Preparation method of benzodiazothioketone serving as intermediate of alprazolam
A technology of alprazolam and heterothione, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of unfavorable industrial production product quality, large dosage, expensive sodium iodide, etc. The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
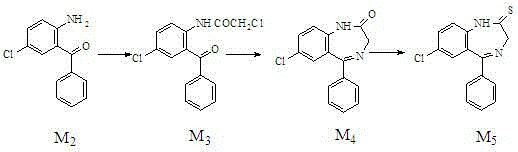
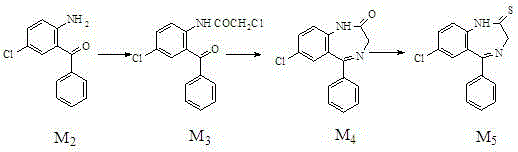
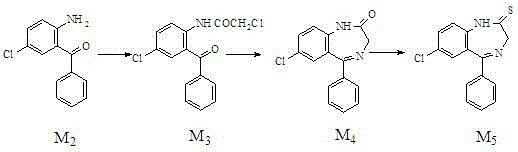
[0018] A kind of preparation method of alprazolam intermediate benzodiazepine thione,
[0019]
[0020] Specific steps are as follows:
[0021] 1. Acylation reaction
[0022] In the reaction flask, add 2-amino-5-chloro-benzophenone (M 2 ) 250g (1.08mol), cyclohexane 1750g, stirred and heated to 60°C, 160g (1.36mol) of chloroacetyl chloride was added dropwise, then heated to 81°C, refluxed for 4h (hydrogen chloride was released during the reaction). After the reaction was completed, it was cooled to 10°C, and light yellow crystals were precipitated. Filter and dry to obtain light yellow crystal M 3 320g, yield 95%, HPLC content>98%, mp117~119℃.
[0023] 2. Cyclization reaction
[0024] Add M to the reaction vial 3 320g (1.03mol), 3200g absolute ethanol, 260g urotropine (that is, hexamethylenetetramine), heat and stir to 50°C, add 142g (1.80mol) of ammonium bicarbonate, ammonium bicarbonate in 3 hours Average added. Reacted at 40°C for 6 hours, followed by HPLC until ...
Embodiment 2
[0030] A kind of preparation method of alprazolam intermediate benzodiazepine thione, comprises the steps:
[0031] 1. Acylation reaction
[0032] In the reaction flask, add 2-amino-5-chloro-benzophenone (M 2 ) 250g (1.08mol), cyclohexane 1750g, stirred and heated to 59°C, 160g (1.36mol) of chloroacetyl chloride was added dropwise, then heated to 70°C, and reacted for 6h (hydrogen chloride was released during the reaction). After the reaction was completed, the material was discharged while it was hot, cooled to 10°C, and light yellow crystals were precipitated. Filter and dry to obtain light yellow crystal M 3 305g, yield 91%, HPLC content > 98%. mp117~119℃.
[0033] 2. Cyclization reaction
[0034] Add M to the reaction vial 3 305g (0.98mol), 3050g absolute ethanol, 190g urotropine, heated and stirred to 60°C, added 237g (3mol) of ammonium bicarbonate, and the ammonium bicarbonate was added evenly within 3 hours. React at 60°C for 5 hours, and track and detect by HPLC...
Embodiment 3
[0040] A kind of preparation method of alprazolam intermediate benzodiazepine thione, comprises the steps:
[0041] 1. Acylation reaction
[0042] In the reaction flask, add 2-amino-5-chloro-benzophenone (M 2 ) 250g (1.08mol), cyclohexane 1750g, stirred and heated to 61°C, 160g (1.36mol) of chloroacetyl chloride was added dropwise, then heated to 70°C, and reacted for 6h (hydrogen chloride was released during the reaction). After the reaction was completed, the material was discharged while it was hot, cooled to 10°C, and light yellow crystals were precipitated. Filter and dry to obtain light yellow crystal M 3 320g, yield 91%, HPLC content>98%, mp117~119℃.
[0043] 2. Cyclization reaction
[0044] Add M to the reaction vial 3320g (1.03mol), 3200g absolute ethanol, 260g urotropine, heated and stirred to 60°C, and 166g (2.1mol) of ammonium bicarbonate was added several times. Reacted at 80°C for 4 hours, followed by HPLC detection until the reaction of the raw materials w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com