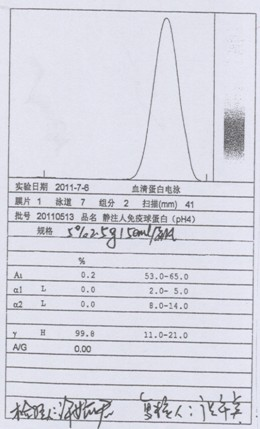
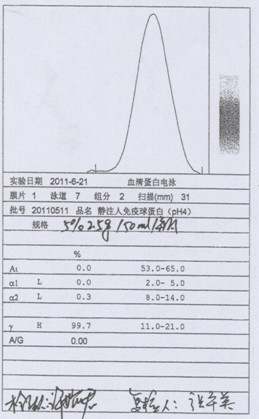
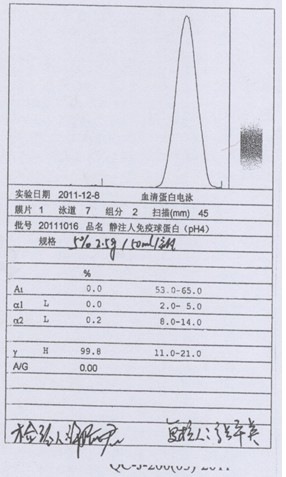
Process for preparing human immunoglobulin for intravenous injection
A technology of human immunoglobulin and preparation process, which is applied in the field of biopharmaceuticals, can solve problems such as the influence of product purity, and the effect of coagulation factors is not obvious, and achieve the effects of improving product quality, improving product biological activity, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: The preparation process is as follows:
[0043] (1) Centrifuge the qualified human plasma during the quarantine period. When centrifuging, the centrifugation speed should not exceed 4L / min / unit, and the temperature of the liquid should be controlled at 0-4°C; the cryoprecipitate should be separated, and the cryoprecipitate should be used for the production of factor VIII. Transfer the centrifuged supernatant protein solution to the protein separation reaction tank, so that the temperature of the protein solution is controlled between 1 and 3°C, and the pH of the protein solution is adjusted by adding pH 4.0 acetic acid buffer at a flow rate of no more than 1.0L / min to adjust the pH of the protein solution to 6.80~ 7.00; add -15°C or colder 95% ethanol, the flow rate does not exceed 1.5L / min, so that the final concentration of ethanol volume ratio reaches 8%, the temperature is controlled at -1 ~ -3°C, and the pH value is 6.80 after adding ethanol ~7.20; th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com