Hydrogel-forming sustained-release preparation
a technology of sustained release and hydrogel, which is applied in the direction of pill delivery, pharmaceutical non-active ingredients, pharmaceutical delivery mechanism, etc., can solve the problems of little research on the general consideration of drug release, and the inability to anticipate drug release in the colon, etc., to achieve rapid drug release, reduce the amount of hydrogel-forming base, and increase the amount of drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0117]
9 AAP 100 (Parts by weight) PEG6000 400 POLYOX303 300
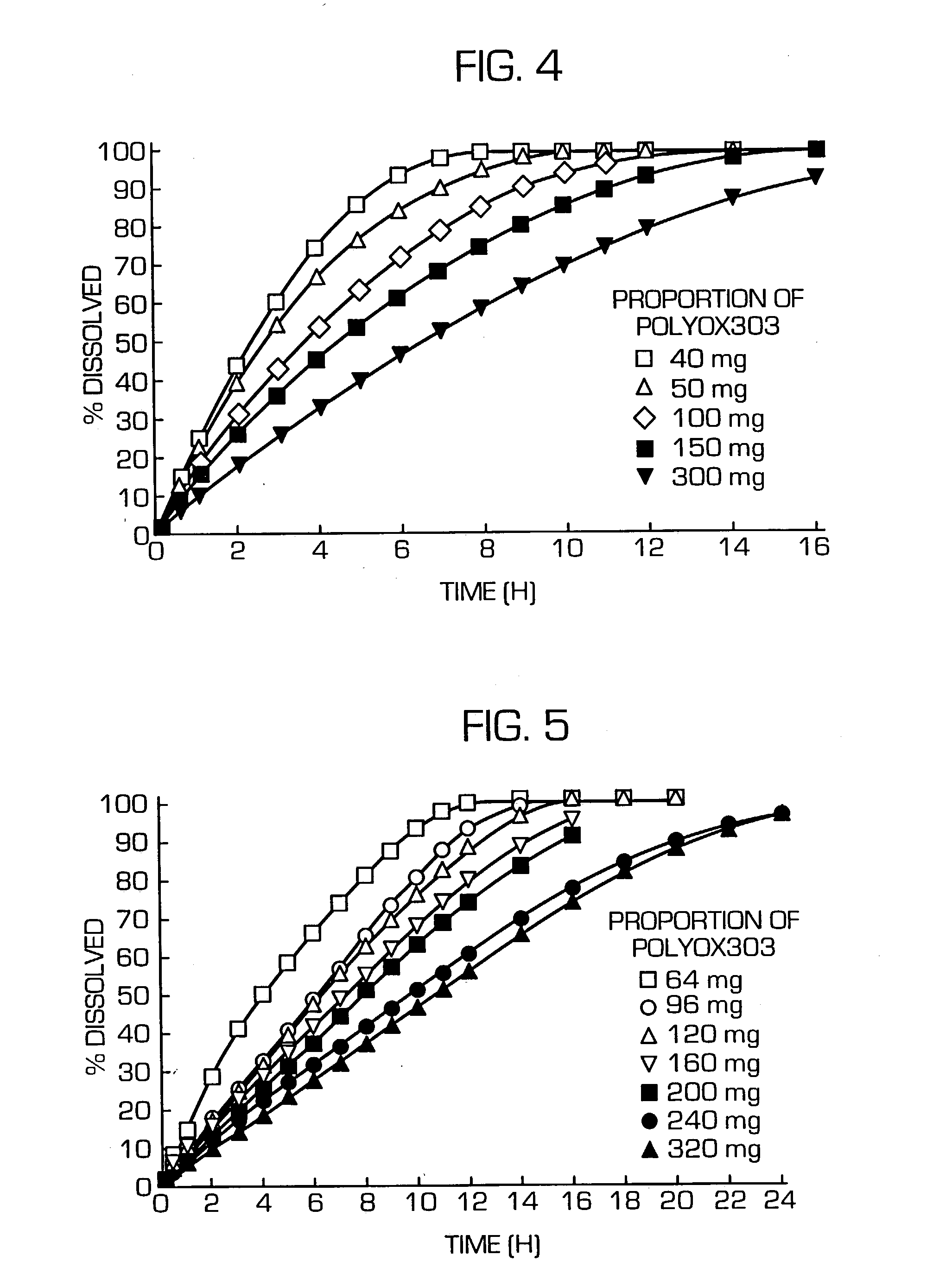
[0118] Acetaminophen (AAP) and PEG6000 were melted at 80.degree. C., then cooled to solidify, and pulverized. The pulverizate and POLYOX303 were mixed in a mortar and the resulting composition was compression-molded using an oil press at a compression pressure of 1 ton / punch to provide tablets each measuring 9 mm in diameter and weighing 400 mg (AAP content: 50 mg).
example 2
[0136]
16 Pd 160 (Parts by weight) HCO-60 80 TC-5E 160 PEG6000 400 POLYOX303 240
[0137] Nicardipine hydrochloride (Pd), HCO-60, TC-5E and PEG6000 were dissolved in a solvent mixture (dichloromethane-methanol) and the solution was spray-dried using a spray dryer. This dry preparation was mixed with POLYOX303 in a mortar and the resulting composition was compression-molded using an oil press at a compression pressure of 1 ton / punch to provide tablets each measuring 9.0 mm in diameter and weighing 346.7 mg (Pd content: 53.3 mg).
example 3
[0149]
21 Pd 65 (Parts by weight) Tween 80 13 Sustained-Release (SR) Component CMEC 65 PEG6000 65 POLYOX303 65 Pd 15 Immediate-Release (QR) Component
[0150] Nicardipine hydrochloride (Pd), Tween 80 and CMEC were dissolved in a solvent mixture (dichloromethane-methanol) and the solution was spray-dried using a spray dryer. The dried mixture was blended with PEG6000 and POLYOX303 and the resulting composition was compression-molded using an oil press at a compression pressure of 1.0 ton / punch to provide tablets (SR) each measuring 8.5 mm in diameter and weighing 273 mg (QR; Pd content: 65 mg). For use as the immediate-release (QR) component, tablets each containing 15 mg of Pd were separately prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com