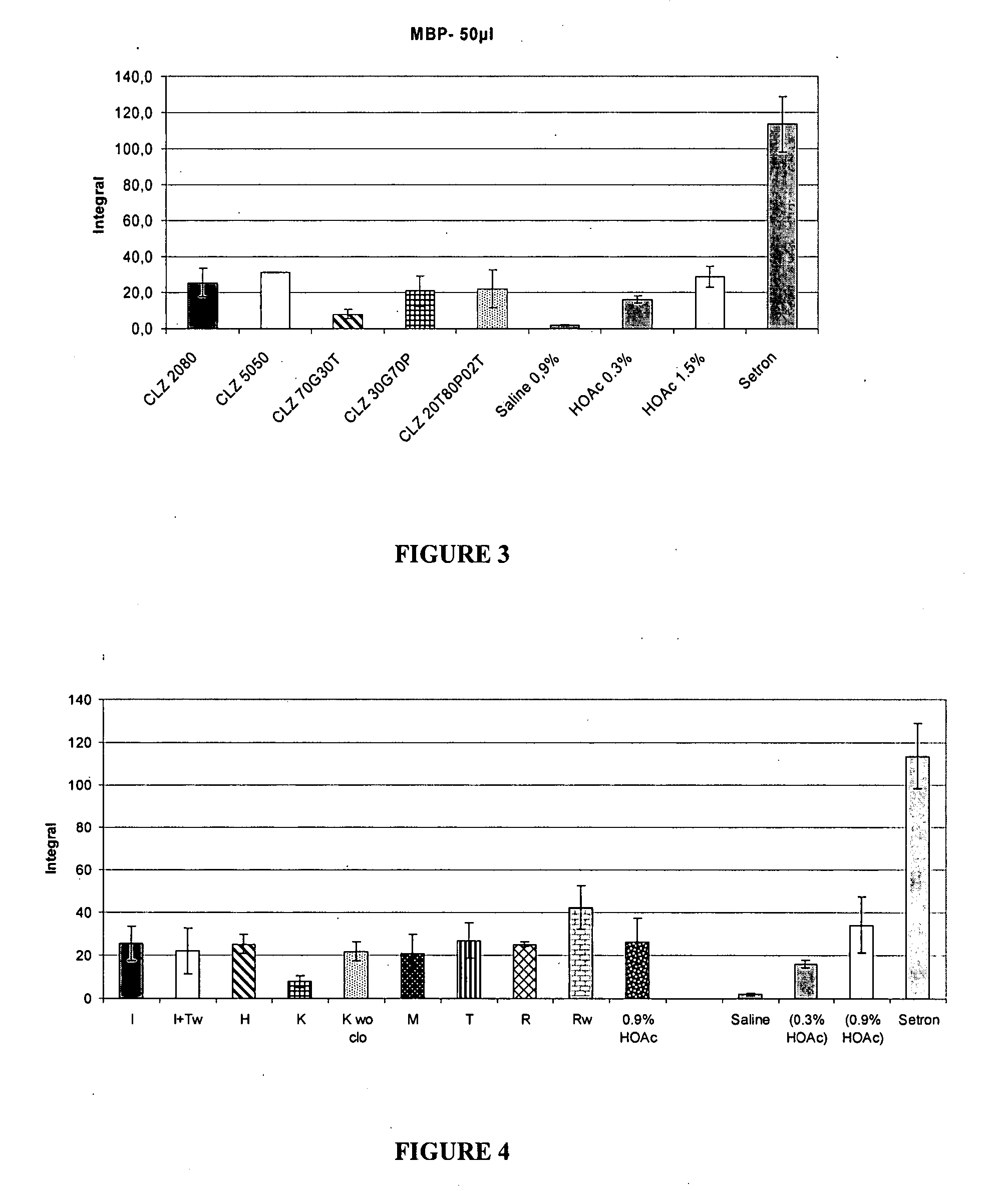
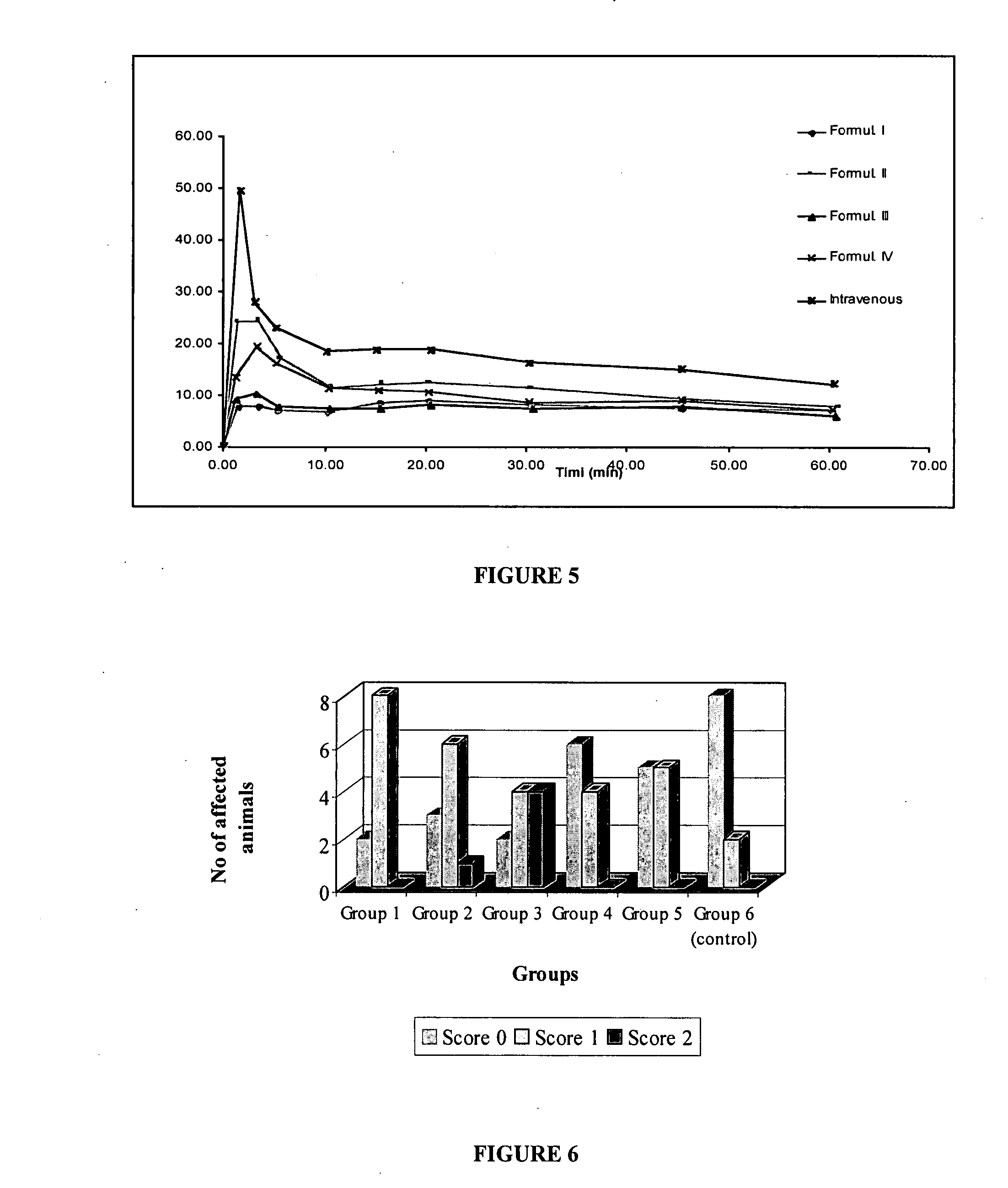
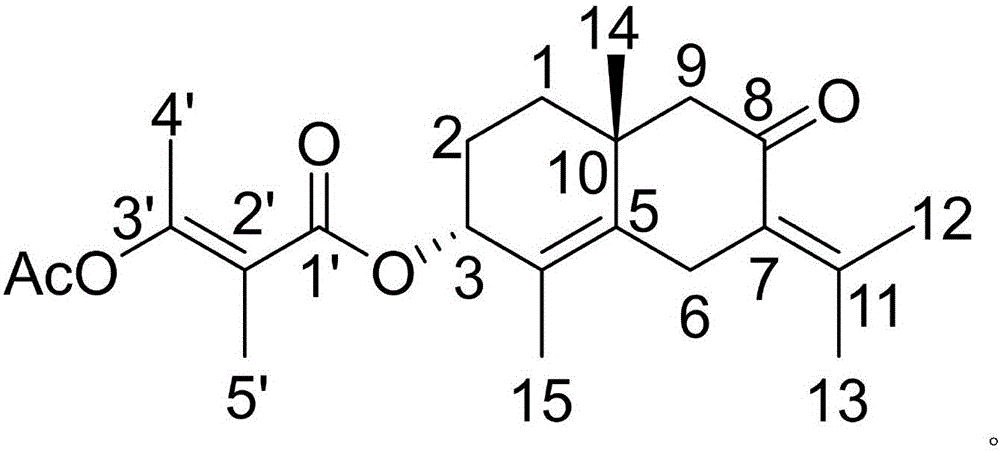
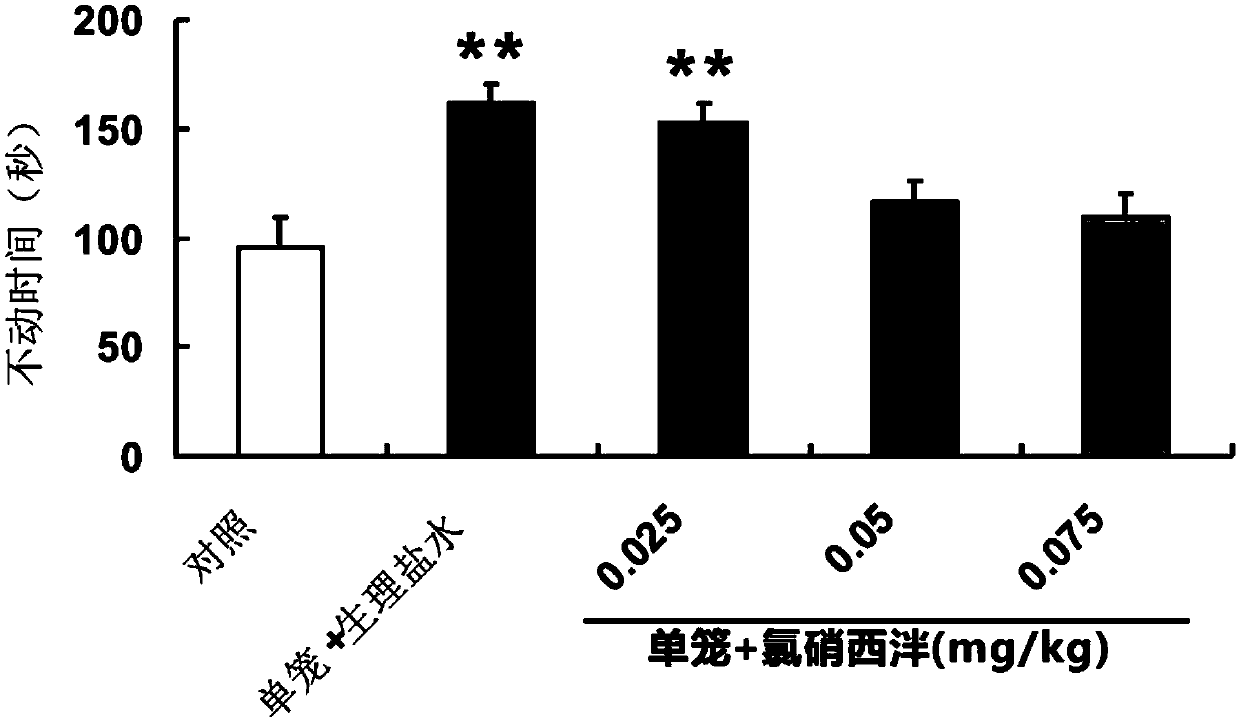
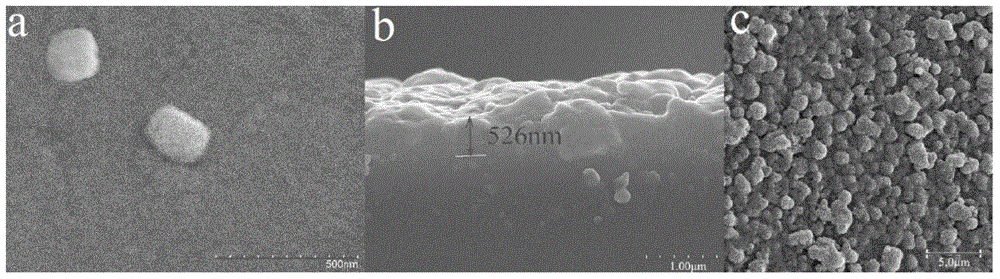
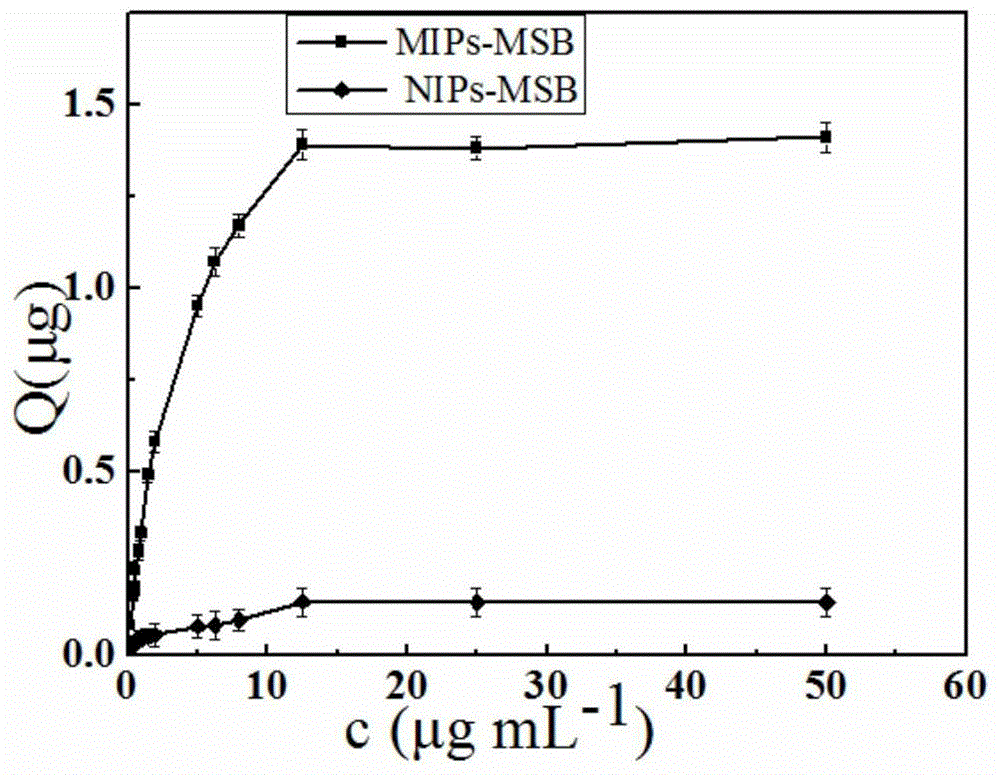
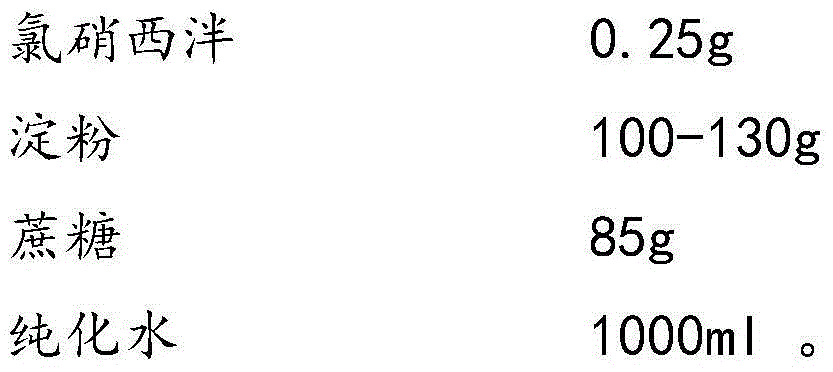Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
47 results about "Clonazepam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clonazepam is used to prevent and control seizures.
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Pharmaceutical compositions of benzodiazepines and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA
Pharmaceutical compositions of clonazepam and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
Pharmaceutical compositions of benzodiazepines and method of use thereof
The present invention includes benzodiazepine compositions formulated for intranasal administration, comprising a binary solvent system comprising a first solvent in which the benzodiazepine is soluble, the first solvent capable of penetrating nasal mucosal tissue, and a second solvent in which the benzodiazepine in less soluble. The compositions of the present invention may be used to treat a variety of disorders including, but not limited to, panic attacks, muscle spasms, anxiety, and seizures. In one aspect, the present invention relates to a fast-acting, clonazepam composition for transnasal administration that can be used for the treatment of seizure clusters.
Owner:JAZZ PHARMA INC
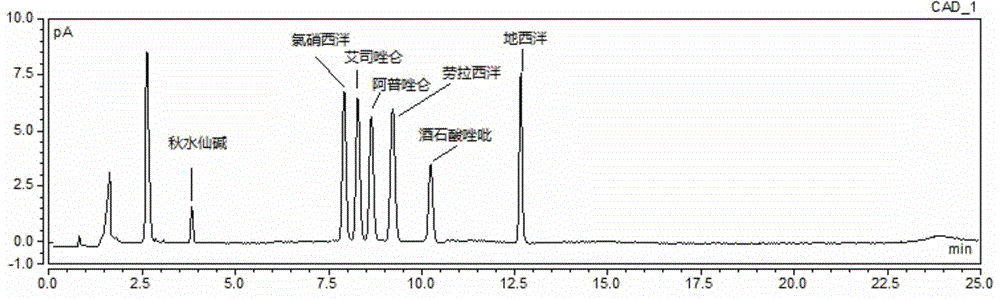
Method for simultaneous determination of contents of 6 kinds of sedative-hypnotic drugs in serum by UHPLC-CAD technology
ActiveCN104133030ASimultaneous measurementShort analysis timeComponent separationSide effectSedative/hypnotic
The invention relates to a method for simultaneous determination of the contents of 6 kinds of sedative-hypnotic drugs in serum by a UHPLC-CAD combined technology, wherein the 6 kinds of sedative-hypnotic drugs are diazepam, lorazepam, alprazolam, estazolam, clonazepam and zolpidem tartrate. The method is characterized by including the following steps: 1) reference substance preparation, 2) internal standard solution preparation, 3) sample solution preparation, 4) mobile phase solution preparation, 5) chromatographic condition setting, 6) electrospray detector condition optimization, 7) sample determination, 8) gradient-concentration reference substance solution preparation, 9) standard curve preparation, and 10) data calculation and analysis. The method is advanced, has good clinical application prospects and effects, can rationally use drugs for clinic, increases drug curative effects, lowers toxic and side effects and drug costs, and plays a positive role.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
Medication and treatment for disease
Owner:ALTMAN ENTERPRISES
Kit for determining antianxiety/hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085265AReduce matrix effectThe test result is accurateComponent separationBromazepamTandem mass spectrometry
The invention provides a kit for determining antianxiety / hypnotic type drugs in serum and plasma through liquid chromatography tandem mass spectrometry. The kit comprises the following constituents: drug standards comprising bromazepam, clonazepam, diazepam, lorazepam, midazolam, nitrazepam, oxazepam and Temazepam; drug internal standard compounds comprising alprazolam-d5, clonazepam-d4, diazepam-d5, lorazepam-d4, midazolam-d4, nitrazepam-d5, oxazepam-d5 and Temazepam-d5; drug extraction compositions comprising, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropanol solution and 10% of purified water; negative plasma; and diluent: 50% of carbinol water solution. The kit can be used for simultaneously determining antianxiety / hypnotic type drugs and active metabolites thereof, and has the advantages of short determination time and high flux.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Method for determining residual sedative type veterinary medicaments in mutton
InactiveCN103954721AMeet Residue Analysis RequirementsHigh recovery rateComponent separationPerphenazinePromethazine
The invention relates to a method for determining residual medicaments in mutton, and in particular relates to a method for determining multiple sedative type medicaments in mutton at the same time. The residual sedative type veterinary medicaments refer to zolpidem, haloperidol, chlordiazepoxide, promethazine, nitrazepam, chlorpromazine hydrochloride, perphenazine, fluphenazine hydrochloride, clonazepam, xylazine hydrochloride, propionylpromazine, carazolol, acepromazine, droperidol and azaperone. The method has the advantage that the residual amounts of 15 types of sedative type medicaments in mutton are determined through high-resolution liquid chromatography-tandem mass spectrometry. The method is high in sensitivity and high in recovery rate, and can meet the detection requirements on veterinary medicaments.
Owner:GANSU AGRI UNIV
Clonazepam medicinal composition and medicinal application thereof
InactiveCN105663138AHas anti-inflammatory and cough-relieving propertiesGood anti-inflammatory and cough-relieving effectOrganic compound preparationAntipyreticNatural productTraditional medicine
The invention discloses a pharmaceutical composition of clonazepam and its medical use. The pharmaceutical composition of clonazepam provided by the invention contains clonazepam and a natural product compound (I) with a novel structure. The composition of clonazepam and compound (I) has obvious anti-inflammatory and antitussive effects, and is better than the independent anti-inflammatory and antitussive effects of clonazepam or compound (I), and can be developed into an anti-inflammatory and antitussive drug, which is different from existing Compared with the technology, it has outstanding substantive characteristics and significant progress.
Owner:贺玉皓
Clonazepam colloidal gold method detection test paper and preparation method thereof
The invention provides a nitrazepam colloidal gold method detection test paper and a preparation method, belonging to the field of measurement technique of a biological immune method. The detection test paper of the invention consists of a sample pad, a bonded releasing pad, a nitrocellulose film, a water-absorbing pad and a plastic soleplate; the plastic soleplate is sequentially bonded with the sample pad, the bonded releasing pad, the nitrocellulose film and the water-absorbing pad; the bonded releasing pad is wrapped by an anti-nitrazepam multi-resistance-colloid golden marking; the nitrocellulose film is sequentially wrapped by nitrazepam coupling antigen and goat anti-rabbit IgG antibody; the nitrazepam coupling antibody is used as a testing line and the goat anti-rabbit IgG antibody is used as a quality control line. The preparation method of the invention has the advantages of convenience, economical and quick usage, easy fabrication and low cost.
Owner:JIANGNAN UNIV
Medication and Treatment for Disease
ActiveUS20110217278A1Shorten the progressPromote formationOrganic active ingredientsNervous disorderDiseaseVitamin B12
A treatment is described for diseases with symptoms that can include fatigue, muscle aches and spasms, weakness, demylenation, and nerve pain. Diseases can include fibromyalgia, depression, and auto-immune and immuno-suppressive diseases, such as MS. The treatment comprises about 1-10 mg naltrexone, at least about 20 μg vitamin B12, at least about 5 mg vitamin B6, at least about 2 mg coenzyme Q, and preferably at least one ancillary medication selected from the group consisting of diazepam, cyclcobenzaprine, clonazepam, alprazolam, 9-tetrahydrocannibinol, fumarate, caffeine, and combinations thereof. The treatment can be administered orally, and can decrease mental and physical symptoms such as, for example, fatigue, gait problems, visual dysfunction, and pain while improving cognitive skills.
Owner:ALTMAN ENTERPRISES
Method and kit for detecting 19 drugs and metabolites thereof in blood by liquid chromatography-tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 drugs and metabolites thereof in blood through liquid chromatography-tandem mass spectrometry. The substances to be detected comprise sulpiride, pentafluridol, mianserin, buspirone, tandospirone, hydroxyazine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, reboxetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, toluenesulfobutyl urea, glimepiride, 1-pyrimidinepiperazine, desmethylvenlafaxine, 6-hydroxy buspirone and normipramine, and the substances to be detected are selected from the group consisting of sulpiride, pentafluridol, mianserin, venlafaxine, metandospirone, metandospirone, hydroxazine, diazepam, venlafaxine, moclobemide, the pharmaceutical composition is prepared from noramitriptyline, nordiazepam and clopidogrel metabolite; the detection method comprises the following steps: calibrating a standard solution, treating a to-be-detected sample, and detecting the to-be-detected sample by adopting high performance liquid chromatography-mass spectrometry. The embodiment of the invention can quickly and accurately measure the content, and the sample treatment method is simple and easy to implement, high in sensitivity and accurate in quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Clonazepam colloidal gold method detection test paper and preparation method thereof
InactiveCN101315365AMeet the needs of residue detectionAccurate detectionBiological testingAntigenCellulose
A clonazepam colloidal gold method test paper and a preparation method belong to the testing technology field of the biological immunity method. The test paper is composed of a sample pad, a combined release pad, a cellulose nitrate membrane, a water absorbing pad and a plastic base plate; the sample pad, the combined release pad, the cellulose nitrate membrane and the water absorbing pad are sequentially stuck to the plastic base plate; the combined release pad is coated with an anti-clonazepam multi-resistance colloidal gold label; the cellulose nitrate membrane is sequentially coated with clonazepam coupling antigen and goat anti-rabbit IgG antibody, wherein the clonazepam coupling antigen is taken as the test line and the goat anti-rabbit IgG antibody is taken as the quality control line. The clonazepam colloidal gold method test paper and the preparation method have the characteristics of convenient use, economy, rapidness, simple preparation and low cost.
Owner:JIANGNAN UNIV
Online solid phase extraction liquid chromatography method for detecting clonazepam content in blood
ActiveCN108445113AReduce stepsImprove accuracyComponent separationWorking fluidSolid phase extraction
The invention discloses an online solid phase extraction liquid chromatography method for detecting clonazepam content in blood. The method is to calibrate a standard solution by using high performance liquid chromatograph and a DVD detector, a standard curve equation obtained is y=a*x+b, a blood sample to be tested is taken, after the blood to be tested is processed, the sample to be tested is also detected by using the high performance liquid chromatograph and the DVD detector, a blood y value to be tested is obtained, the blood y value to be tested is substituted into the standard curve equation, the relative concentration x of clonazepam is calculated in the blood sample to be tested, and the concentration of internal standard working fluid is known, thereby calculating the concentration of the clonazepam in the blood to be tested in the sample; the serum of the method is directly injected after sedimentation and centrifugation, which makes the detection process simple and rapid, the experimental cost is reduced, and the method is more conducive to monitoring the blood concentration of the clonazepam in a patient during clinical treatment.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
ELISA detection reagent kit suitable for clonazepam relict analysis
InactiveCN101315373AReduce testing costsLong storage timeMaterial analysisBenzodiazepineRetention time
The invention relates to an enzyme-linked immune detection kit suitable for the analysis of clonazepam residues, and belongs to the technology field of the enzyme-linked immune adsorption analysis kit. The kit comprises an enzyme label plate coated with the envelope antigen of the clonazepam, a sponge support, a clonazepam standard, a clonazepam polyclonal antibody, an enzyme sign second antibody, a concentrating and washing liquid, a colored solution and a reaction stopping solution; the envelope antigen of the clonazepam is the coupling compound of 7-amino clonazepam and an egg-white protein; and the enzyme sign second antibody is a horseradish peroxidase labeled goat anti-rabbit antibody. The kit adopts the clonazepam polyclonal antibody, and can accurately and sensitively detect the clonazepam residues or other structurally similar benzodiazepine residues in urine or tissue; the process of pretreating samples is simple; the time consumption is low; a large quantity of samples can be tested simultaneously; furthermore, the cost for sample detection is lower than that of the traditional instrument detection method; the kit has a long retention time, no radioactive pollution, and practical significance for realizing on-site monitoring of the clonazepam residues of the large quantity of samples.
Owner:JIANGNAN UNIV
Carbostyril derivatives and mood stabilizers for treating mood disorders
The pharmaceutical composition of the present invention comprises a carbostyril derivative which is a dopamine-sero-tonin system stabilizer and a mood stabilizer in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof. The mood stabilizer may include but is not limited to lithium, valproic acid, divalproex sodium, carbamaza-pine, oxcarbamazapine, zonisamide, lamotragine, topiramate, gabapentin, levetiracetam or clonazepam. These compositions are used to treat patients with mood disorders, particularly bipolar disorder with or without psychotic features, mania or mixed episodes. Methods are provided for separate administration of a carbostyril derivative and a mood stabilizer to a patient with a mood disorder.
Owner:OTSUKA PHARM CO LTD
Novel method for preparing 7-amino clonazepam compound
The invention discloses a novel method for preparing a 7-amino clonazepam compound, and belongs to the technical field of organic synthesis. The preparation method comprises the steps that 2-amino-4-nitrobenzoic acid serves as an initial raw material, and a target compound is obtained through the processes of acetyl-lactonization, Grignard reaction, amide hydrolysis, intramolecular condensation reaction, reduction and the like. The method provided by the invention is safe to operate, avoids the use of heavy metals, is simple and convenient in post-treatment, can obtain the product through direct filtration and recrystallization, and does not need other purification. Only conventional acid, alkali and solvents are used in the whole reaction process, so that the method is less in environmental pollution, low in cost and higher in yield.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Method for synthesizing 7-amino clonazepam compound
ActiveCN113072509AShort synthetic stepsOperational securityOrganic chemistryWittig reactionOrganic synthesis
The invention discloses a method for synthesizing a 7-amino clonazepam compound, and belongs to the technical field of organic synthesis. The preparation method comprises the steps that 2-cyano-4-nitroaniline serves as an initial raw material, and a target compound is obtained through oxidative coupling, amidation, affinity substitution reaction, intramolecular Wittig reaction, reduction reaction and other processes. According to the invention, a brand new synthetic route is provided for 7-amino nitrazepam, the method has the advantages of short synthetic steps, safe operation and simple post-treatment, only conventional acid-base and solvent are used in the whole reaction process, the cost is low, and the yield is increased by more than 20%.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
LC (liquid chromatography) for concentration determination of clonazepam in blood
InactiveCN108303488AMeet the requirements of blood drug concentration monitoringHigh extraction rateComponent separationWorking fluidUltraviolet detectors
The invention provides a method for concentration determining concentration of clonazepam in blood. The method comprises the following steps: a liquid chromatograph and an ultraviolet detector are used for calibrating a standard solution, fitting is performed, a standard curve equation of y = a*x+b is obtained, a to-be-detected blood sample is taken, treated and then detected by the liquid chromatograph and the ultraviolet detector, y of the to-be-detected blood sample is obtained and substituted into the standard curve equation, relative concentration x of a target in the to-be-detected bloodsample is obtained by calculation, the concentration of internal standard working fluid is known, and accordingly, the concentration of clonazepam in the to-be-detected blood sample is calculated. The method has the advantages of being rapid, high in extraction rate, good in reproducibility and specificity, high in separation efficiency and accurate in quantitative analysis, can meet the monitoring requirements of plasma concentration of the anti-epilepsy drug clonazepam, and provides a basis for individualized administration of clinical clonazepam.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
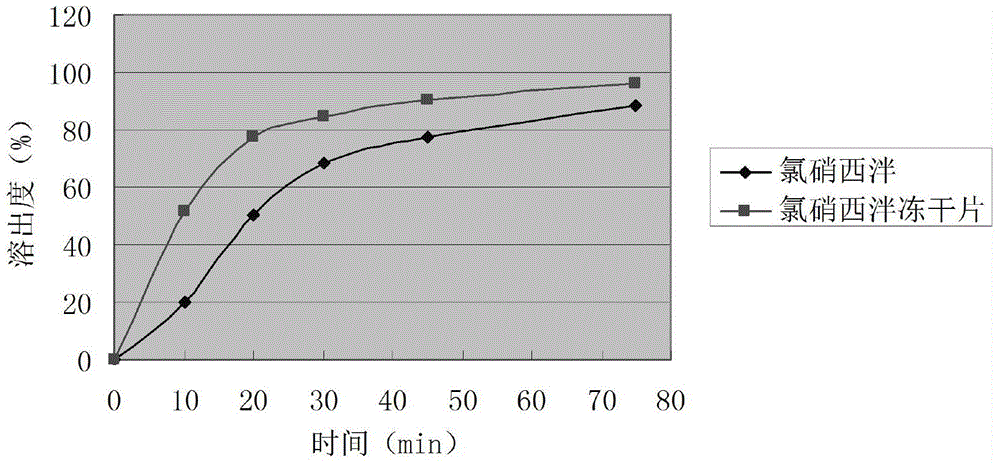
Clonazepam composition freeze-dried tablet and preparation method thereof
InactiveCN104546673AGood molding effectHigh dissolution rateOrganic active ingredientsNervous disorderSucroseMedicine
The invention provides a clonazepam composition freeze-dried tablet and a preparation method thereof, and relates to the technical field of medicines and medicine production. The clonazepam composition freeze-dried tablet is prepared from clonazepam, starch and cane sugar, starch and cane sugar are used as auxiliary materials, and heating process treatment is performed on the ordinary corn starch, so that the bonding and disintegration functions of starch in the tablet are improved, the formability of the tablet is improved, and the clonazepam composition freeze-dried tablet only needs the two auxiliary materials namely starch and cane sugar. The clonazepam composition freeze-dried tablet is prepared by the freeze-drying process that the temperature is increased and decreased for two times; the process that the temperature is increased and decreased for two times enables the formability of the tablet to be better and increases the dissolution rate of the tablet, so as to improve the bioavailability of the tablet; the tablet overcomes the defect of the common clonazepam, is high in dissolution rate and high in bioavailability, and ensures the curative effect and the safety of the clinical medication; the varieties and the dosage of the auxiliary materials in clonazepam are reduced.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
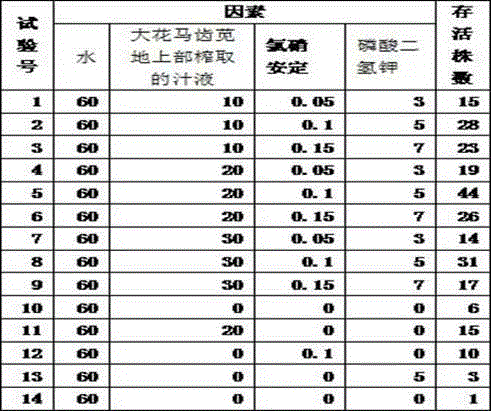
Liquid matrix for artificial cultivation of piper bambusaefolium
InactiveCN104620961AImprove the survival rate of cultivationPromotes rooting of cuttingsAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersMonopotassium phosphateHorticulture
The invention discloses liquid matrix for artificial cultivation of piper bambusaefolium, belonging to the field of artificial cultivation of piper bambusaefolium. According to the artificial cultivation of the piper bambusaefolium, a cutting is difficult to root in an environment with relatively low temperature; the cutting does not root, so that an obstacle is brought for artificial cultivation. The liquid matrix for artificial cultivation of the piper bambusaefolium disclosed by the invention is characterized by being prepared by mixing the following raw materials in parts by weight: 60 parts of water, 20 parts of juice squeezed from the overground part of portulaca grandiflora, 0.1 part of clonazepam and 5 parts of monopotassium phosphate. According to the liquid matrix, the cultivating survival rate of the piper bambusaefolium can be improved; and rooting of the piper bambusaefolium cutting can be promoted.
Owner:王信云
Diagnostic agent, diagnostic method and therapeutic agent for fibromyalgia
A diagnostic agent and a diagnostic method for appropriately diagnosing fibromyalgia of a specific type, and a therapeutic agent for the aforesaid type include a diagnostic agent for fibromyalgia relating to an anti-voltage-gated potassium channel complex antibody (anti-VGKC complex antibody), said diagnostic agent containing a reagent for detecting the anti-VGKC complex antibody; a diagnostic method using the diagnostic agent; and a therapeutic agent for fibromyalgia relating to the anti-VGKC complex antibody. A diagnostic agent for fibromyalgia relating to an antibody against a voltage-gated potassium channel complex includes a reagent for detecting the antibody against the voltage-gated potassium channel complex; and a therapeutic agent for fibromyalgia relating to an antibody against voltage-gated potassium channel contains an effective amount of an anticonvulsant drug (gabapentine or clonazepam) as the active ingredient.
Owner:AXIS INC
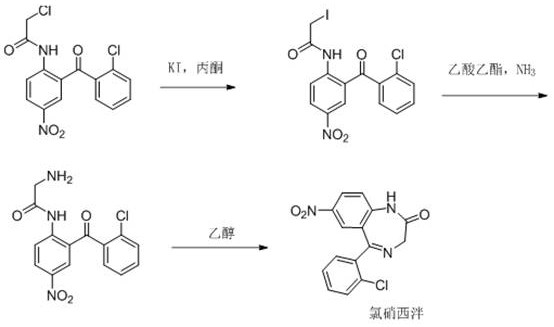
Preparation method for continuous flow synthesis of clonazepam
The invention belongs to the technical field of medicine preparation, and particularly relates to a preparation method for continuous flow synthesis of clonazepam. The method comprises the following steps: (1) pumping an acetone solution of a starting material chloride into a heat exchanger, then flowing into a potassium iodide fixed bed, and reacting to obtain an intermediate iodide; and (2) enabling the iodide to flow into a reactor, introducing ammonia gas into the reactor at the same time, continuously collecting the reaction liquid, carrying out vacuum concentration on an organic phase, and thus obtaining a crude product clonazepam. The preparation method has the beneficial effects that (1) compared with a traditional method in the prior art, the clonazepam prepared by the method disclosed by the invention has the advantages that the generation of impurity dimers is greatly reduced, and the purity of the product is remarkably improved; (2) the method disclosed by the invention is short in reaction time and time-saving; and (3) the yield of the product is high, the highest yield of the product can reach 88% or above, and the highest purity can reach about 99.8%.
Owner:济南同路医药科技发展有限公司
Black/white preparation recipe for treating psychosis
InactiveCN1857271ASimple ingredientsEasy to manufactureOrganic active ingredientsPowder deliveryCurative effectActive ingredient
The present invention relates to a kind of psychosis treating black / white medicine preparation. The psychosis treating black / white medicine preparation includes one black preparation for night taking and one white preparation for day time taking, and each of the black preparation and the white preparation consists of antipsychotic components and clonazepam. The black preparation with the functions of treating psychosis, dissipating anxiety, tranquilizing and promoting sleep, and the white preparation with the functions of treating psychosis, dissipating anxiety and treating depression cooperate to reach raised psychosis treating effect.
Owner:陈彦方
Method and kit for the detection of 19 drugs and their metabolites in blood by liquid chromatography tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 kinds of drugs and their metabolites in blood by liquid chromatography tandem mass spectrometry. Substances to be tested include sulpiride, penfluridol, mianserin, buspirone, tandospirone, hydroxyzine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, rebo Cetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, tolbutamide, glimepiride, 1-pyrimidine piperazine, desvenlafaxine, 6- Hydroxybuspirone, norimipramine, noramitriptyline, nordiazepam, clopidogrel metabolites; detection methods include: calibrating standard solutions, processing samples to be tested, using high performance liquid chromatography‑ Mass spectrometry detects the sample to be tested. The embodiment of the present invention can quickly and accurately measure the content, the sample processing method is simple and easy, and has high sensitivity and accurate quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Monoclonal antibody, ELISA method and kit for detecting benzodiazepines
ActiveCN104530240BIncreased cross-reactivityExcellent cross-reactivity rateMicroorganism based processesTissue cultureBenzodiazepineNitrazepam
Owner:HUAZHONG AGRI UNIV
Preparation method of clonazepam molecularly imprinted stirring rod coating
InactiveCN105688857BImprove stabilityImprove bindingOther chemical processesSolid sorbent liquid separationMagnetite NanoparticlesSolvent
The invention discloses a preparation method of a clonazepam molecular imprinting stirring rod coating. The method comprises the following processes of preparation and activation of a magnetic stirring rod; preparation of magnetic nanoparticles modified by diaminobenzene carboxyl in the tail end; preparation of a magnetic field induced self-assemble molecular imprinting stirring rod. The out-field self-assemble molecular imprinting stirring rod prepared through the method provided by the invention has specific recognition capability on benzodiazepines compounds, compared with the traditional stirring rod, polymerization steps are reduced, the adsorption amount is increased, a molecular recognition mode with multiple functions is provided, and the adsorption efficiency in a polar solvent is obviously increased. The accuracy of the method when being applied to a practical sample test can be improved; the coating has no breakage or falling off phenomenon after being used repetitively, and is coupled with chromatography so as to be applied to separation and concentration of the benzodiazepines compounds in complex matrixes such as health food, feed and biological samples.
Owner:NANJING MEDICAL UNIV
Application of clonazepam to preparation of medicine for treating adolescent depression
InactiveCN108992449AHas anti-adolescent depression effectsOrganic active ingredientsNervous disorderClonazepamDrug
The invention discloses application of clonazepam to preparation of a medicine for treating adolescent depression. Experiment researches prove that, the clonazepam has the effect of resisting adolescent depression. Therefore, the clonazepam can be used for preparing the adolescent depression, and has wide application prospects.
Owner:SOUTHERN MEDICAL UNIVERSITY
Preparation method of clonazepam molecular imprinting stirring rod coating
InactiveCN105688857AImprove stabilityImprove bindingOther chemical processesSolid sorbent liquid separationMagnetite NanoparticlesBiological activation
The invention discloses a preparation method of a clonazepam molecular imprinting stirring rod coating. The method comprises the following processes of preparation and activation of a magnetic stirring rod; preparation of magnetic nanoparticles modified by diaminobenzene carboxyl in the tail end; preparation of a magnetic field induced self-assemble molecular imprinting stirring rod. The out-field self-assemble molecular imprinting stirring rod prepared through the method provided by the invention has specific recognition capability on benzodiazepines compounds, compared with the traditional stirring rod, polymerization steps are reduced, the adsorption amount is increased, a molecular recognition mode with multiple functions is provided, and the adsorption efficiency in a polar solvent is obviously increased. The accuracy of the method when being applied to a practical sample test can be improved; the coating has no breakage or falling off phenomenon after being used repetitively, and is coupled with chromatography so as to be applied to separation and concentration of the benzodiazepines compounds in complex matrixes such as health food, feed and biological samples.
Owner:NANJING MEDICAL UNIV
Clonazepam tablet and preparation method thereof
ActiveCN110420190AGood formabilityHigh dissolution rateOrganic active ingredientsNervous disorderFiller ExcipientDissolution
The invention relates to a clonazepam tablet. The clonazepam tablet is prepared from the following raw materials in parts by weight: 0.5-1.5 parts of clonazepam, 25-50 parts of a first filling agent,5-50 parts of a second filling agent, 0.05-1.5 parts of a binding agent, 0.05-1 part of a first lubricating agent, 0.05-0.5 part of a second lubricating agent and appropriate amount of purified water;the first lubricating agent is talcum powder, and the second lubricating agent is magnesium stearate. The preparation process comprises the working procedures of raw material weighing and preparation, material mixing, mucilage dispensing, wet granulation, straightening granulation, total mixing, tableting, packaging and the like. According to the clonazepam tablet and the preparation method thereof, the preparation method is suitable for large-scale and industrial generalizing and production, a prepared product is better in compactibility, better in dissolution rate, higher in chemical stability, and the safety of drug use and the effectiveness are effectively improved.
Owner:HUNAN DONGTING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com