Polypeptide for inhibiting activity of bacillus subtilis transglutaminase (BTG) and its screening method and use
A technology of transglutaminase and Bacillus subtilis, which is applied in the biological field, can solve the problems of toxicity, low enzyme yield, and no patent publications have been found, and achieve the effects of high-efficiency expression, release of inhibitory effects, and mitigation of toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be further described in detail below through the specific examples, the following examples are only descriptive, not restrictive, and cannot limit the protection scope of the present invention with this.
[0030] The methods in the following examples are conventional methods unless otherwise specified.
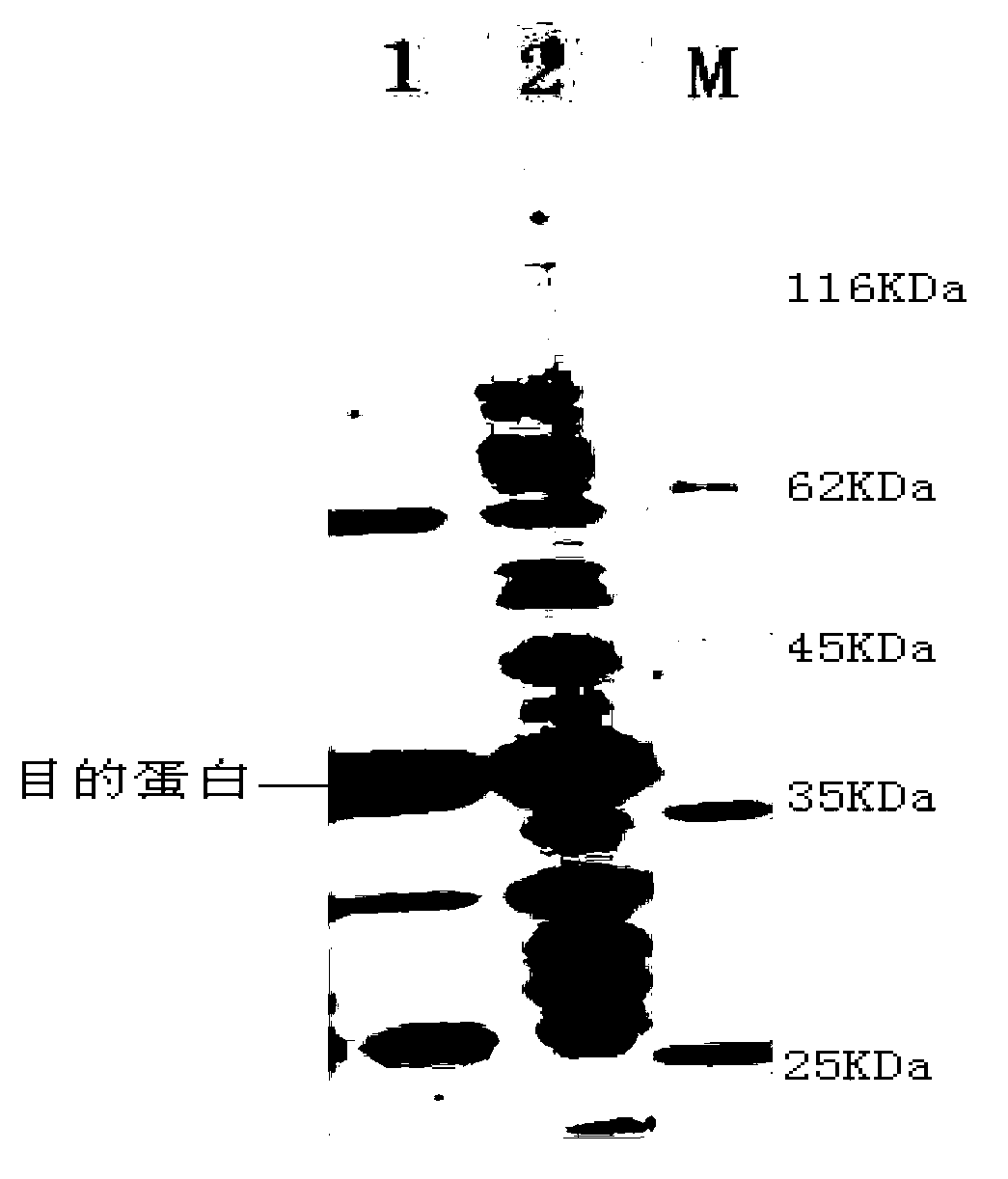
[0031] TG derived from Streptomyces has a signal peptide and a propeptide. Due to the action of the propeptide, TG derived from Streptomyces is inactive in cells and cannot function as a cross-linking protein, thereby not damaging cells. However, BTG derived from Bacillus subtilis has no signal peptide and propeptide, and only has an active mature peptide. Therefore, it is hoped that the activity of BTG can be inhibited by the propeptide of Streptomyces TG.
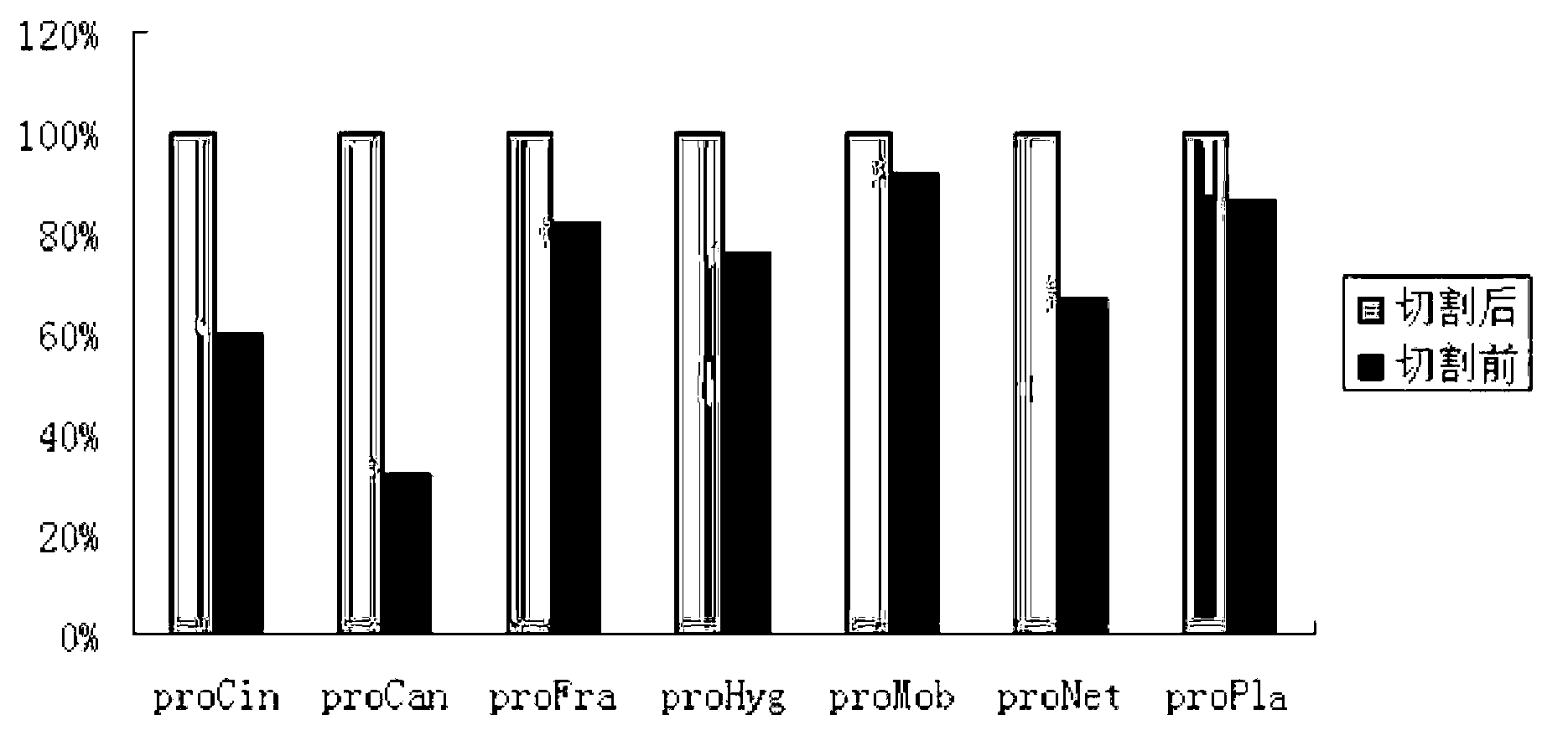
[0032] Selected and compared the TG propeptide sequences from 7 different Streptomyces (S.cinnamoneus, S.caniferus, S.fradiae, S.hygroscopicus, S.mobaraense, S.netropsis, S.platensis), re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com