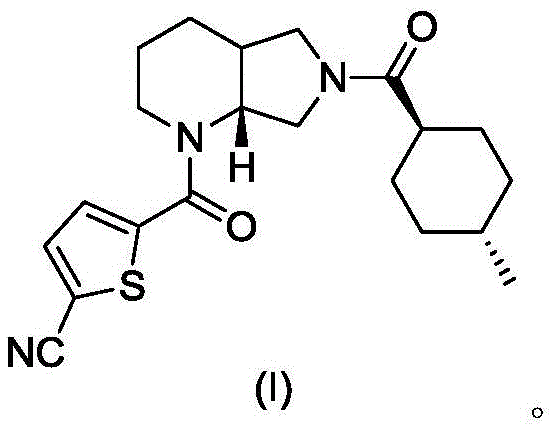
A blood coagulation factor Xa inhibitor containing bicyclic amide structure, its preparation method and use
One aspect, the technology of venous thrombosis, applied in blood diseases, extracellular fluid diseases, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthesis of embodiment 1 compound I [0001]
[0018]
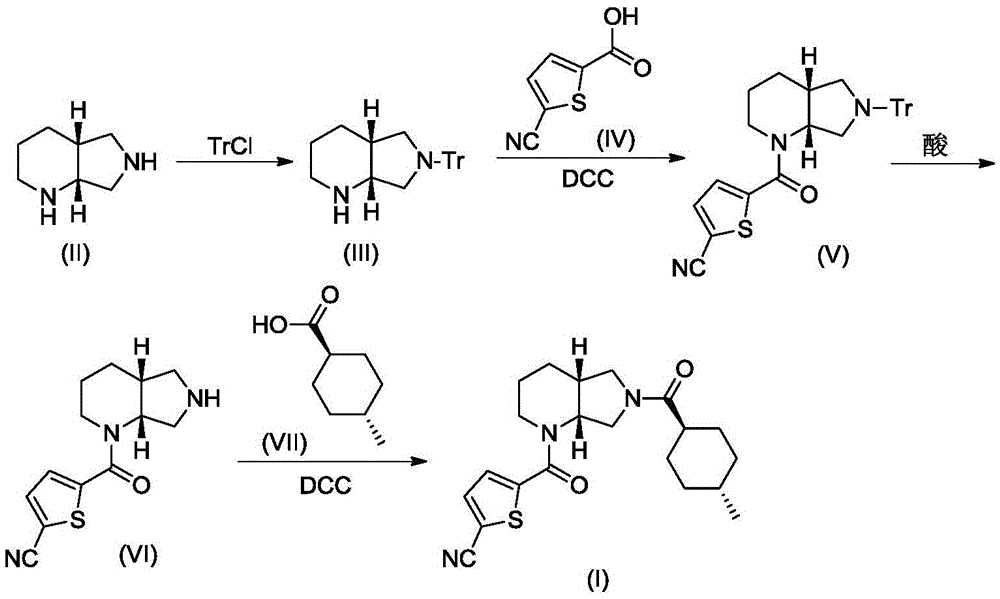
[0019] A. Synthesis of Compound III
[0020] Compound II (1.26g, 10mmol) and triethylamine (3.04g, 30mmol) were dissolved in 10mL of dry DMF, stirred under cooling in an ice-water bath, slowly added dropwise from triphenylchloromethane (TrCl; 3.07g, 11mmol) and 10 mL of dry DMF solution. After the addition was complete, the resulting reaction mixture was stirred at room temperature for 3 hours, and TLC showed that the reaction was complete. The reaction mixture was poured into 120 mL of ice water, CH 2 Cl 2 (50mL×3) was extracted, and the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound III, a white solid, ESI-MS, m / z=369 ([M+H] + ).
[0021] B. Synthesis of Compound V-1 ...
Embodiment 2
[0027] The synthesis of embodiment 2 reference compound D-1
[0028] Compound D-1 is also a compound designed by the present inventors in the course of research (not yet published).
[0029]
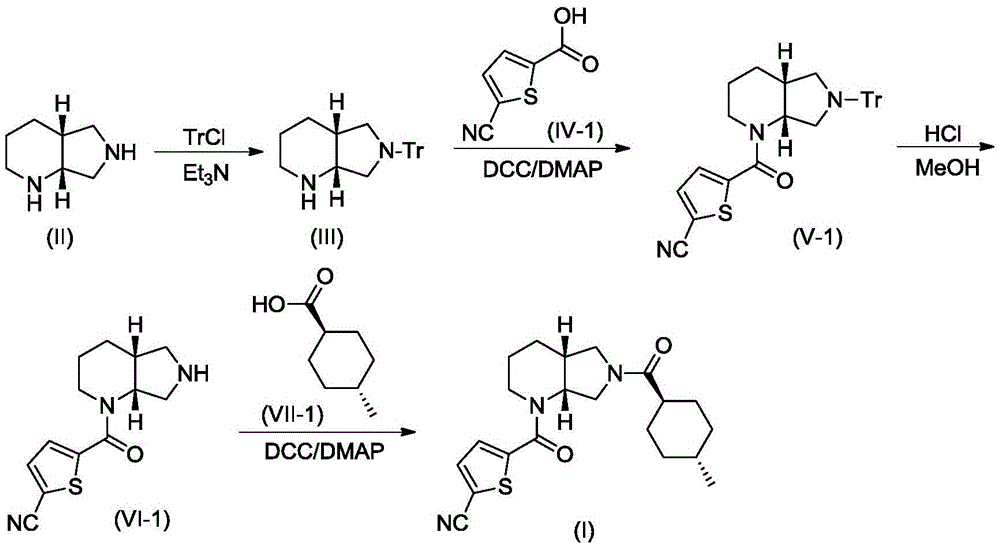
[0030] A. Synthesis of Compound III
[0031] Compound II (1.26g, 10mmol) and triethylamine (3.04g, 30mmol) were dissolved in 10mL of dry DMF, stirred under cooling in an ice-water bath, slowly added dropwise from triphenylchloromethane (TrCl; 3.07g, 11mmol) and 10 mL of dry DMF solution. After the addition was complete, the resulting reaction mixture was stirred at room temperature for 3 hours, and TLC showed that the reaction was complete. The reaction mixture was poured into 120 mL of ice water, CH 2 Cl 2 (50mL×3) was extracted, and the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by ...
Embodiment 3
[0038] Inhibitory test of embodiment 3 compound to FXa in vitro
[0039] 5% DMSO solution (10 μ L) of the embodiment compound to be tested and positive drug EDOXABAN (its concentration is set appropriately step by step), Tris buffer (100 mM Tris, 200 mM potassium chloride, 0.2% BSA, pH 7.4) (40 μ l) and 0.0625U / mL human FXa (Enzyme Research Laboratories, Inc., dissolved and diluted (10 μl) in Tris buffer were placed in the wells of a 96-well microplate, and a 750 μM aqueous solution (40 μl) of S2222 (Chromogenix Co.) was added, The increase in absorbance (ΔOD / min) was measured by measuring the absorbance at 405 nm at room temperature for 10 minutes. As a negative control, Tris buffer was used instead of the test compound.
[0040] According to the following formula, the percent inhibitory rate (%) at the initial concentration of the test compound and the final concentration of the test compound are respectively plotted on the ordinate and abscissa of the logarithmic orthogon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com