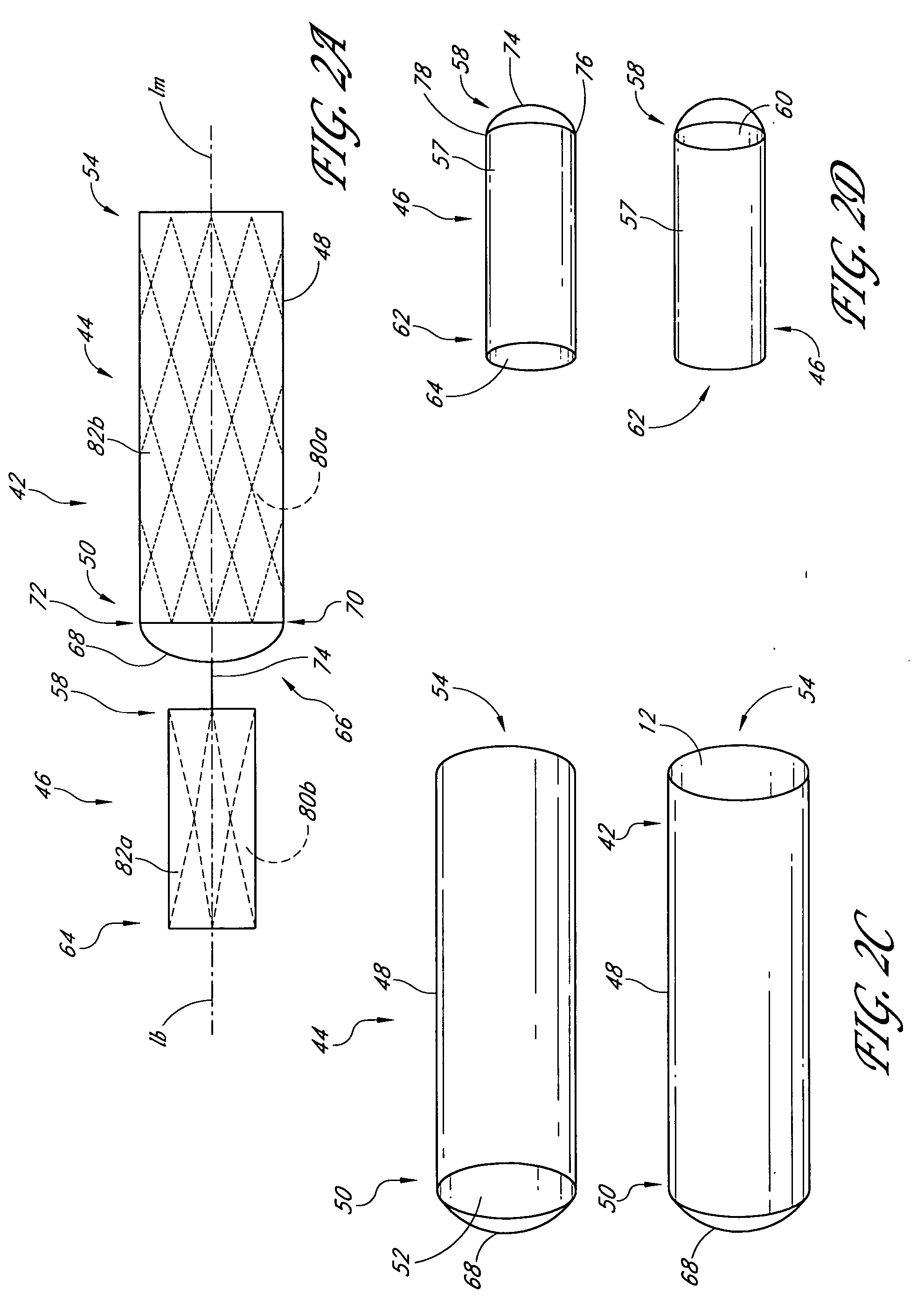
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Vascular grafting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
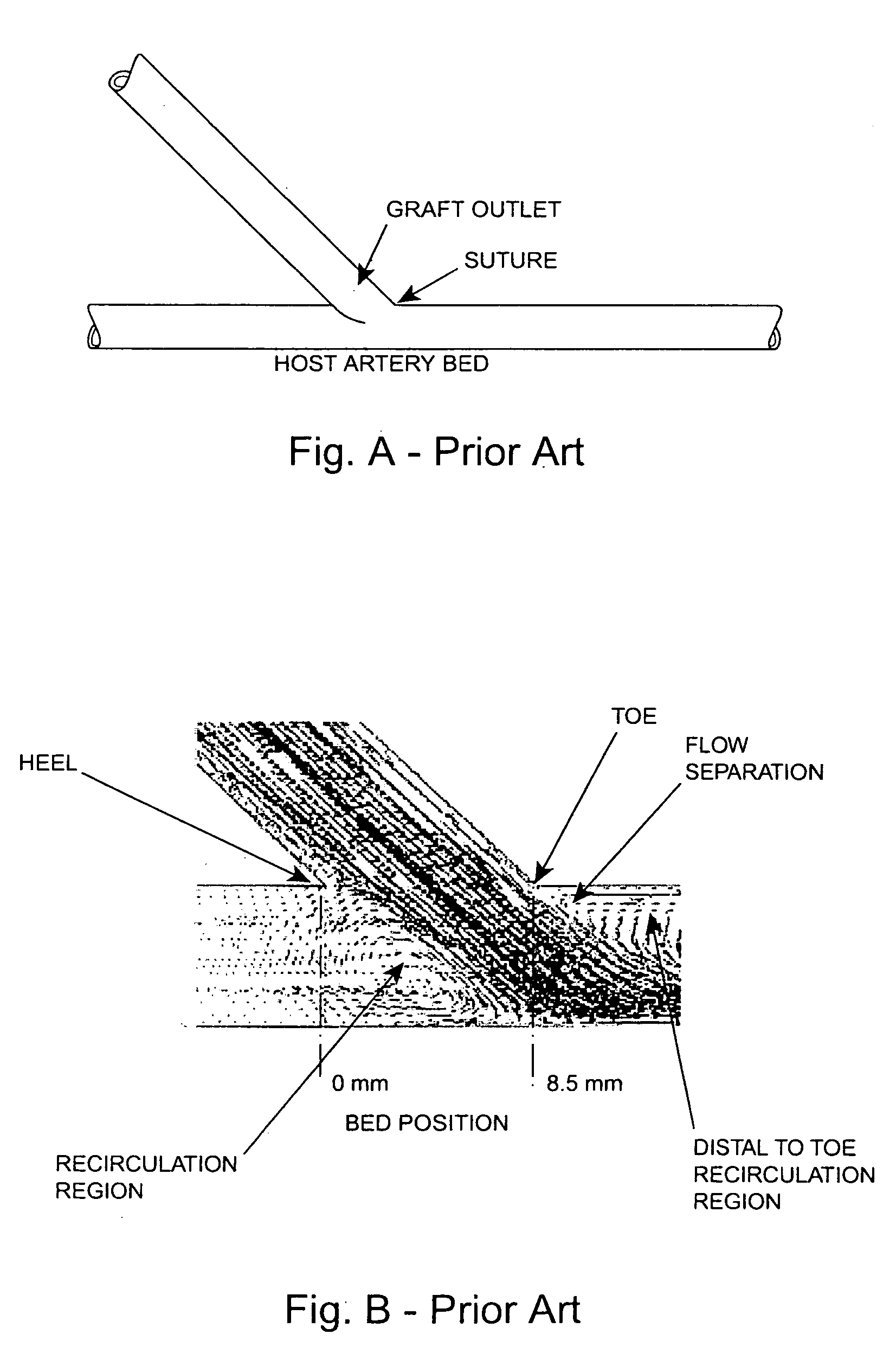
Vascular grafting is the use of transplanted or prosthetic blood vessels in surgical procedures. PTFE and Dacron are some of the most commonly used grafts. Grafts can be used for the aorta, femoral artery or in the forearm. Coronary artery bypass graft is used for people with occluded coronary arteries, and often the saphenous vein or left internal thoracic artery are used in this procedure.
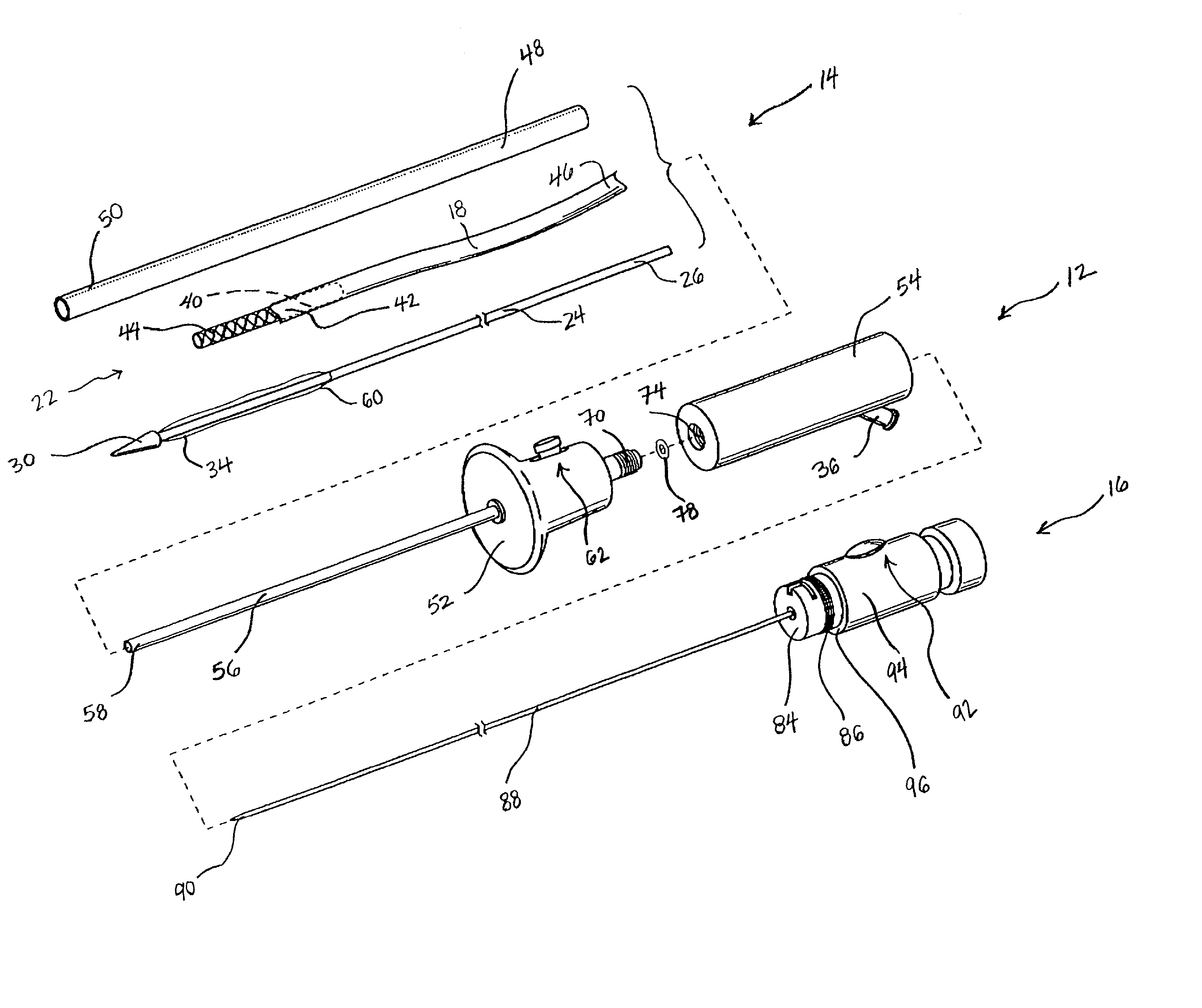
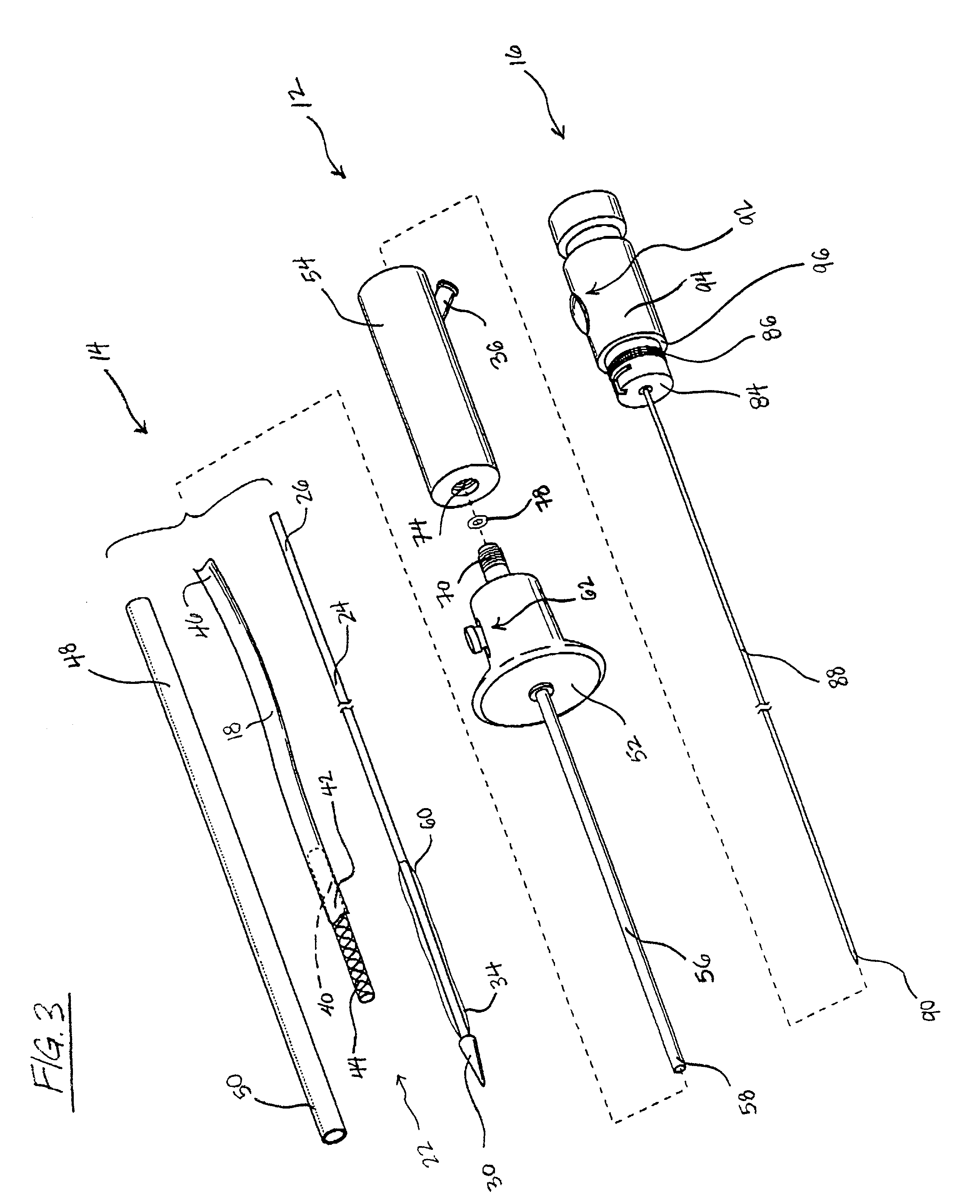
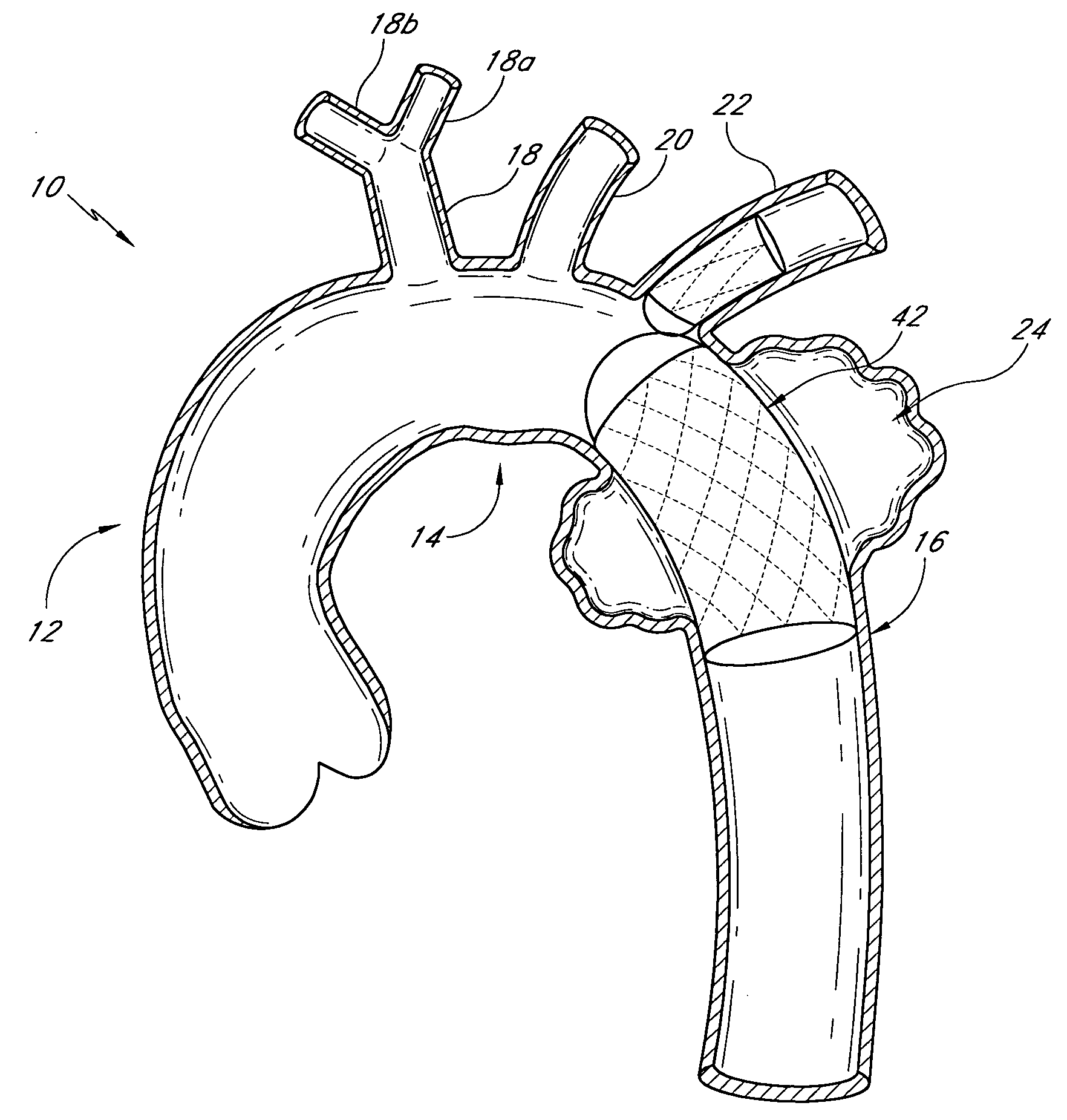
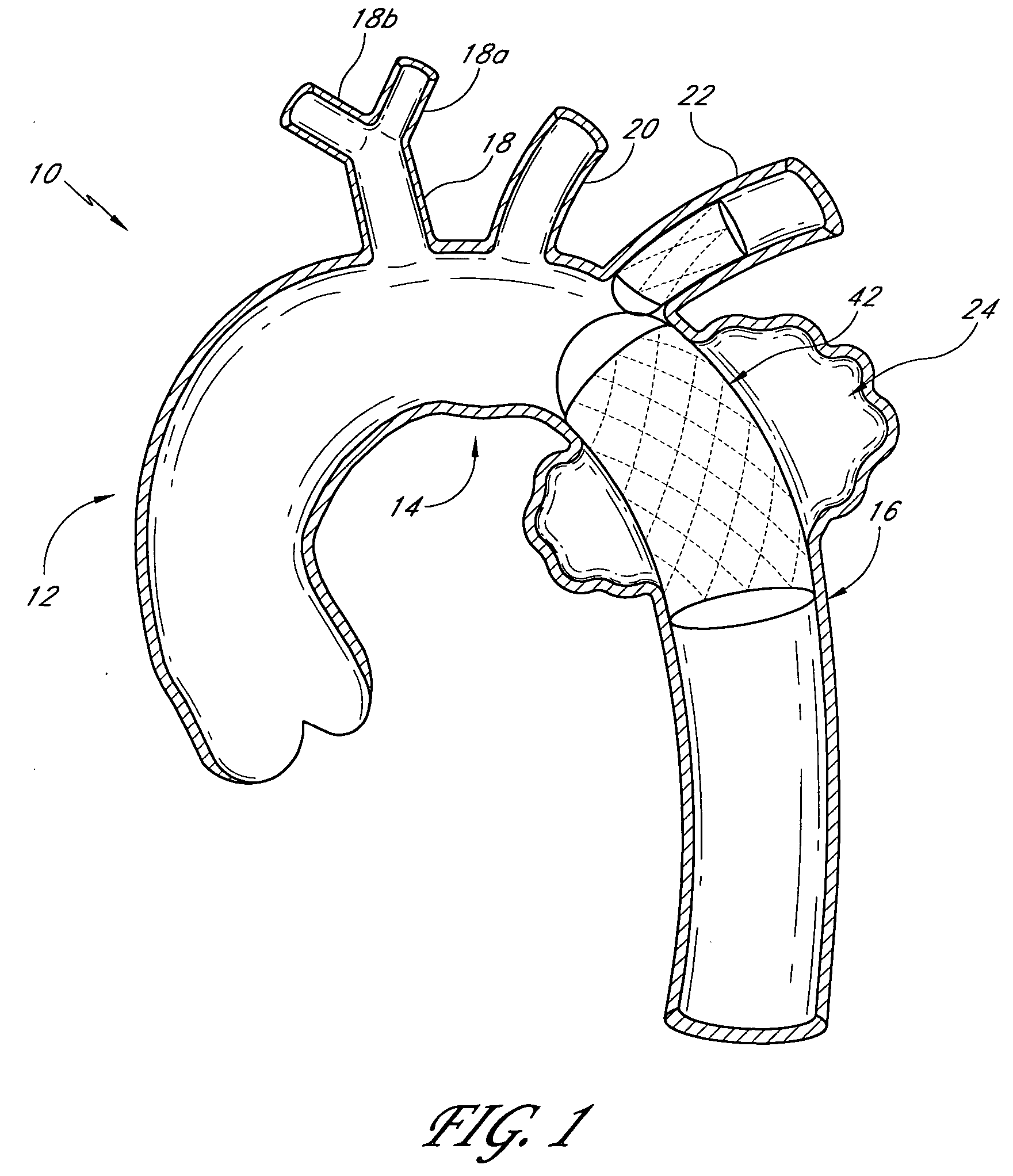
Methods and devices for bypassing an obstructed target vessel by placing the vessel in communication with a heart chamber containing blood
Methods and devices for forming an anastomosis during a bypass procedure utilize a graft vessel secured to a vessel coupling adapted to be fixed to a target vessel without using suture. The graft vessel is placed in fluid communication with a heart chamber containing blood. The vessel coupling may be collapsed for introduction into the target vessel and then expanded to fix the coupling thereto. The vessel coupling may be a stent with the graft vessel secured thereto to form a stent-graft assembly. The anastomosis is carried out to place the graft and target vessels in fluid communication while preserving native proximal flow through the target vessel, which may be a coronary artery. As a result, blood flowing from the aorta and past an obstruction in the coronary artery is not blocked by formation of the anastomosis; rather, such proximal blood flow is free to move past the vessel coupling and the anastomosis.
Owner:MEDTRONIC INC
Vascular graft and deployment system
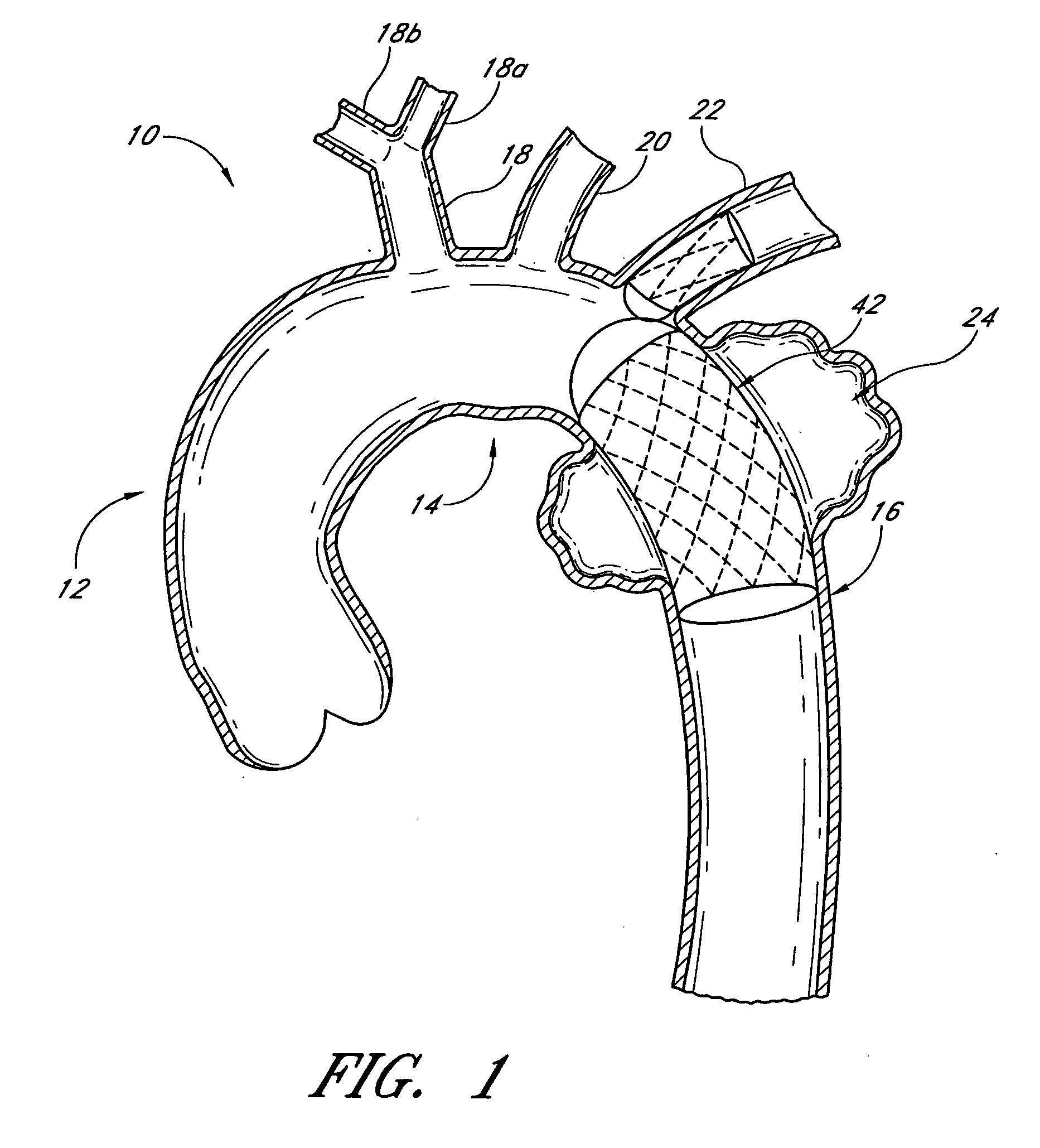
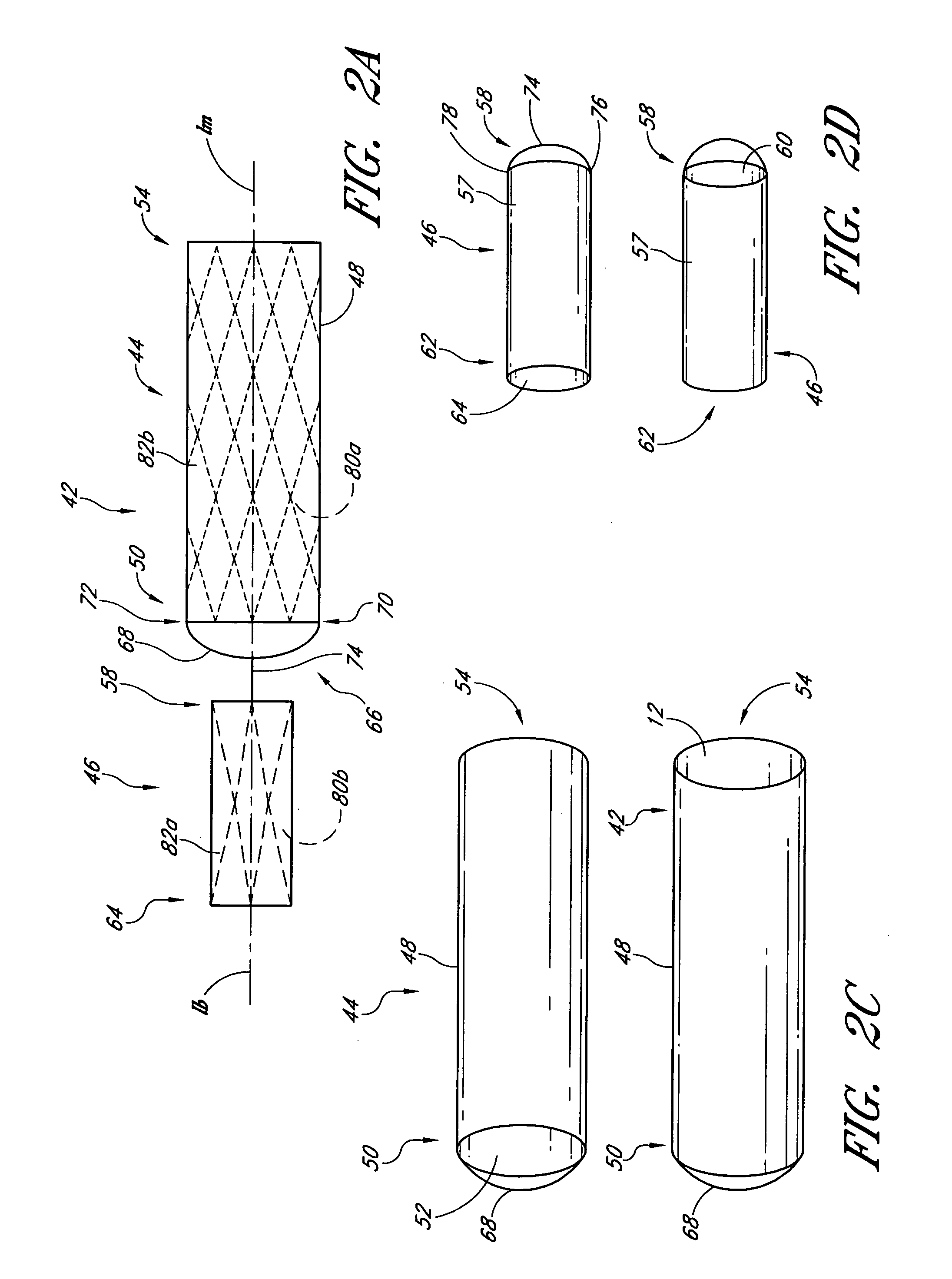
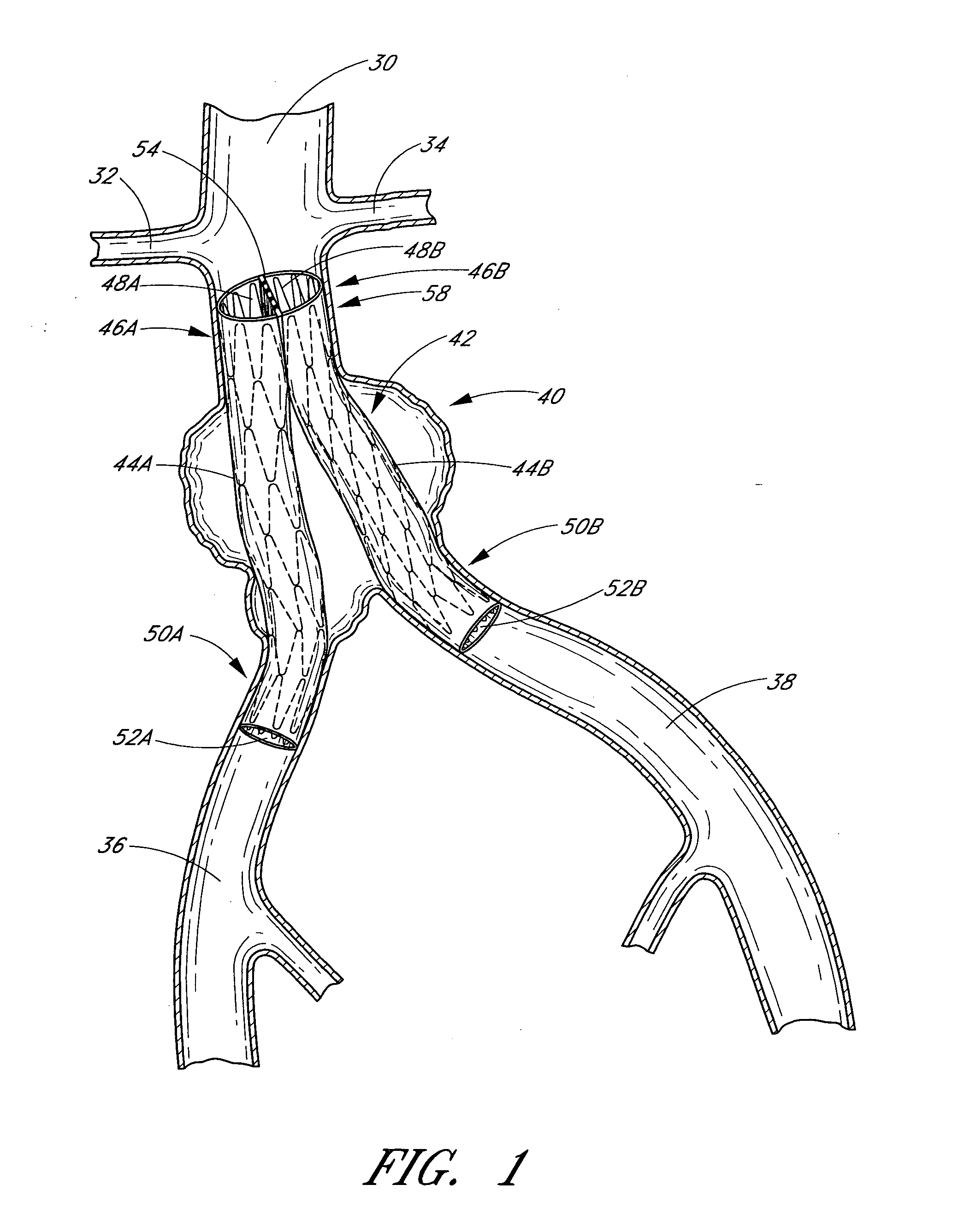
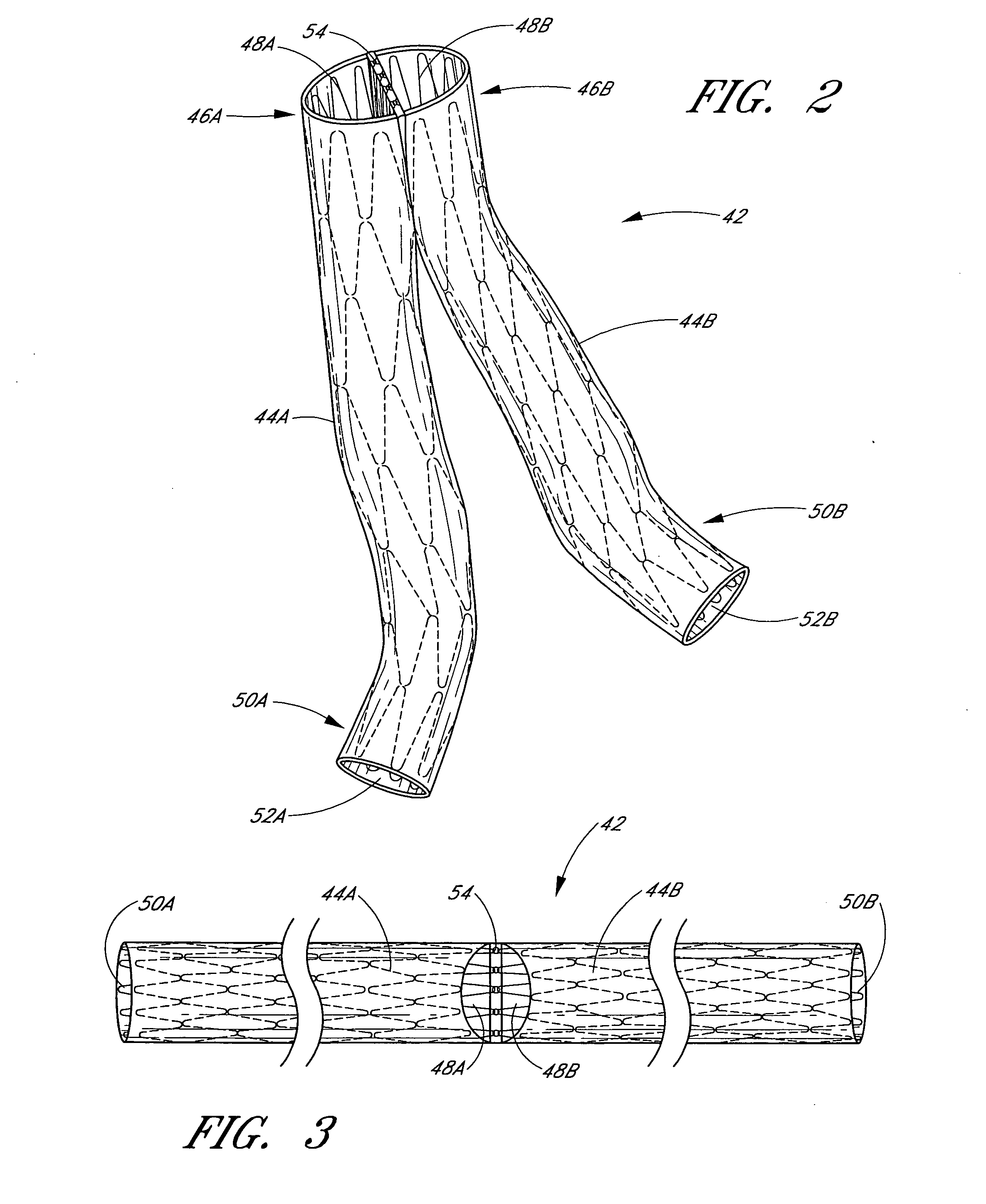
Disclosed is a method and apparatus for treating bifurcations of the vascular system, such as abdominal aneurysms at the bifurcation of the aorta and iliac arteries. A tubular implant having a first section, a second section and a magnetic connection therebetween is positioned across the bifurcation such that the proximal ends of the first and second sections extends into a first iliac and a second iliac respectively. Deployment catheters are also disclosed.
Owner:JACQUES SEGUIN
Vascular graft and deployment system
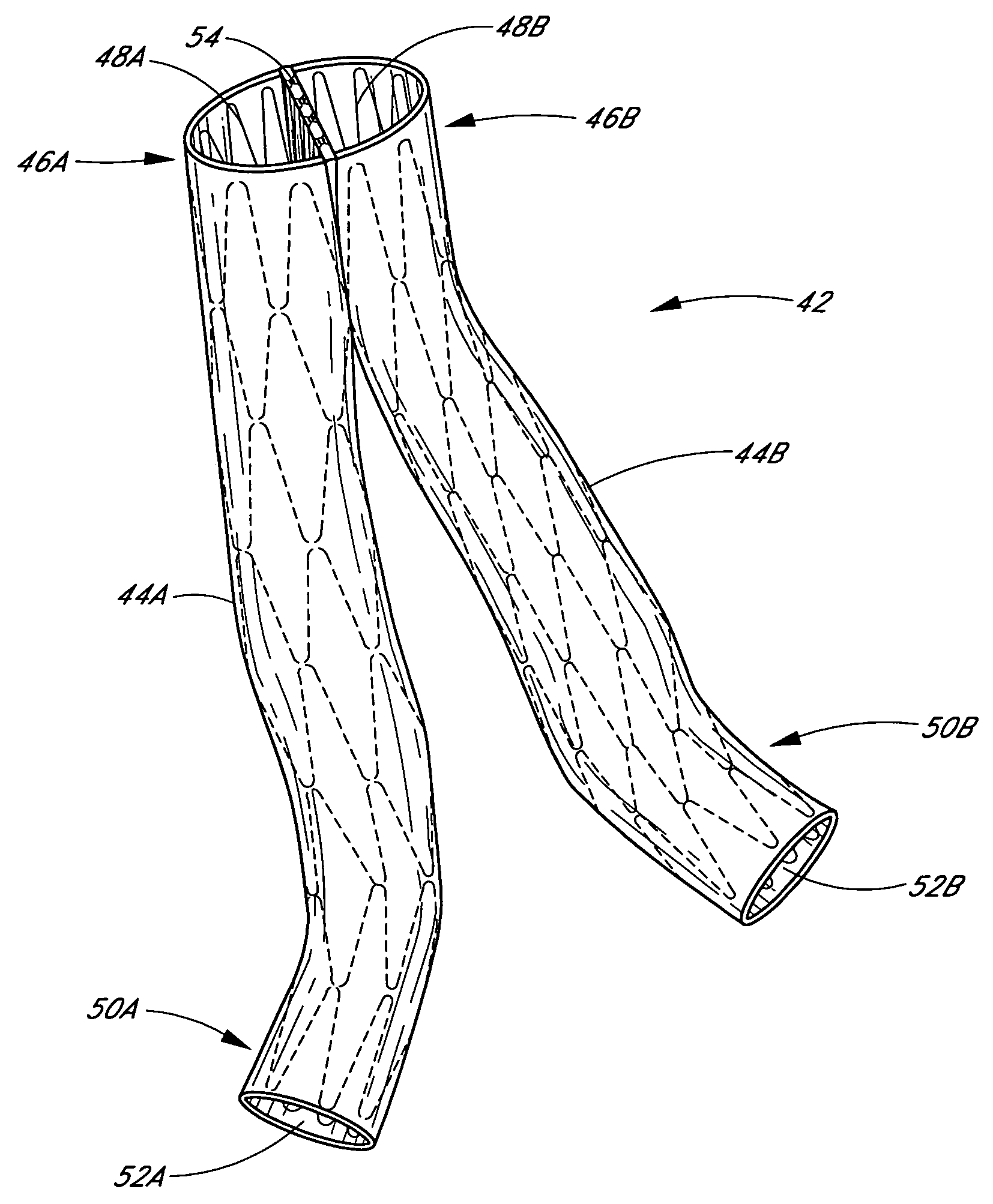
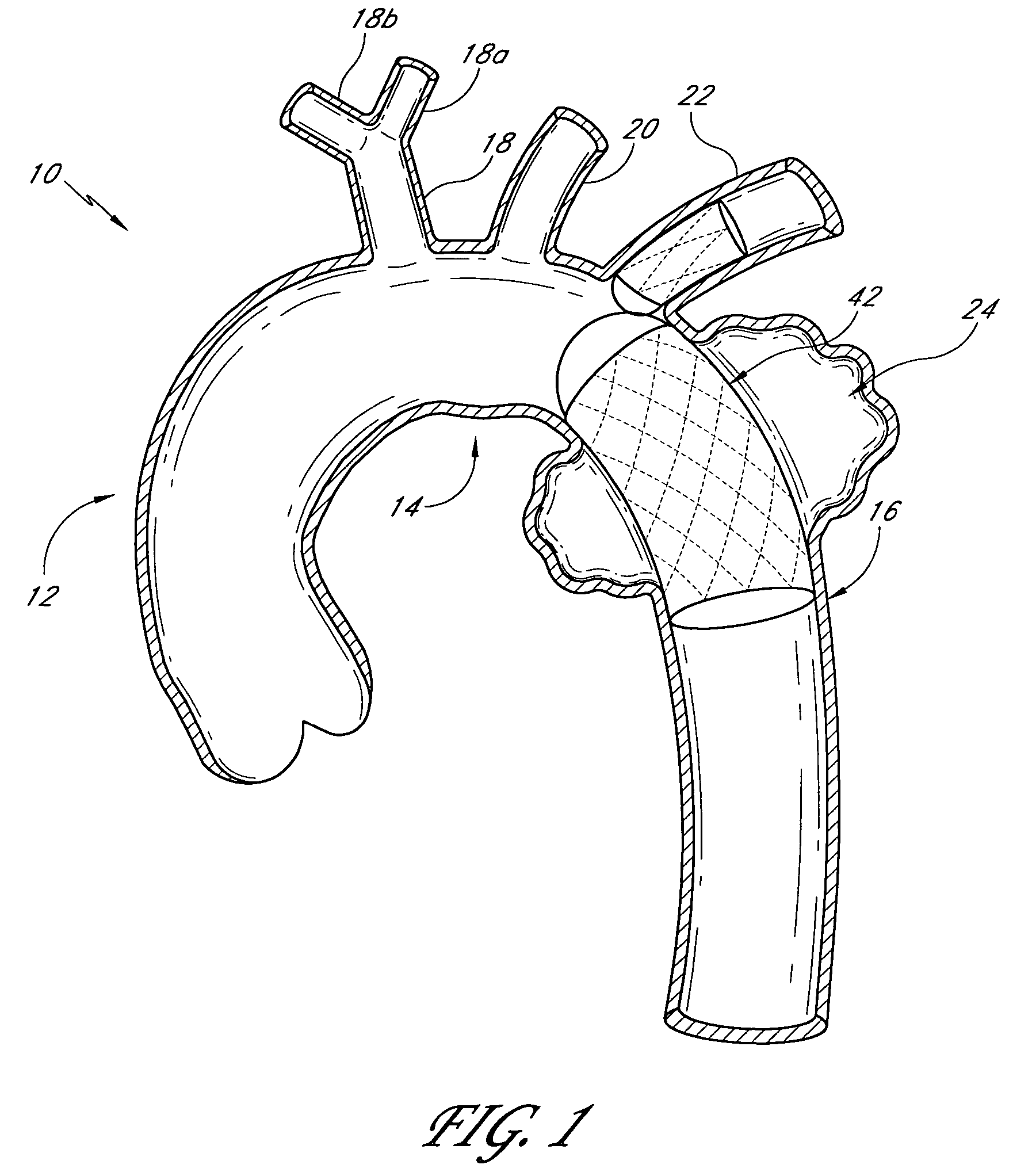
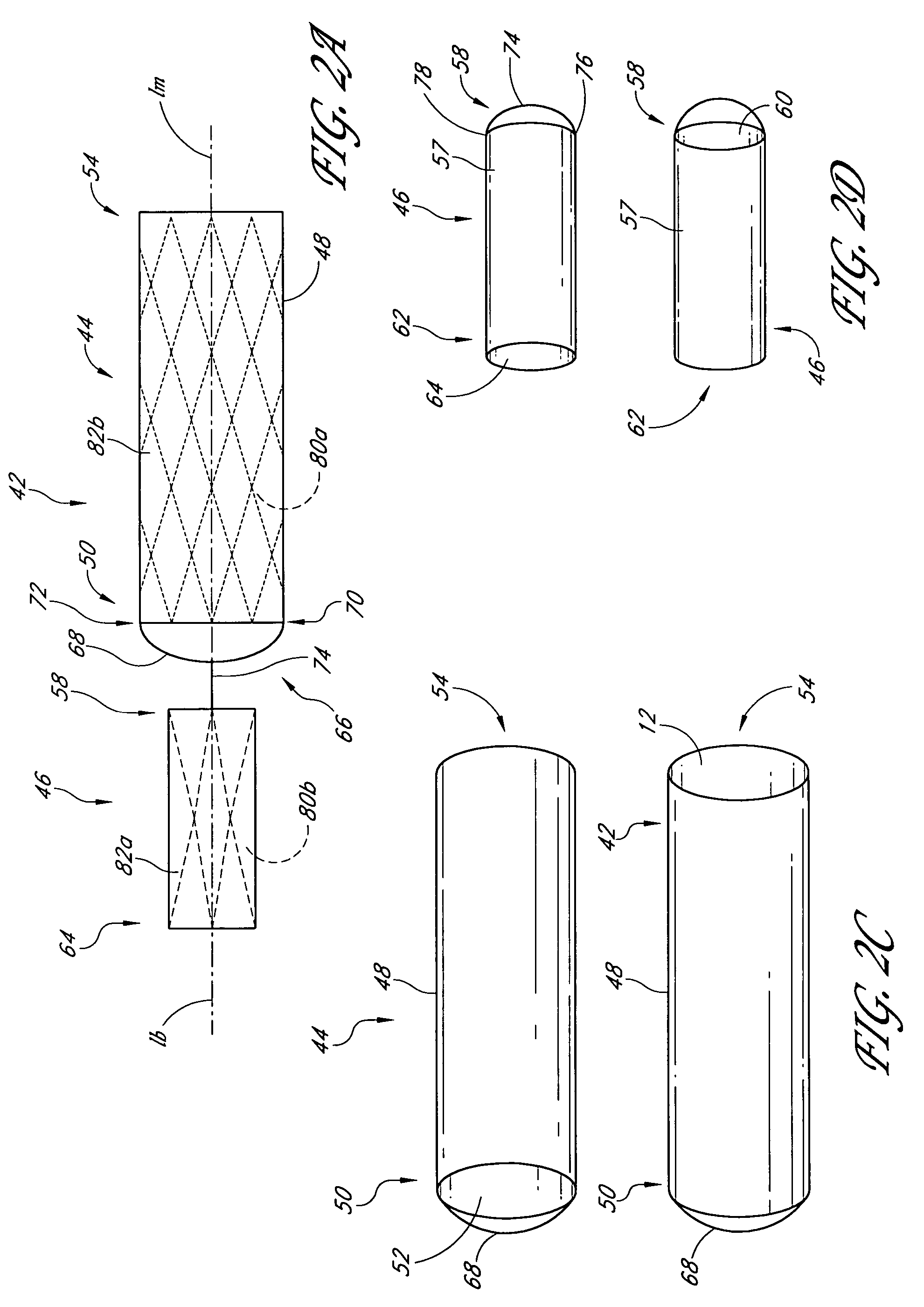
A vascular graft includes a main portion and a branch portion that is coupled to the main portion by an articulating joint. The vascular graft may be inserted into the thoracic aorta with the branch portion positioned within a branch vessel and the main portion positioned within the thoracic aorta. The graft may be deployed within a deployment apparatus comprising an outer member and an inner member and a pusher. The main graft portion may be housed within the inner member while the branch graft portion is housed within the space between the inner and outer members. The inner member may have a longitudinal groove for allowing the articulating joint to pass by when the branch graft portion is deployed.
Owner:DOUGLAS MYLES
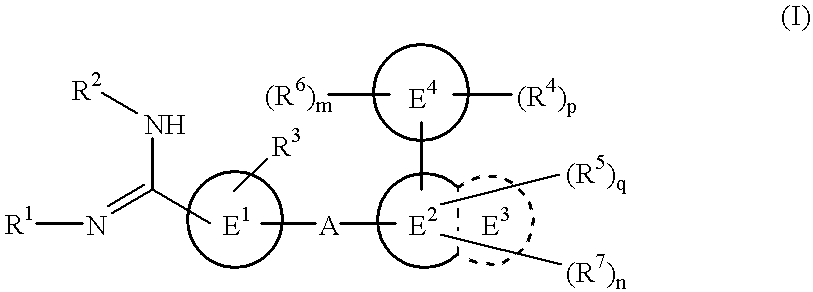
Amidino derivatives and drugs containing the same as the active ingredient
InactiveUS6358960B1BiocideGroup 5/15 element organic compoundsExtracorporeal circulationDisseminated coagulopathy
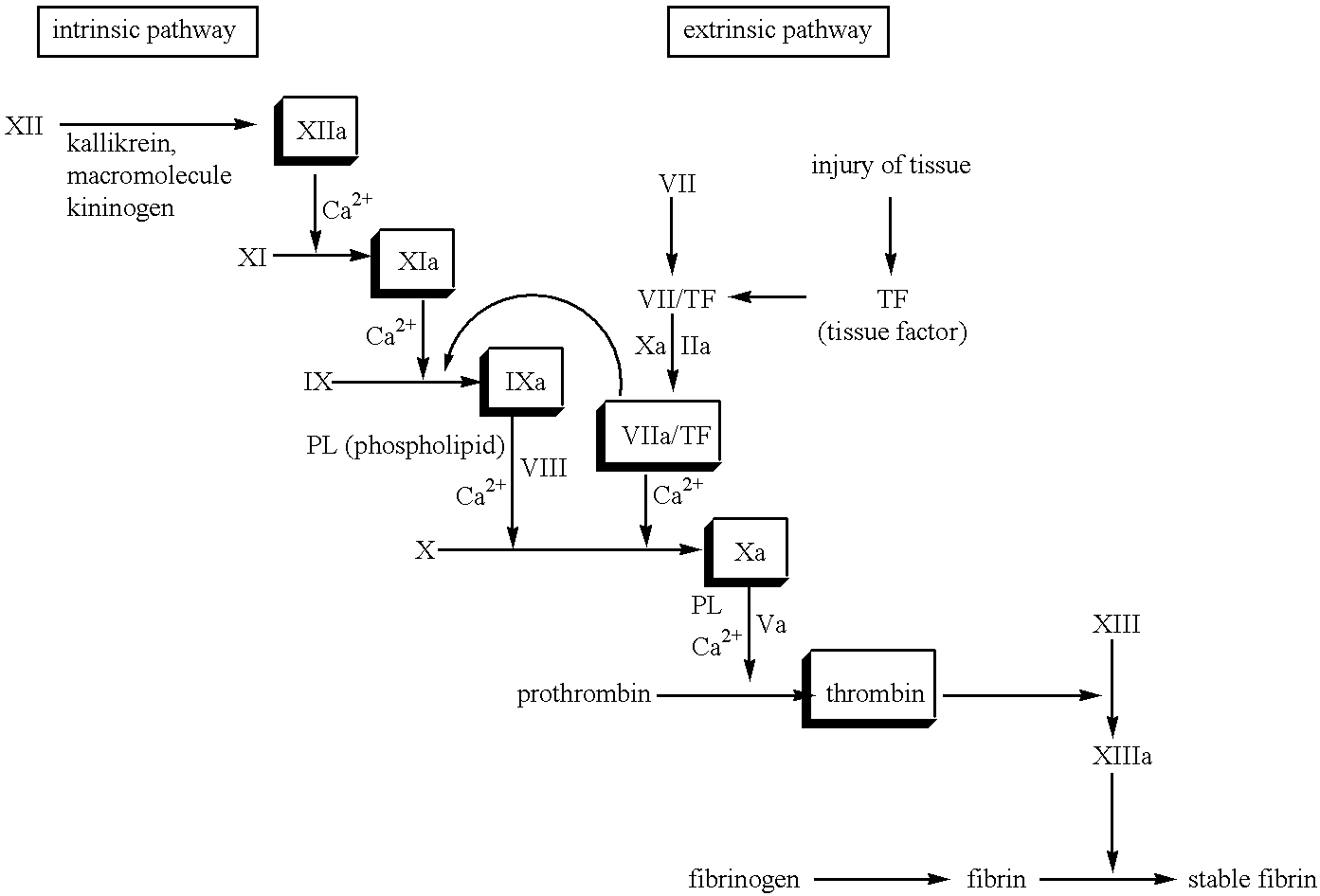
The novel amidino derivatives of the formula (I):wherein all the symbols are as in specification defined;have an inhibitory activity of a blood coagulation factor VIIa and are useful for treatment and / or prevention of several angiopathy caused by enhancing a coagulation activity, such as disseminated intravascular coagulation, coronary thrombosis, cerebral infarction, cerebral embolism, transient ischemic attack, cerebrovascular disorders, pulmonary vascular diseases, deep venous thrombosis, peripheral arterial obstruction, thrombosis after artificial vascular transplantation and artificial valve transplantation, post-operative thrombosis, reobstruction and restenosis after coronary artery bypass operation, reobstruction and restenosis after PTCA or PTCR, thrombosis by extracorporeal circulation and procoagulative diseases such as glomerlonephriitis.
Owner:ONO PHARMA CO LTD
Vascular graft and deployment system
A vascular graft includes a main portion and a branch portion that is coupled to the main portion by an articulating joint. The vascular graft may be inserted into the thoracic aorta with the branch portion positioned within a branch vessel and the main portion positioned within the thoracic aorta. The graft may be deployed within a deployment apparatus comprising an outer member and an inner member. The outer member may include an area of increased flexibility that corresponds to the articulating joint.
Owner:DOUGLAS MYLES
Vascular graft
InactiveUS20060229709A1Reduce the cross-sectional areaEliminate needStentsBlood vesselsVascular graftingVascular graft
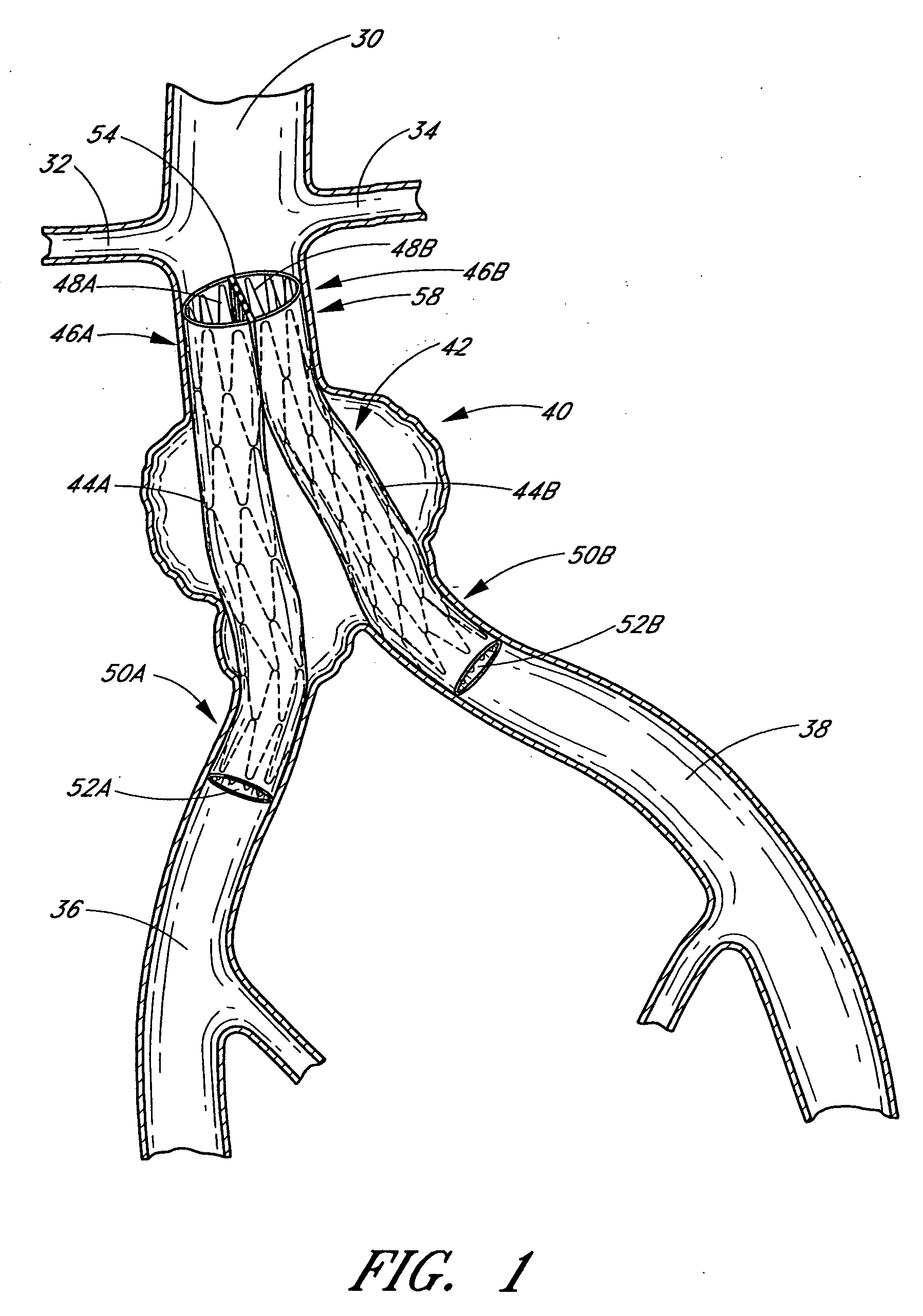
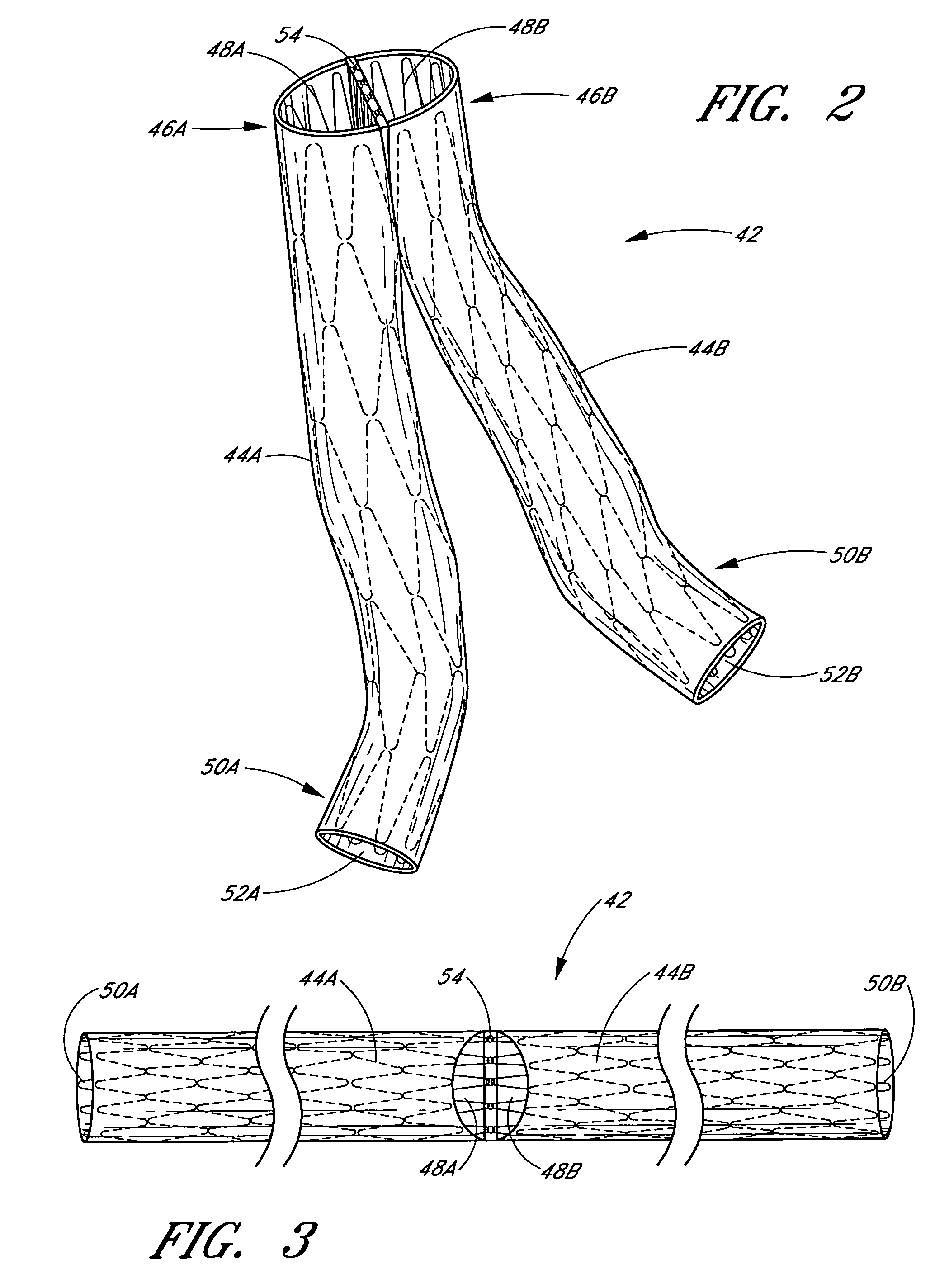
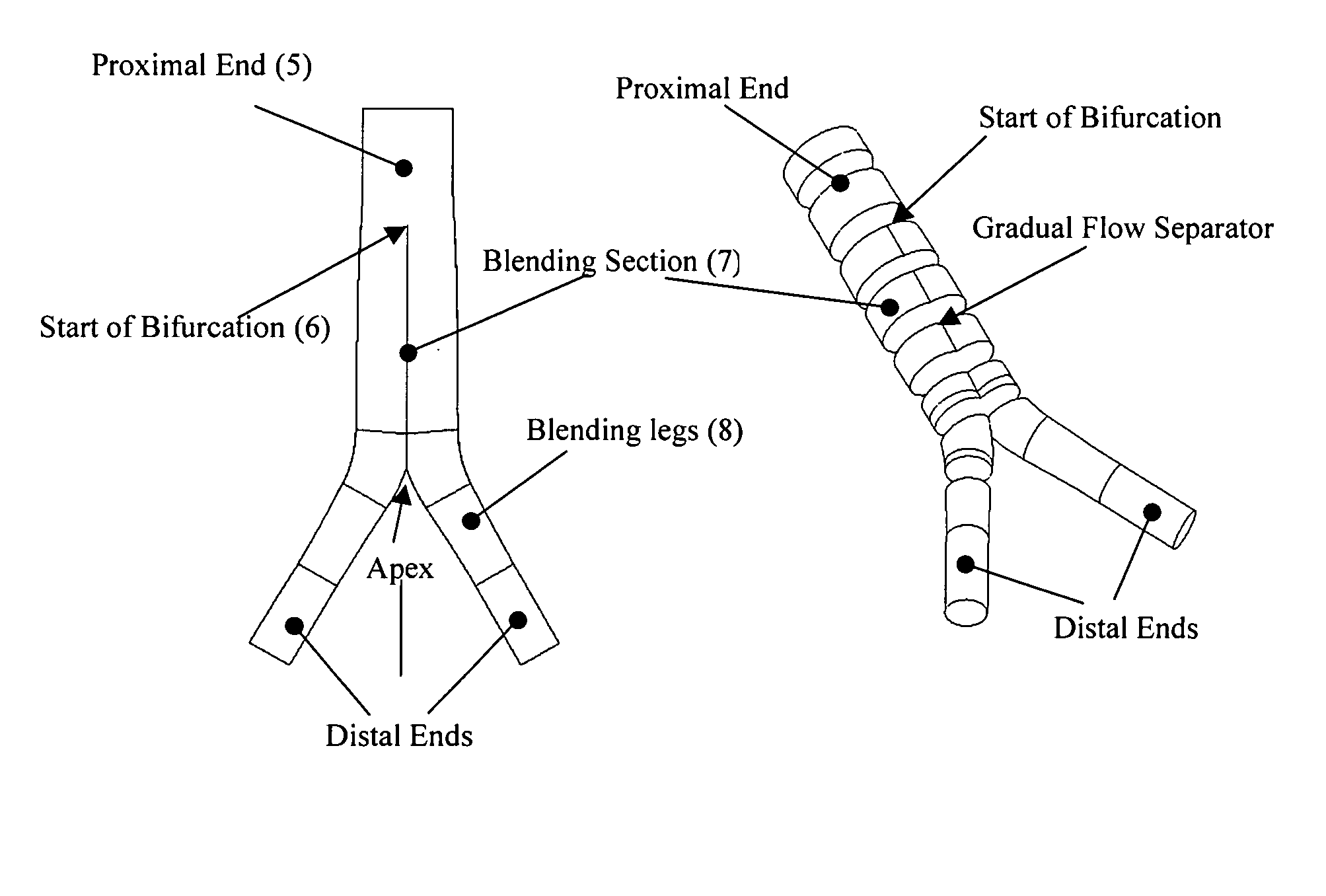
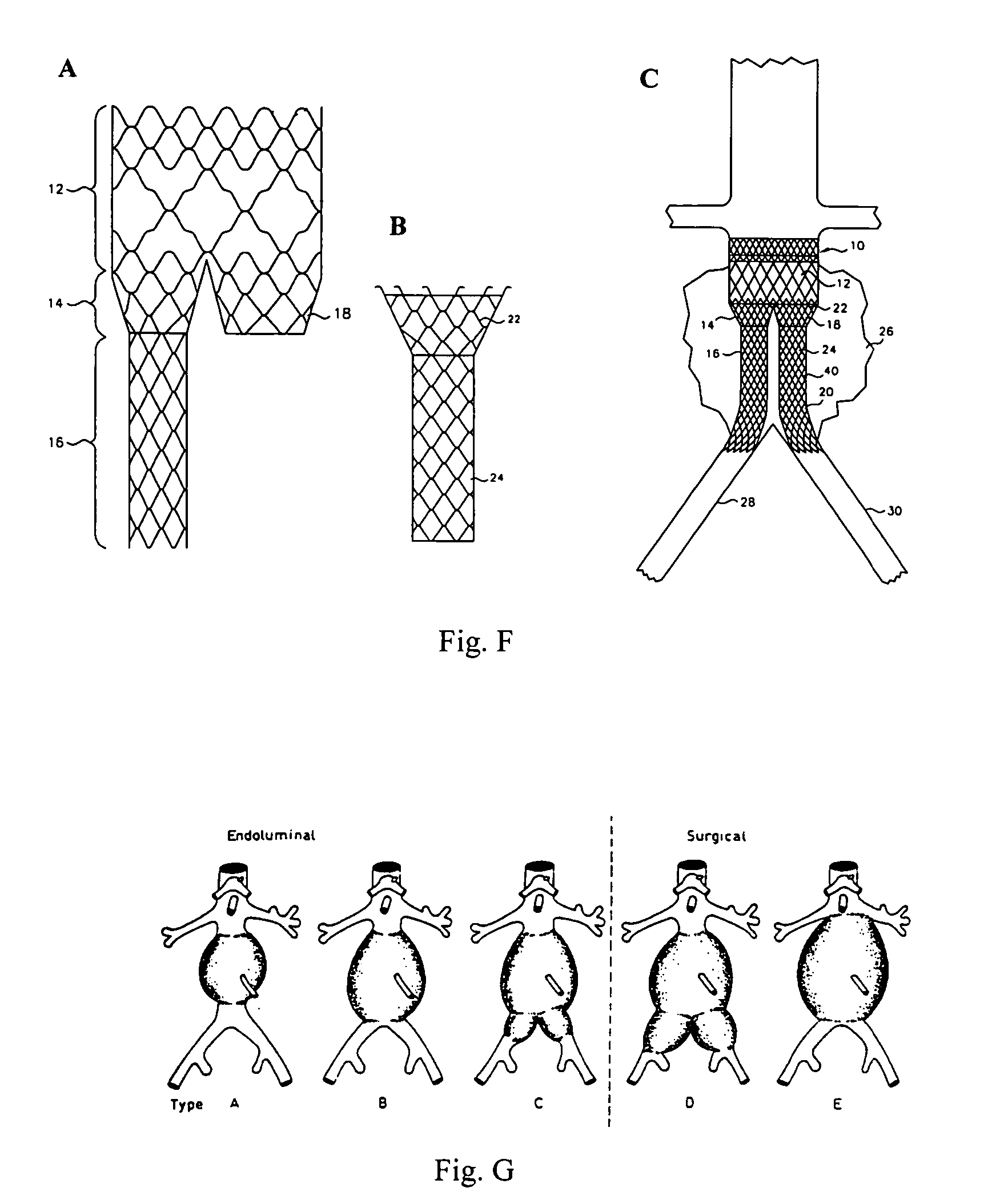
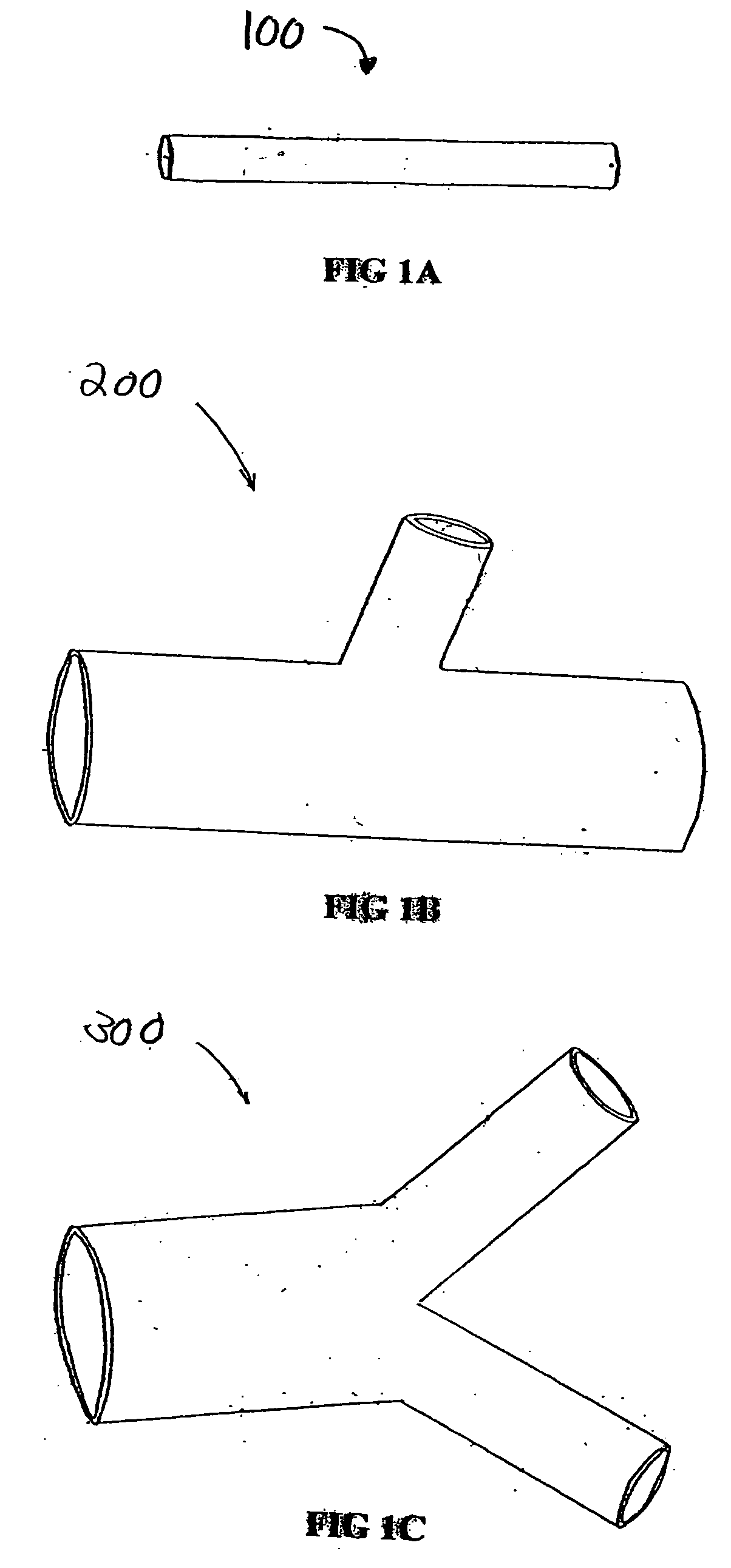
A vascular graft comprising a proximal section, iliac distal legs and a bifurcation blending section (7) between the proximal section and the distal legs. The cross-sectional area of the proximal section at the bifurcation point is less than or equal to the sum of the two cross sectional areas of both iliac legs. The blending section (7) generates a smooth transition from the proximal section to both iliac legs which minimizes wave reflections by ensuring that the area ratio at the bifurcated junction (7) is as close to unity or greater than unity as possible. The blending section (7) defines a first lumen for fluid flow from the proximal section into the first distal leg, and a separate second lumen for fluid flow from the proximal section into the second distal leg. The two lumen are separated by means of a gradual flow which separates the fluid flow from the proximal section into each lumen. The distal legs are connected to the blending section (7) at the bifurcation region to form a substantially “Y”-shaped graft.
Owner:LIMERICK UNIV OF
Process for decellularizing soft-tissue engineered medical implants, and decellularized soft-tissue medical implants produced
InactiveUS7338757B2Mammal material medical ingredientsDead animal preservationMedicineLong term durability
The invention provides methodologies and apparatus for producing acellular soft-tissue implants, both in small quantities and in commercializable quantities. Such soft-tissue implants include vascular graft substitutes. An acellular graft is produced by subjecting the tissue sample to an induced pressure mediated flow of an extracting solution, followed by inducing a pressure mediated flow of a treating solution, then washing the treated tissue to produce the acellular graft. The acellular grafts produced are uniform and non-immunogenic. The inventive method allows for the production of multiple decellularized soft tissue implants, where processing time is significantly less than prior art processes and the number of implants produced per day is increased over prior art processes. In clinical use, the decellularized grafts produced exhibit significantly improved in long-term durability and function.
Owner:LIFENET HEALTH
Process for Decellularizing Soft-Tissue Engineered Medical Implants, and Decellularized Soft-Tissue Medical Implants Produced
The invention provides methodologies and apparatus for producing acellular soft-tissue implants, both in small quantities and in commercializable quantities. Such soft-tissue implants include vascular graft substitutes. An acellular graft is produced by subjecting the tissue sample to an induced pressure mediated flow of an extracting solution, followed by inducing a pressure mediated flow of a treating solution, then washing the treated tissue to produce the acellular graft. The acellular grafts produced are uniform and non-immunogenic. The inventive method allows for the production of multiple decellularized soft tissue implants, where processing time is significantly less than prior art processes and the number of implants produced per day is increased over prior art processes. In clinical use, the decellularized grafts produced exhibit significantly improved in long-term durability and function.
Owner:LIFENET HEALTH
Vascular graft and deployment system
Disclosed is a method and apparatus for treating bifurcations of the vascular system, such as abdominal aneurysms at the bifurcation of the aorta and iliac arteries. A tubular implant having a proximal section, a distal section and a hinged connection therebetween is positioned across the bifurcation such that the proximal section extends into a first iliac and the distal section extends into the second iliac. The proximal and distal iliac sections are both advanced superiorly, causing the implant to fold at the hinge and advance across the aneurysm into the aorta. In one implementation, restraining sleeves are thereafter removed and the implant self expands to place aorta in fluid communication with the first and second iliacs, bypassing the bifurcation. Deployment catheters are also disclosed.
Owner:SEGUIN JACQUES
Film with highly porous vascular graft prostheses
A vascular graft prosthesis having ingrowth-permissive lumenal wall features with a thin film or layer of sealant-like material placed at a lumenal wall location to promote improved transmural tissue growth and healing.
Owner:MEDTRONIC INC
Nanofibrous biocomposite prosthetic vascular graft
The present invention provides a bioactive, small-diameter (typically less than 6 mm in internal diameter) vascular graft prosthesis, and is a textile conduit preferably manufactured using a novel electrospinning perfusion methodology. One preferred embodiment is a nanofibrous biocomposite textile conduit which comprises a prepared liquid admixture of polyester (Dacron), a biodurable implantable synthetic polymer, and Type IV collagen, an extracellular matrix protein. This prepared admixture and blending of diverse fibrous matter is utilized in a novel electrospinning perfusion process to form a small-diameter (less than 6 mm) fabricated textile conduit, a discrete article of manufacture, which then serves as an antecedent tangible workpiece for a subsequently-made prosthetic vascular graft construct. In this manner, after the biocomposite textile conduit has been fabricated as a tangible article, one or more pre-chosen biologically-active compounds are then subsequently permanently bound (covalently or ionically) via a bifunctional linking agent to the wall surface(s) of the textile conduit. These permanently bound compounds retain their characteristic biological activity after becoming permanently immobilized to the textile wall surface; and, via such immobilization, provide the textile conduit wall with many of the highly desired attributes and properties characteristic of naturally occurring blood vessels. Accordingly, after the immobilization of one or more biologically active compounds to the wall surfaces, the completely prepared article is then suitable for use in-vivo as a prosthetic vascular graft construct.
Owner:BIOSURFACES +2
Vascular graft and deployment system
A vascular graft includes a main portion and a branch portion that is coupled to the main portion by an articulating joint. The vascular graft may be inserted into the thoracic aorta with the branch portion positioned within a branch vessel and the main portion positioned within the thoracic aorta. The graft may be deployed within a deployment apparatus comprising an outer member and an inner member and a pusher. The main graft portion may be housed within the inner member while the branch graft portion is housed within the space between the inner and outer members. The inner member may have a longitudinal groove for allowing the articulating joint to pass by when the branch graft portion is deployed.
Owner:DOUGLAS MYLES
Vascular graft
InactiveUS20060085017A1Contact resistanceShorten the timeSurgical staplesWound clampsProsthesisCatheter
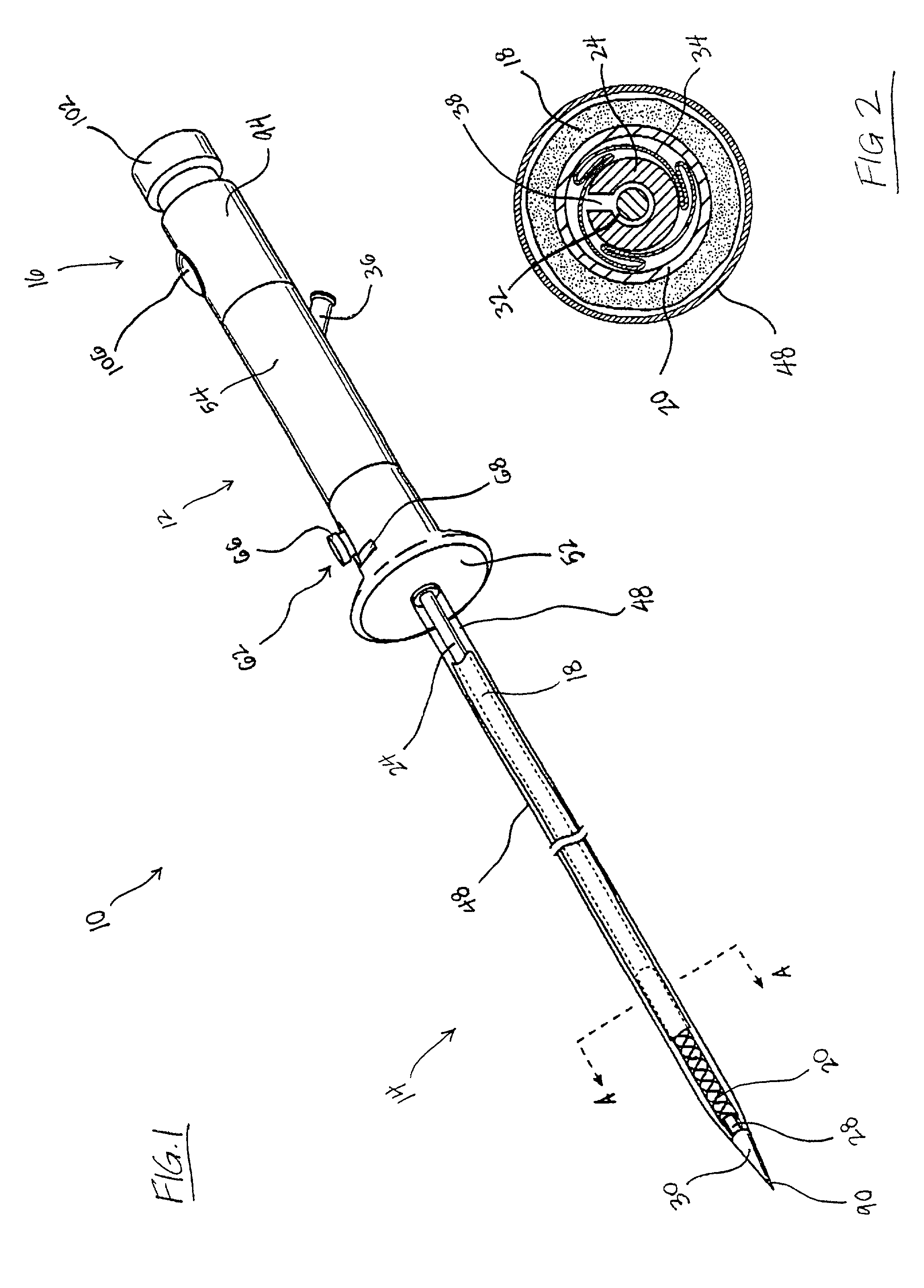
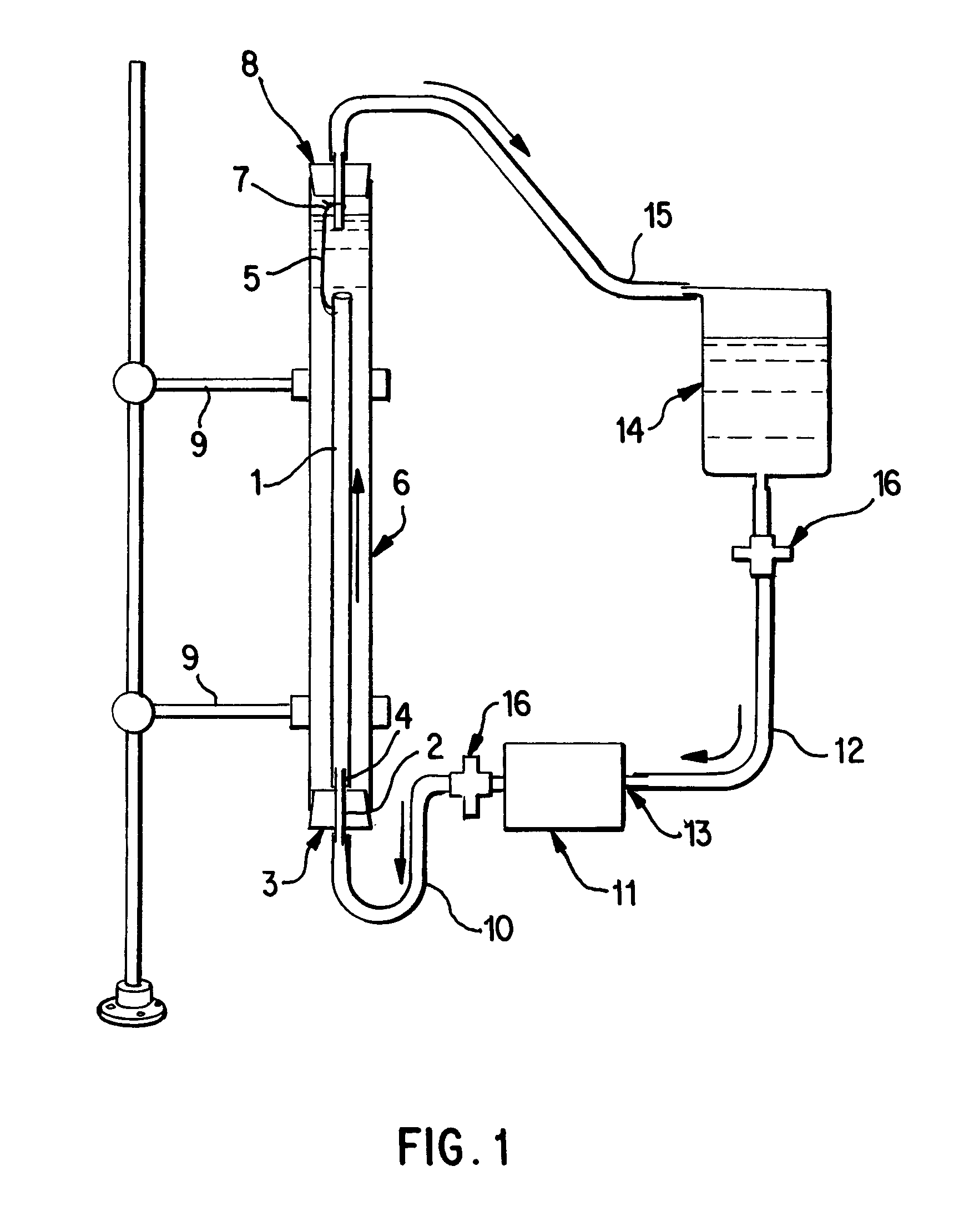
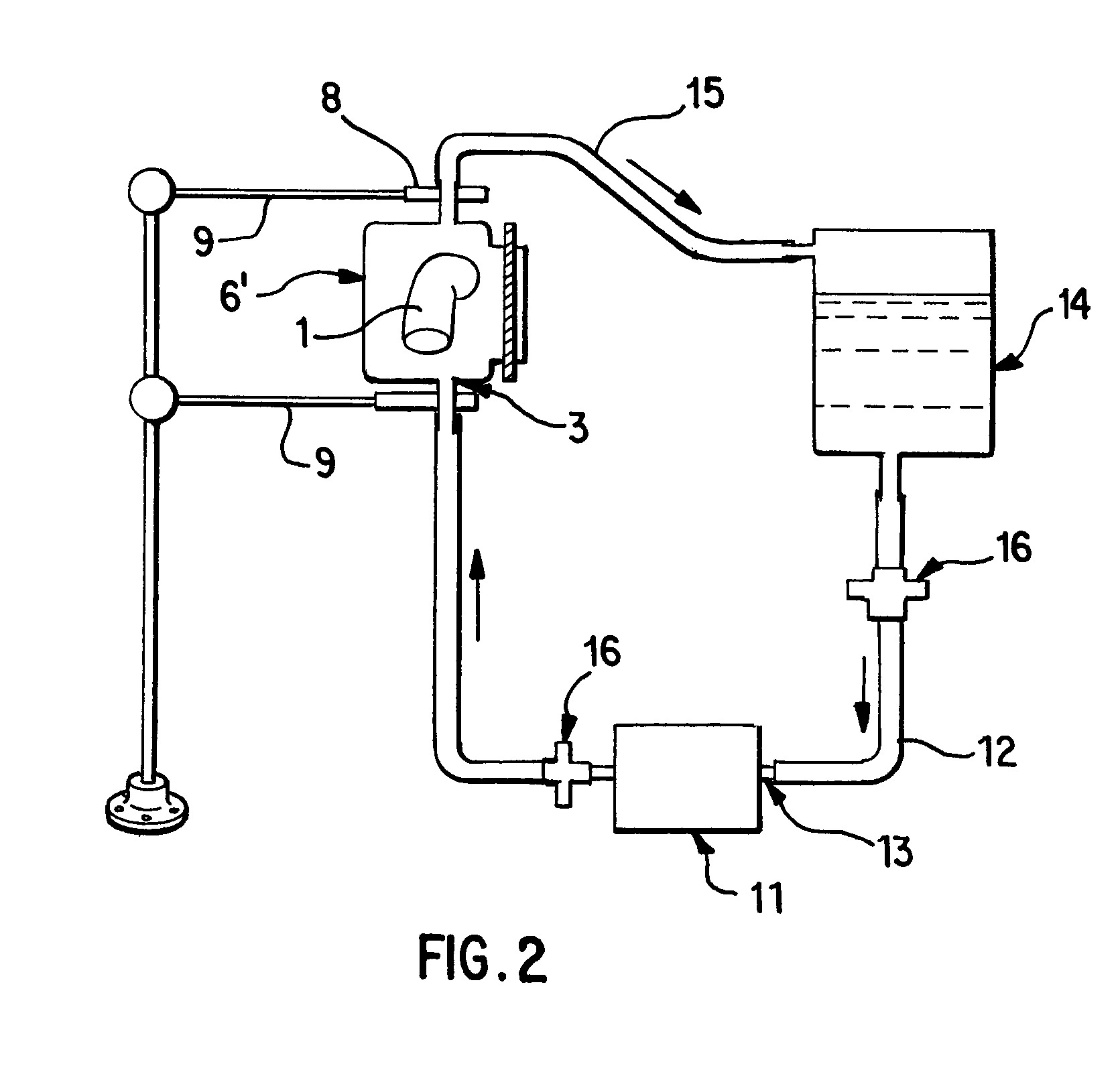
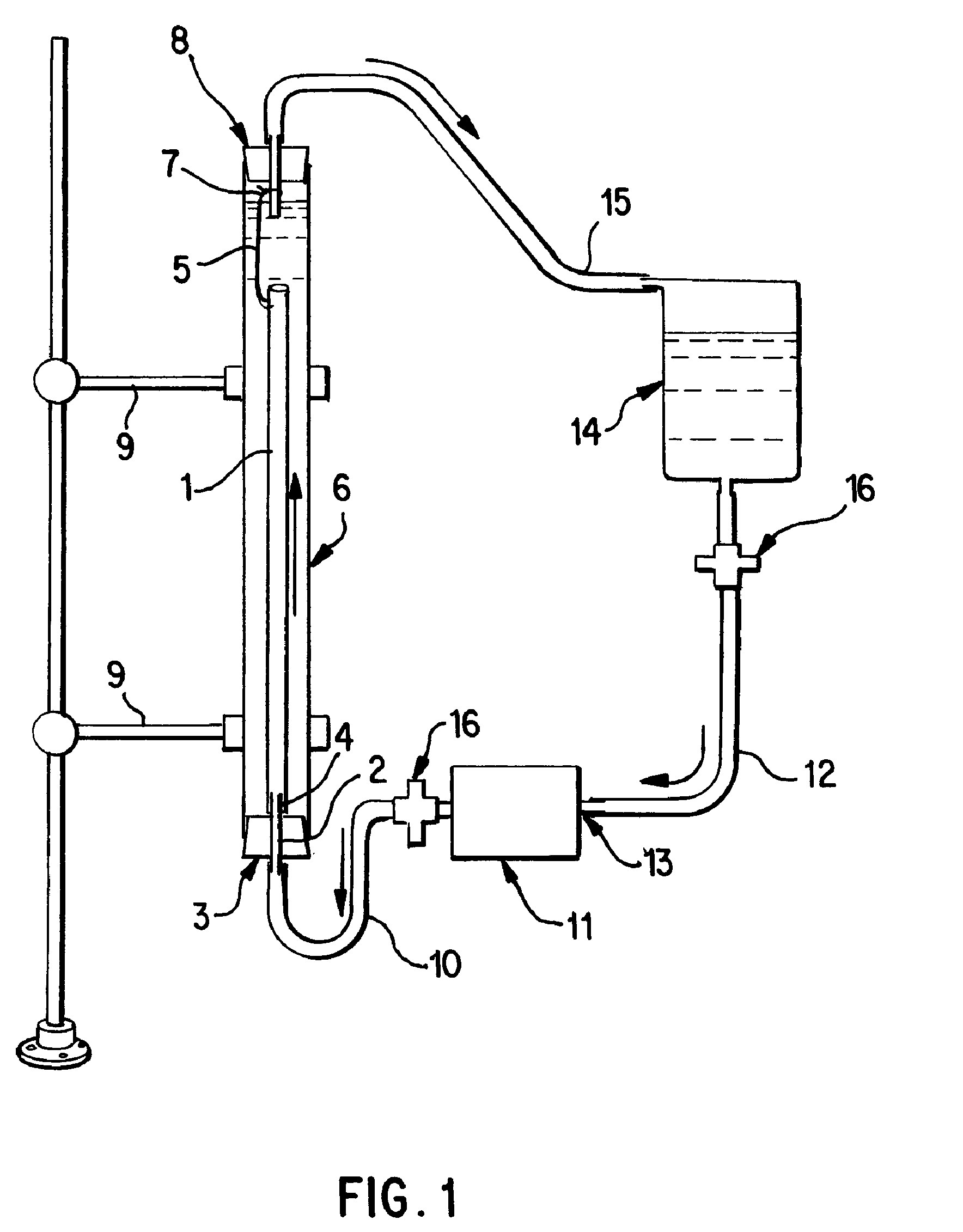
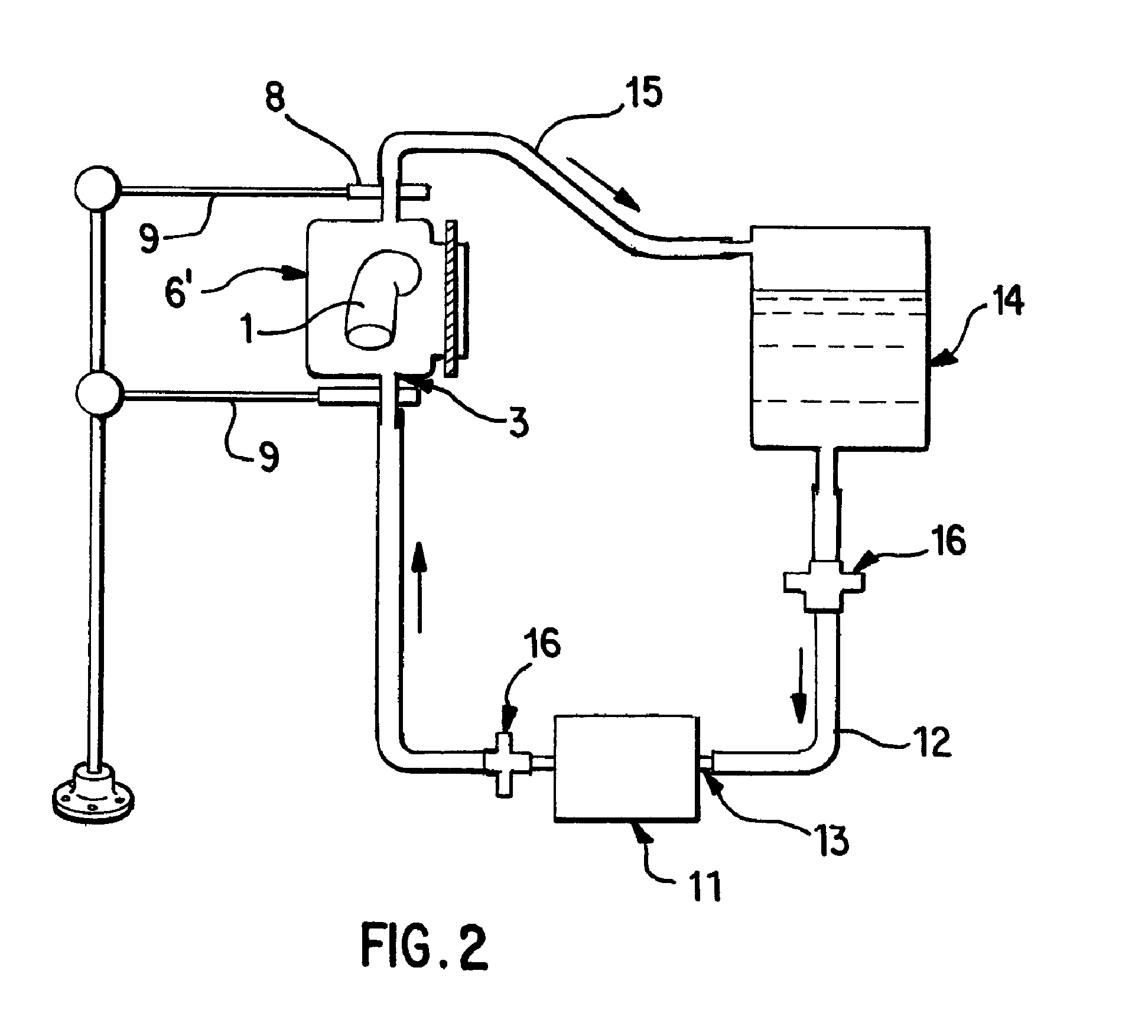
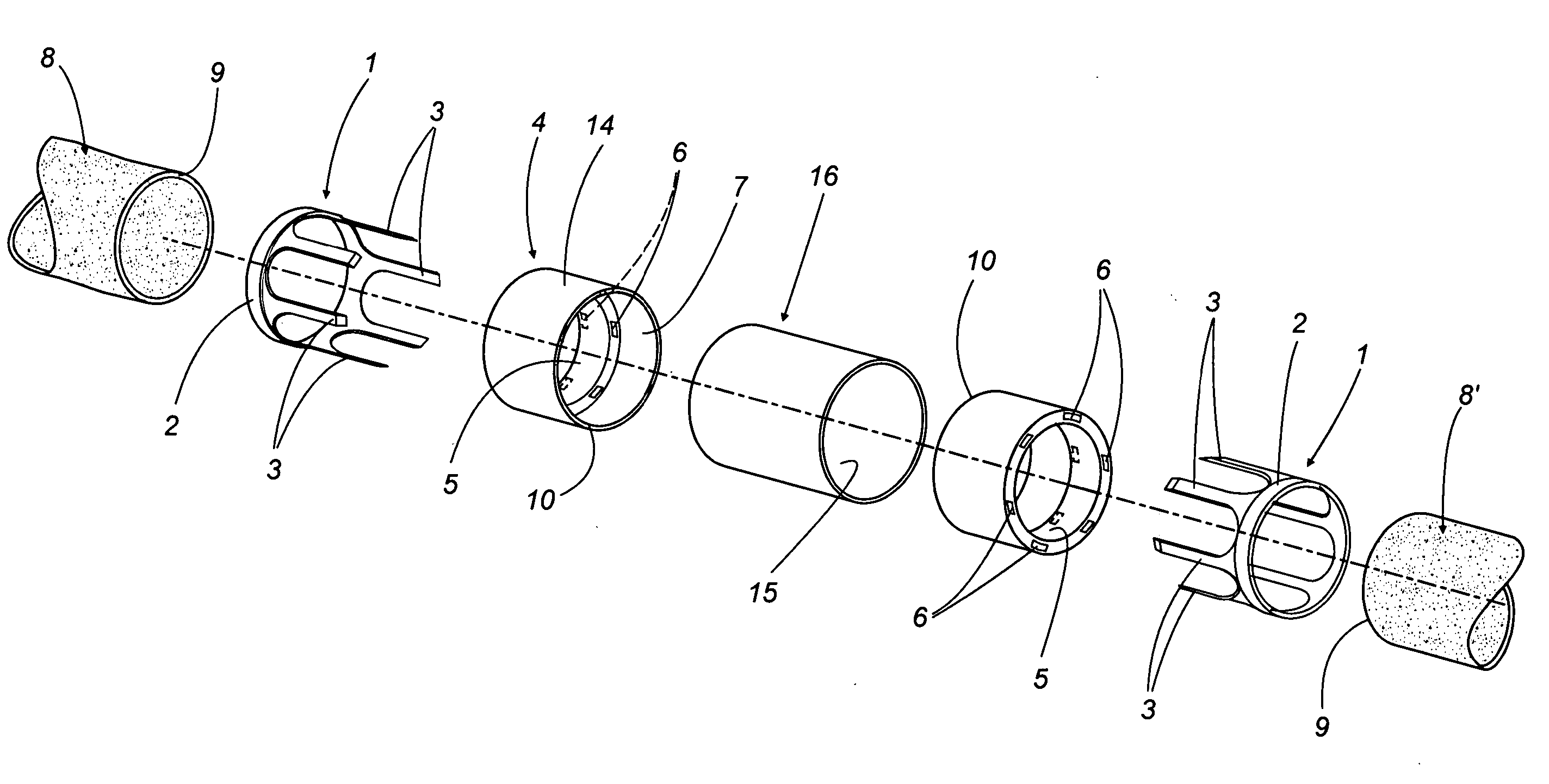
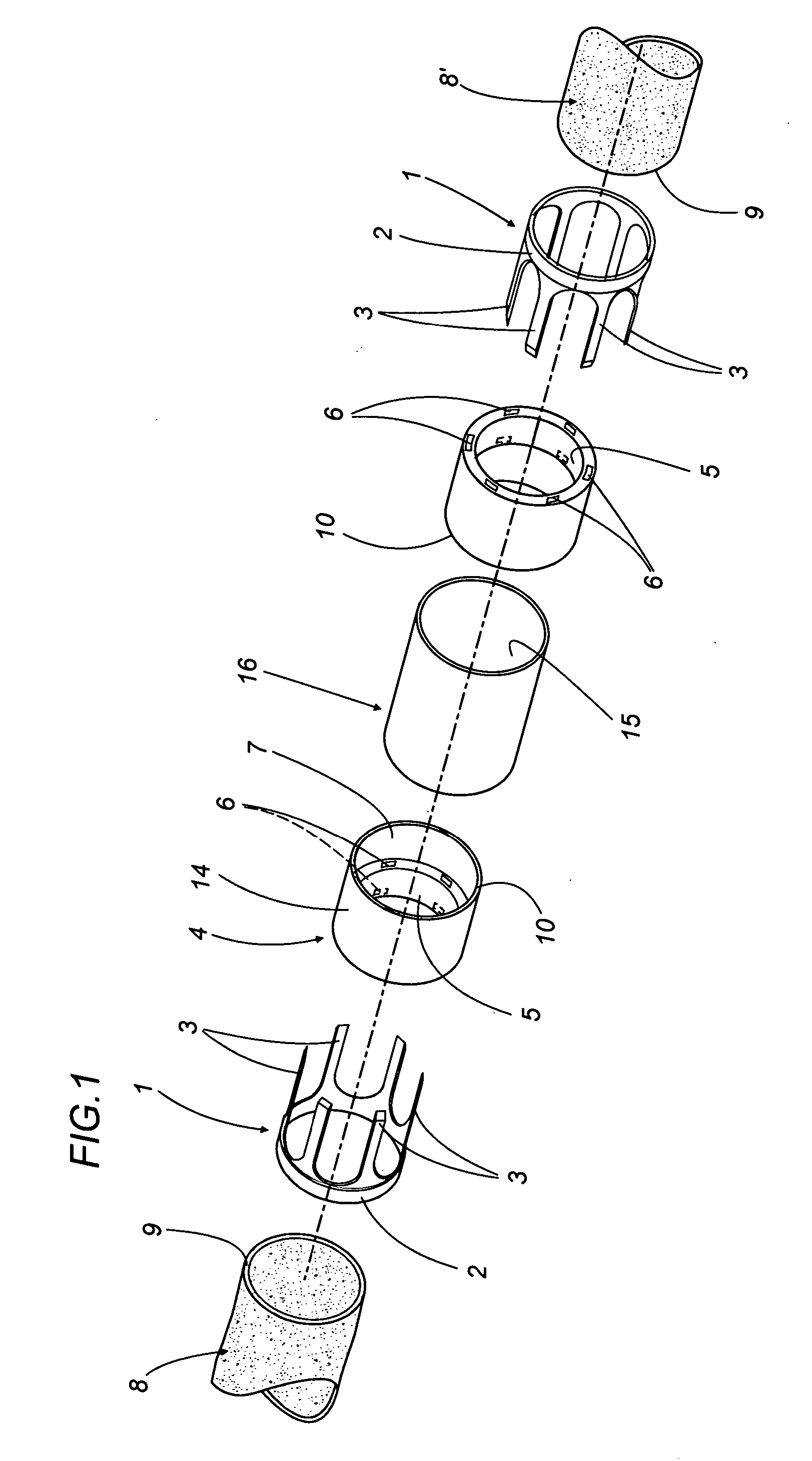
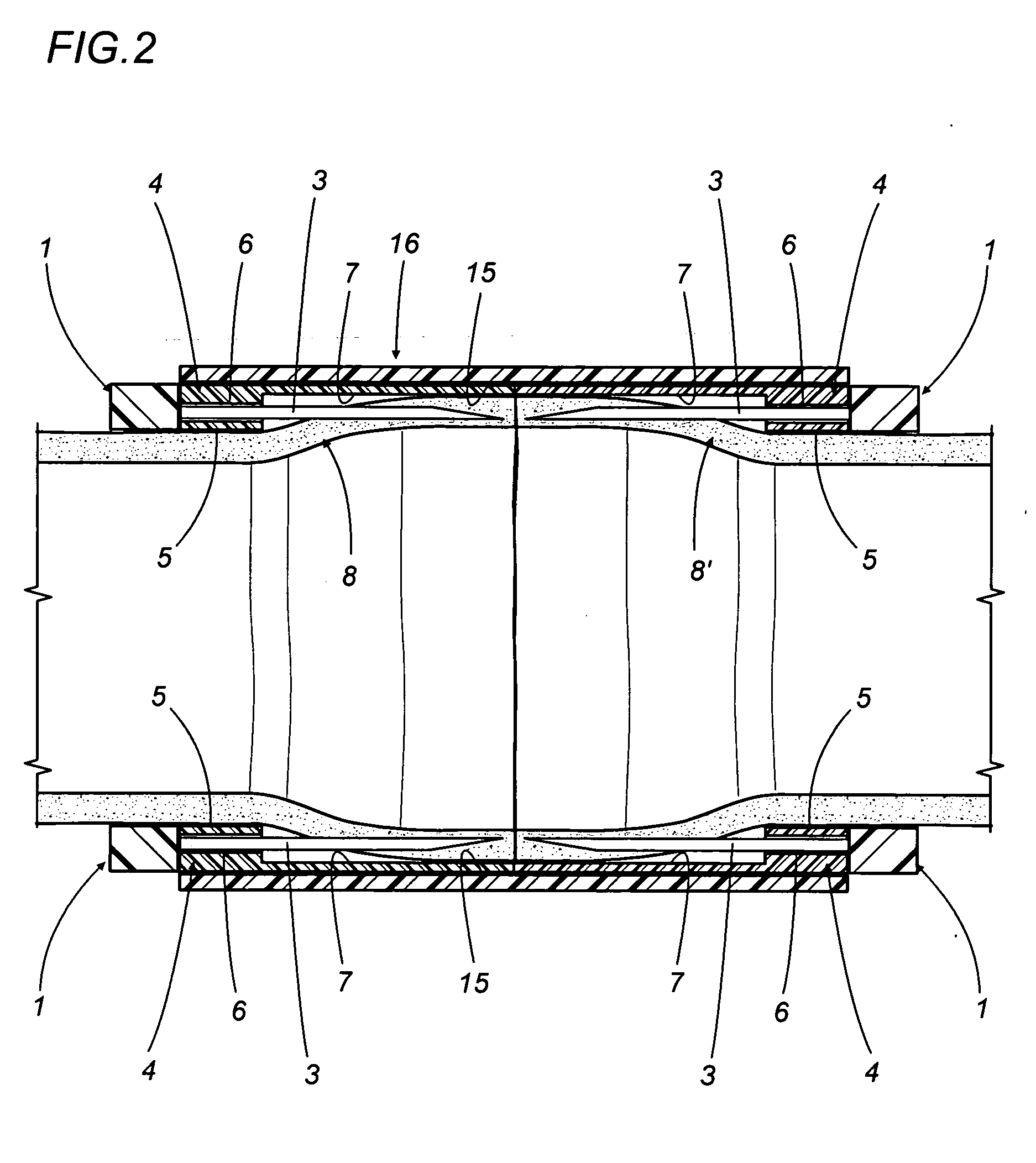
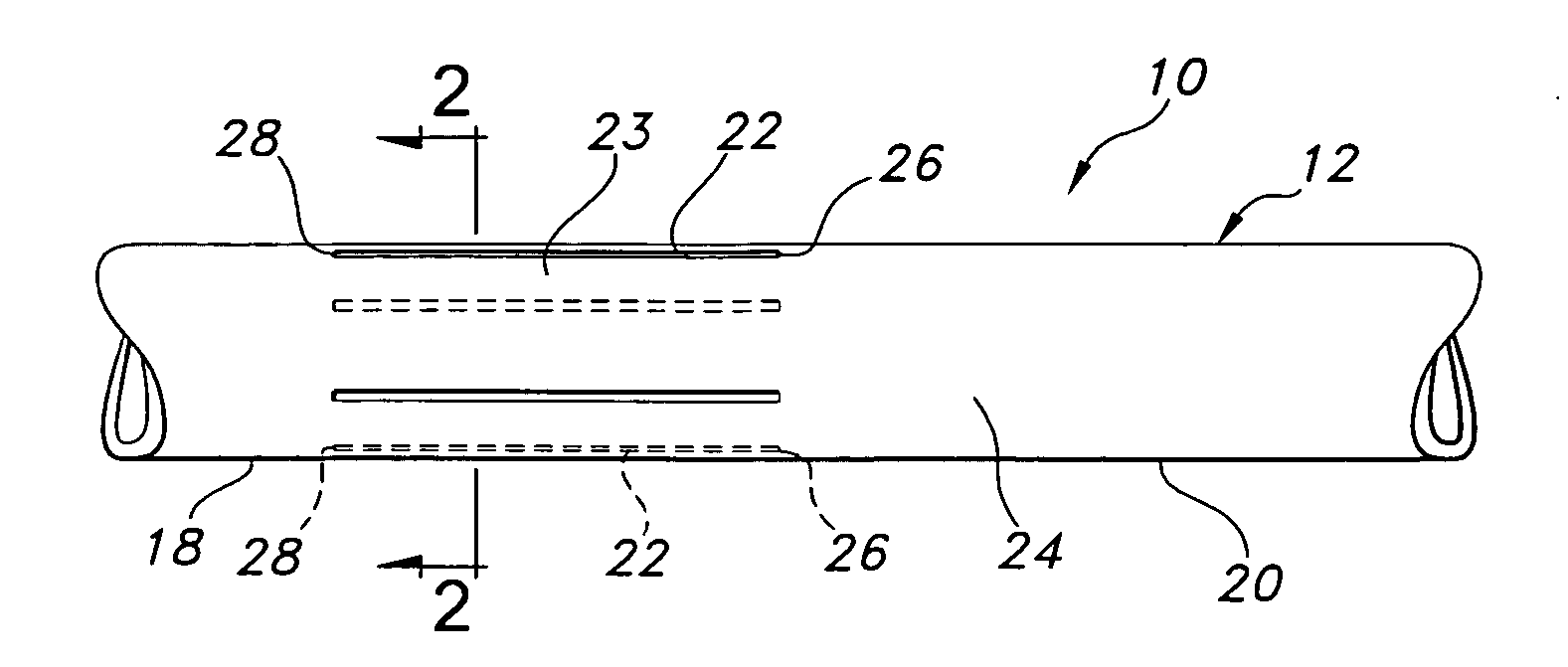
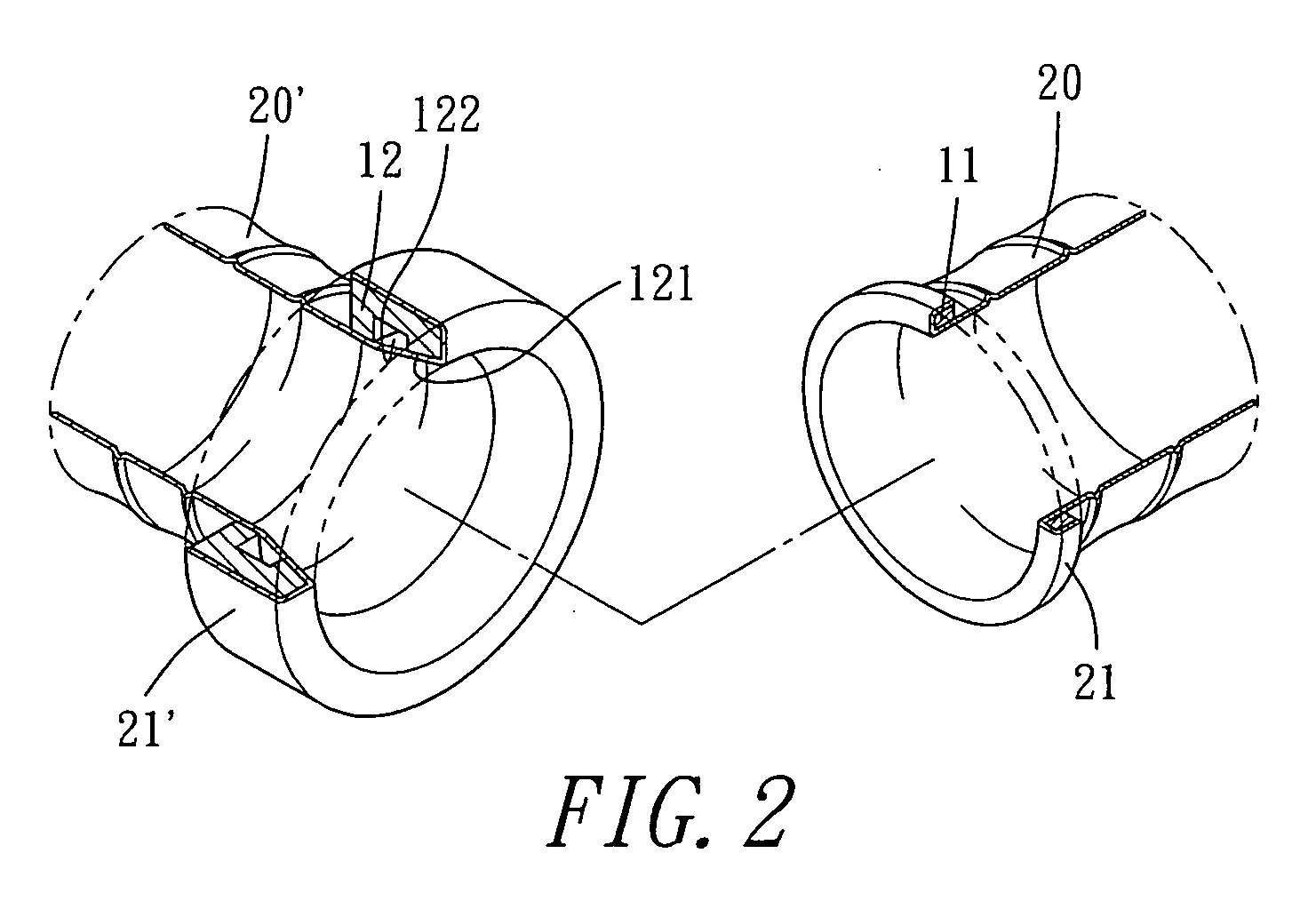
A vascular graft for anastomosis of blood vessels or ducts comprises piercing means (3) for engaging an end portion (8 / 8′) of a blood vessel or prosthesis and means (6, 6′) for precisely guiding one or more piercing elements (3) to pierce the wall of the vessel or prosthesis to a predetermined depth and in a predetermined direction.
Owner:NEWMAN MEDICAL
Process for devitalizing soft-tissue engineered medical implants, and devitalized soft-tissue medical implants produced
InactiveUS8563232B2Alleviate the conditionReduce processing timeCell dissociation methodsDead animal preservationLong term durabilityVascular grafting
The invention provides methodologies and apparatus for producing devitalized soft-tissue implants where the implant retains metabolically non-viable and / or reproductively non-viable cells, and preferably retains large molecular weight cytoplasmic proteins, such implants produced both in small quantities and in commercializable quantities. Such soft-tissue implants include vascular graft substitutes. A devitalized graft is produced by subjecting the tissue sample to an induced pressure mediated flow of an extracting solution, optionally followed by inducing a pressure mediated flow of a salt solution, then washing the tissue to produce the devitalized graft. The devitalized grafts produced are uniform and non-immunogenic. The inventive method allows for the production of multiple devitalized soft tissue implants, where processing time is significantly less than prior art processes and the number of implants produced per day is increased over prior art processes. In clinical use, the devitalized grafts produced exhibit significantly improved in long-term durability and function, and enhanced recellularization post-implantation.
Owner:LIFENET HEALTH
Ex vivo remodeling of excised blood vessels for vascular grafts
InactiveUS7011623B2Increase the diameterIncrease the lengthArtificial cell constructsBlood vesselsBiomechanicsMechanical integrity
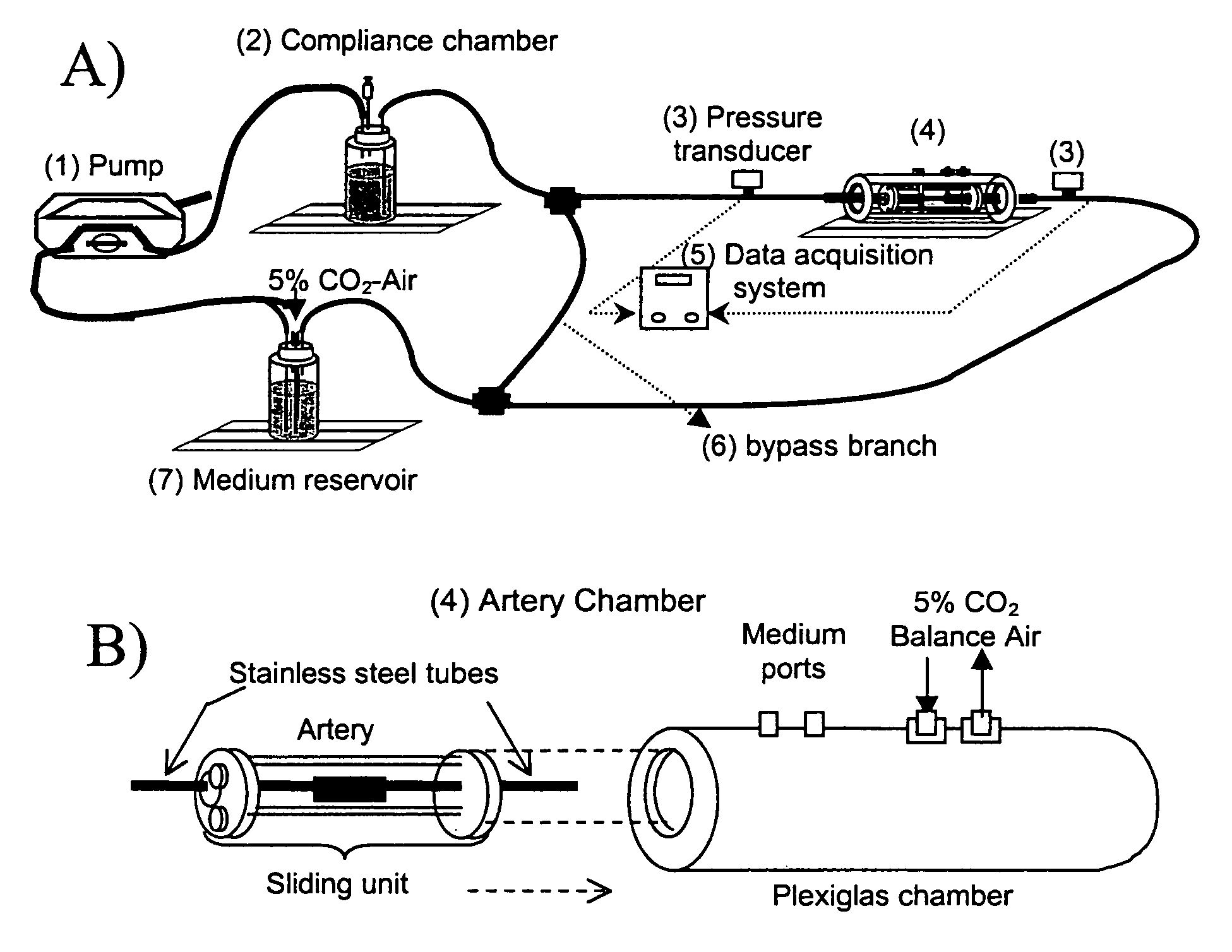
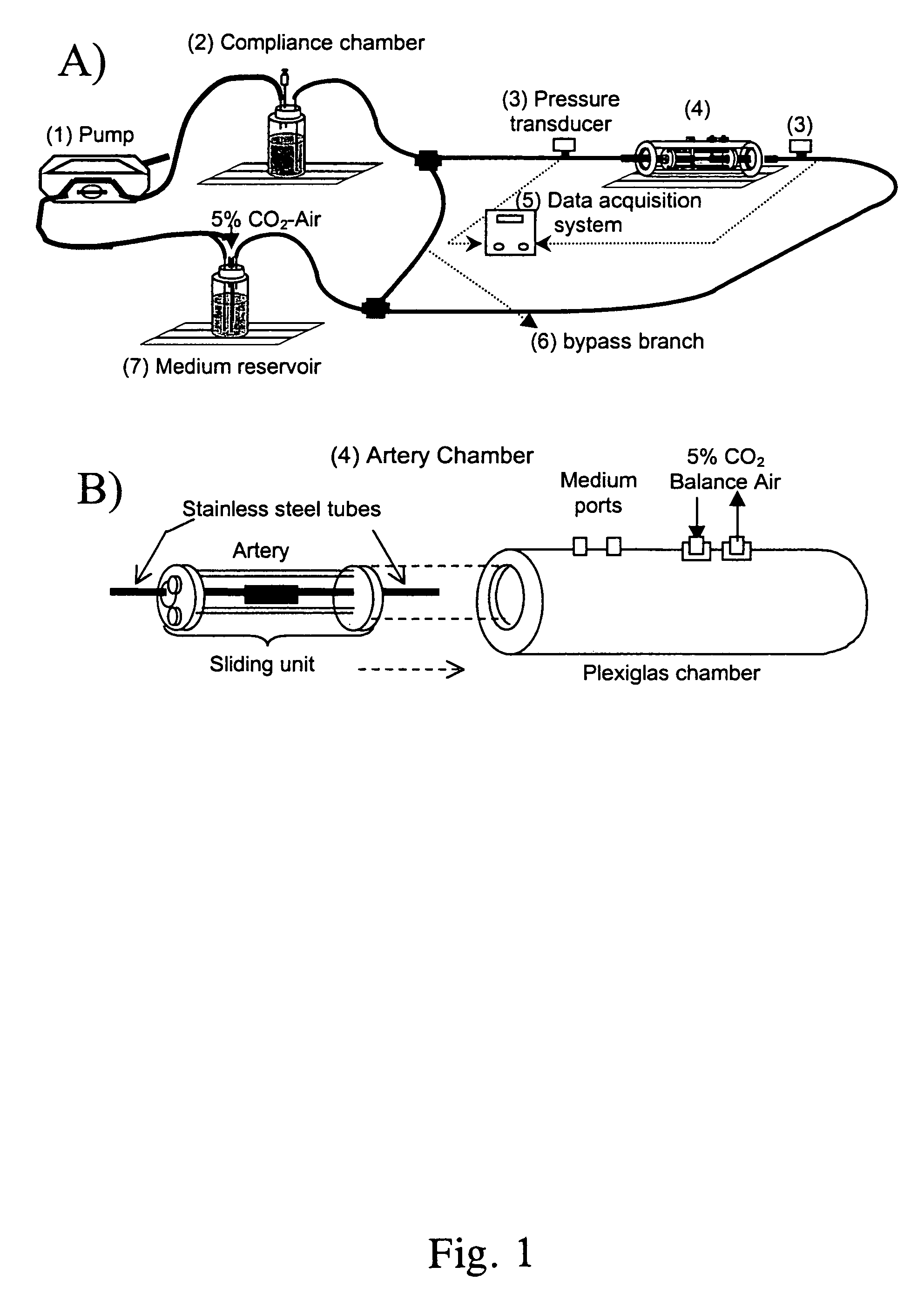
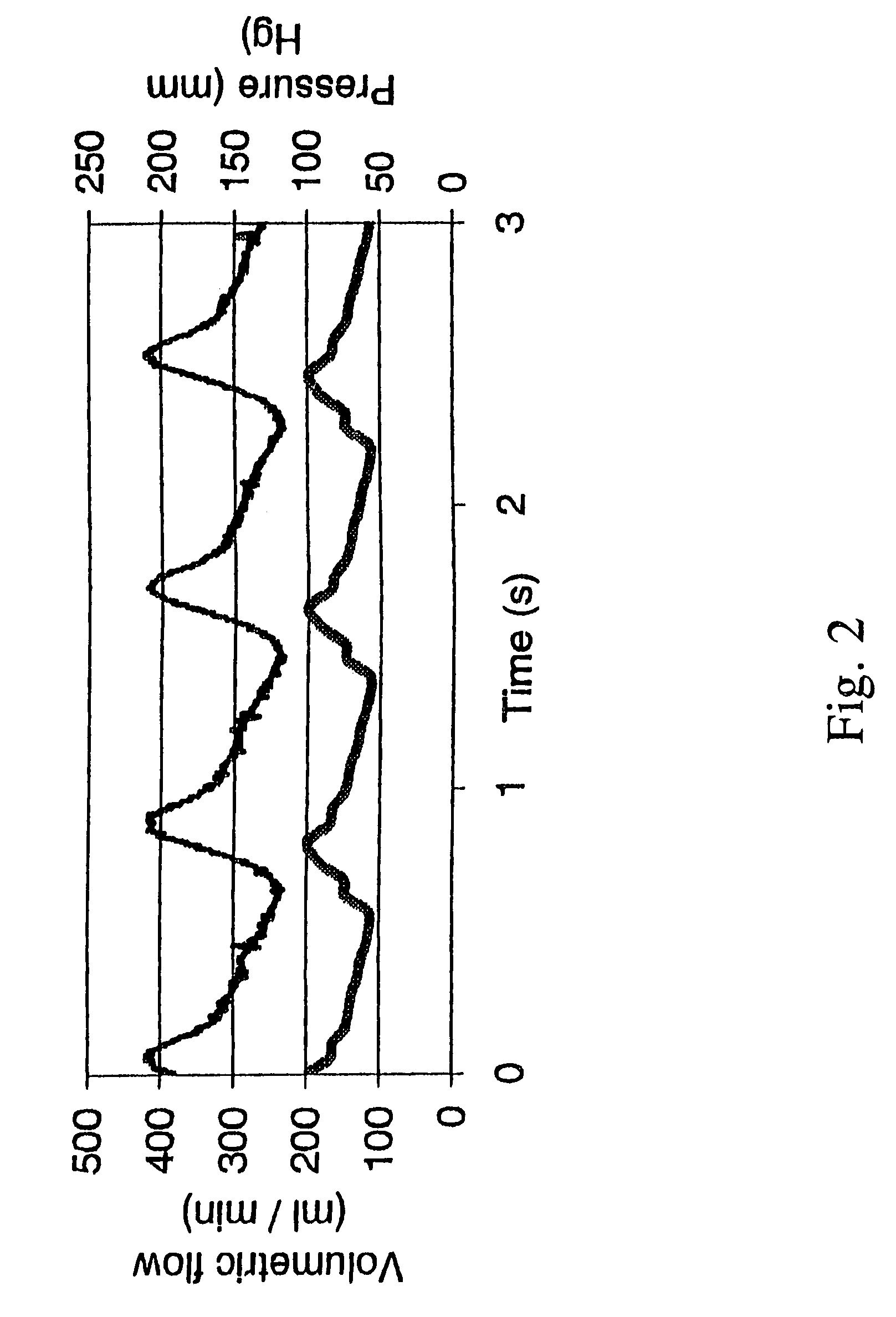
The present invention provides an ex vivo vascular remodeling methods and system by which an excised, small diameter blood vessel can be harvested and expanded to provide viable vascular grafts, as demonstrated at the physical and molecular levels, and as optimized in vivo. The tissue-engineered vessels generated by the present invention closely resemble native vessels in terms of structure, histologically, including endothelial coverage and intricate structural components such as the internal elastic lamina, viability (as measured with the MTT assay and TUNEL analysis), and function (vasoactivity, mechanical and biomechanical properties). Thus, the resulting vascular grafts behave in a manner similar to native arteries in terms of mechanical integrity, and provide clinically relevant patency rates when implanted in vivo. Moreover, the ex vivo methods and system permit the precise control of the mechanical environment involving the excised vessel, while at the same time permitting careful monitoring of the resulting growth / remodeling, thereby opening new avenues of research regarding the mechanical stimuli responsible for specific aspects of remodeling in vivo.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Sintered structures for vascular graft
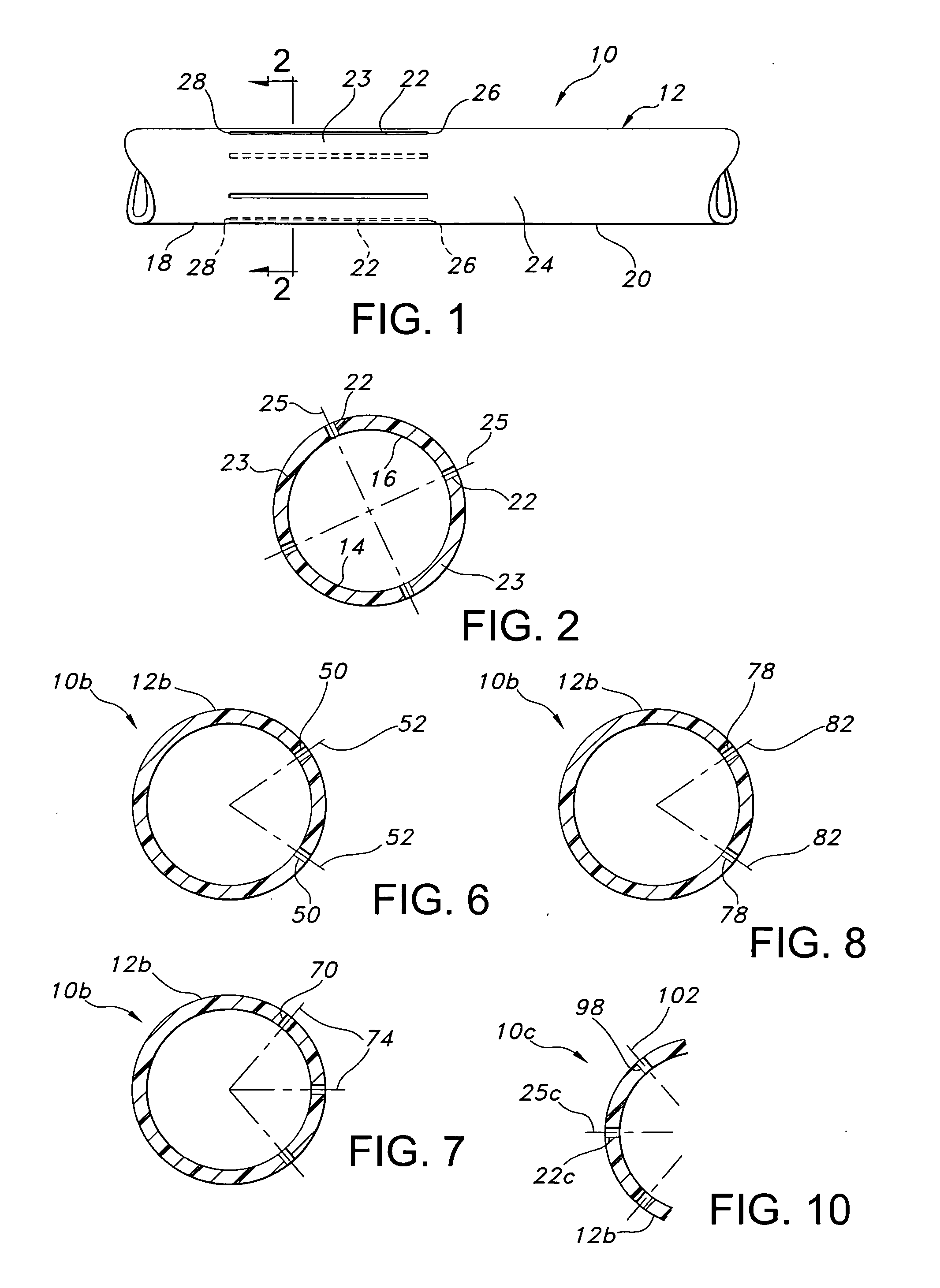
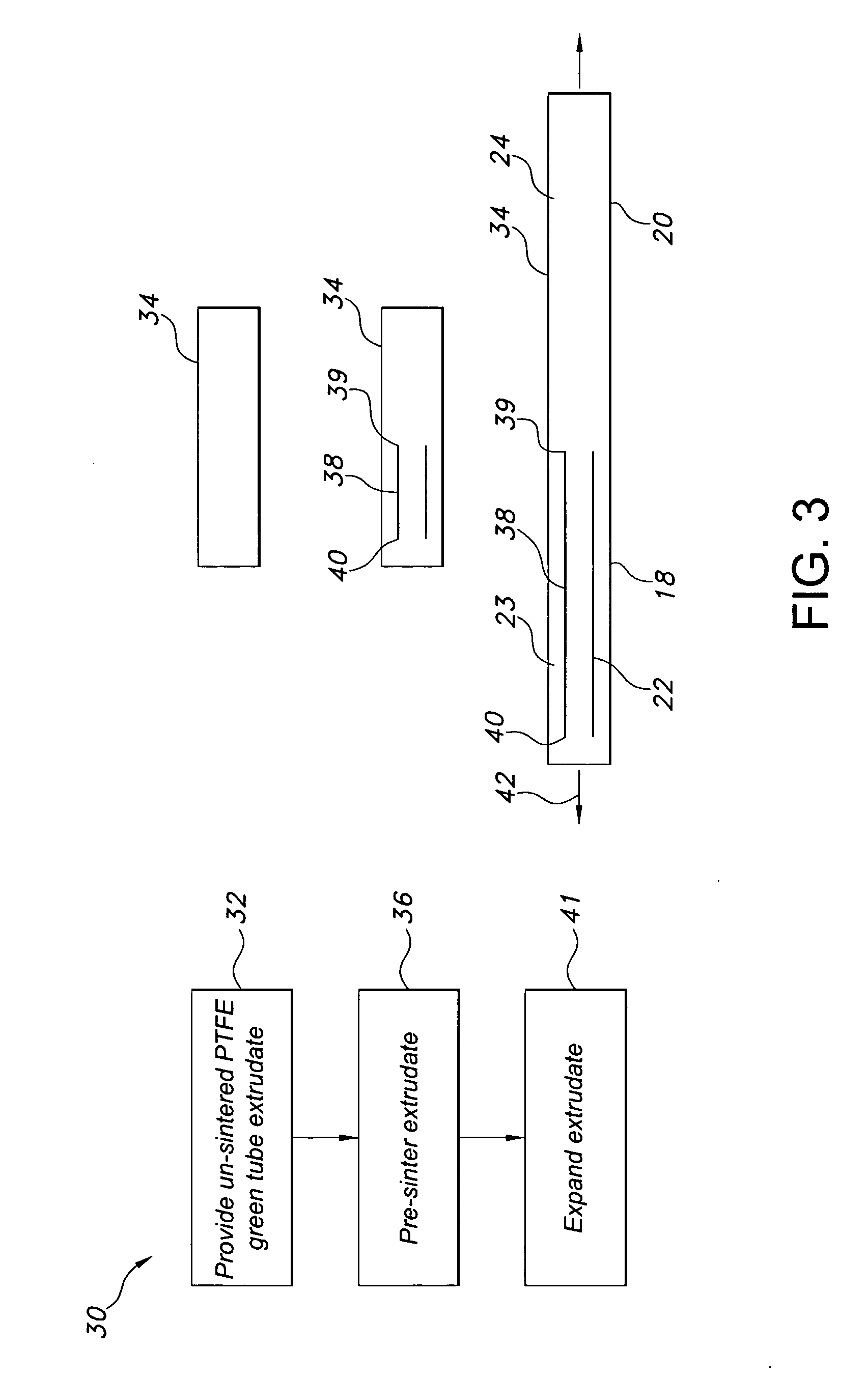
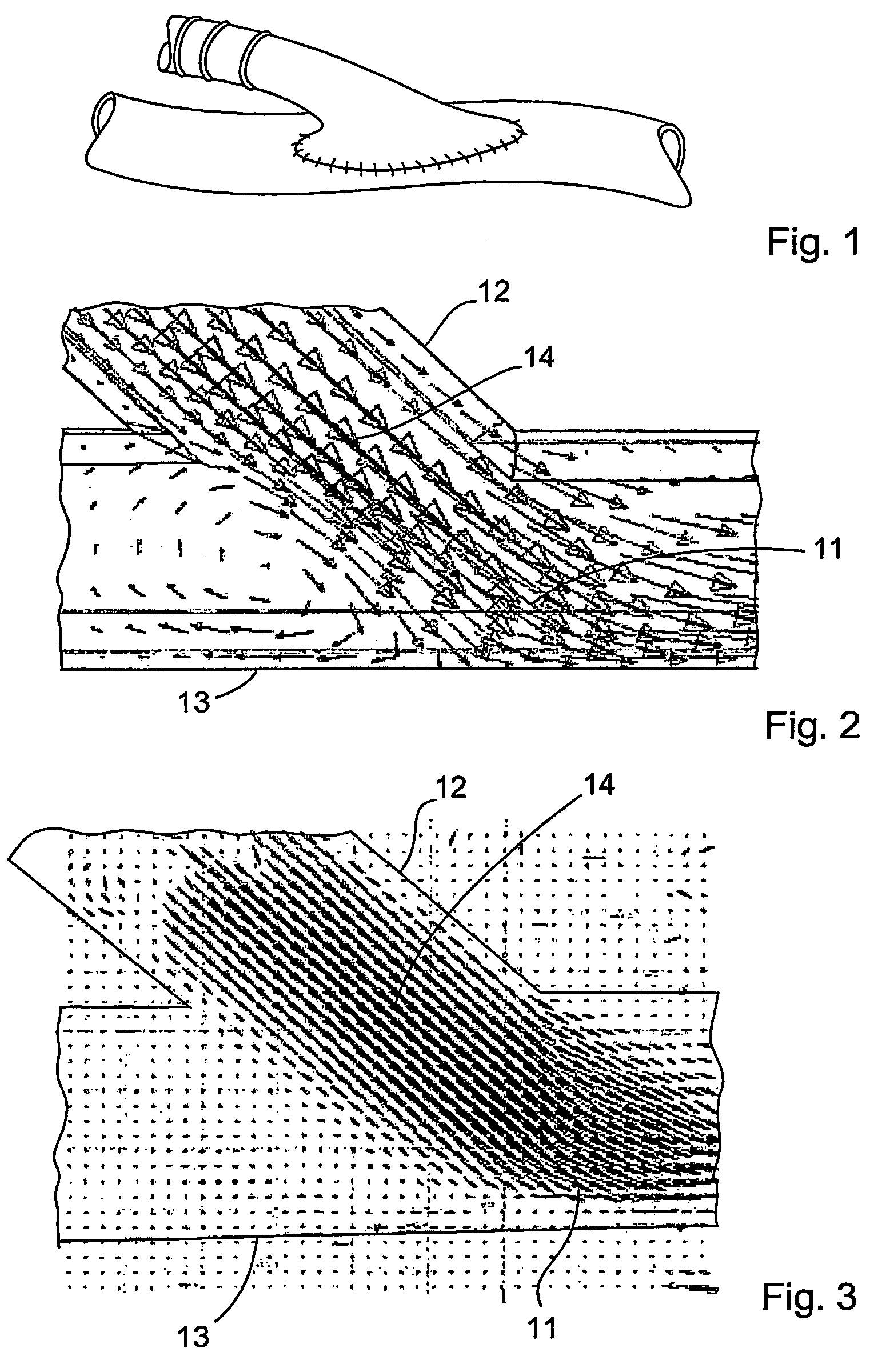
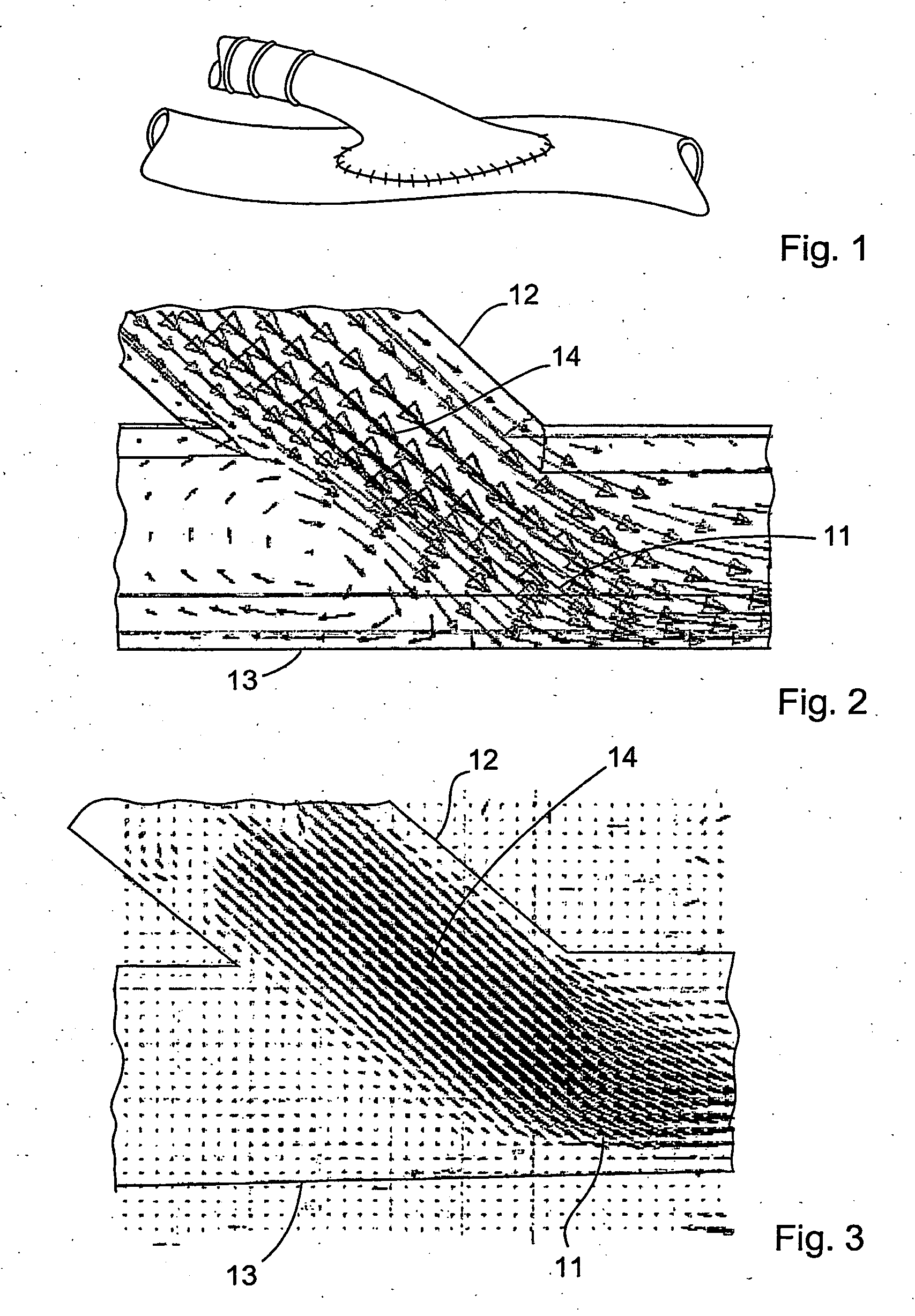
InactiveUS20060149366A1Promote formationImprove performanceCeramic shaping apparatusCork mechanical workingVascular graftingVascular graft
The vascular graft is for implantation within a body and has a PTFE tube structure including a length and inner and outer wall surfaces. The tube structure has a non-expanded portion formed from sintering a PTFE green tube extrudate and an expanded portion formed subsequent to the sintering. The expanded and non-expanded portions are of the same extrudate. The expanded portion has a region which adjoins the non-expanded portion wherein a degree of expansion of the region is limited by the non-expanded portion. The limiting of the expansion by the non-expanded portion is attenuated at a location of the region which is remote from the non-expanded portion. A method for making the vascular graft facilitates the formation of the non-expanded and expanded portions of the PTFE tube structure.
Owner:LIFESHIELD SCI
Vascular graft connector
InactiveUS20100036397A1Easy to operateFirmly connectedBlood vesselsWound clampsProsthesisVascular grafting
The present invention is to provide a prosthetic vascular connector which comprises a male fastener of ring-shaped body and a ring-shaped female fastener; where the outer side of the female fastener is a smooth surface while the inner side, facing the male fastener, is provided with a tapered wall that is gradually contracted inward and an indented catch trough next to the minimum diameter of the tapered wall; when in use, the male fastener and the female fastener each offers the terminal portion of a separate prosthetic blood vessel to pass through its inside, followed by a outward to backward fold around it, and then push the male fastener with the folded terminal portion of the prosthetic blood vessel into the tapered wall of the female fastener covered with the prosthetic blood vessel, where the male fastener is being pushed into a deformed shape to move across the minimum diameter of the tapered wall of the female fastener, and lodged in the catch trough with its original shape restored.
Owner:KANG WEI CHANG +1
Process for Devitalizing Soft-Tissue Engineered Medical Implants, and Devitalized Soft-Tissue Medical Implants Produced
InactiveUS20140154663A1Alleviate the conditionShorten the timeCell dissociation methodsDead animal preservationLong term durabilityVascular grafting
Owner:LIFENET HEALTH
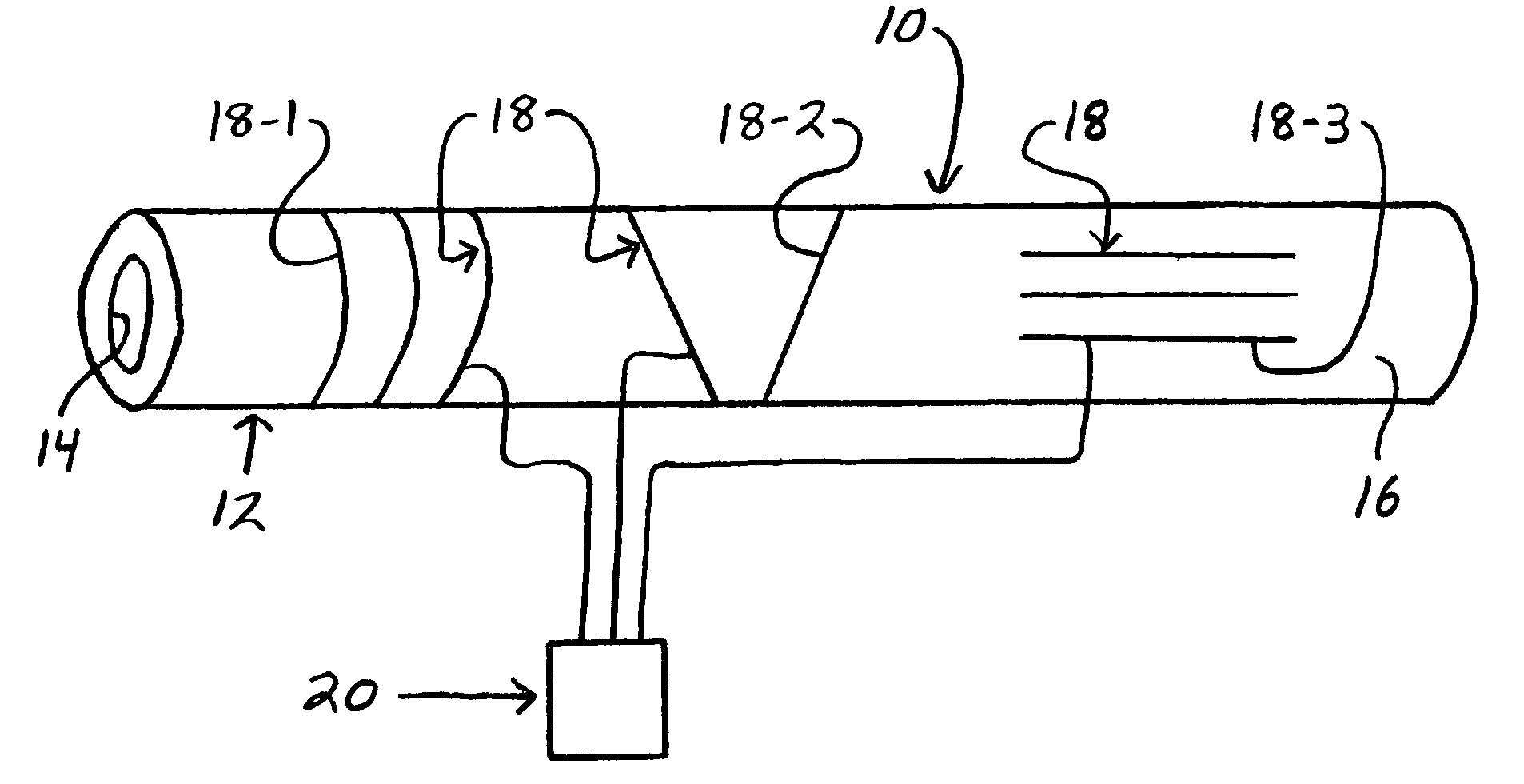
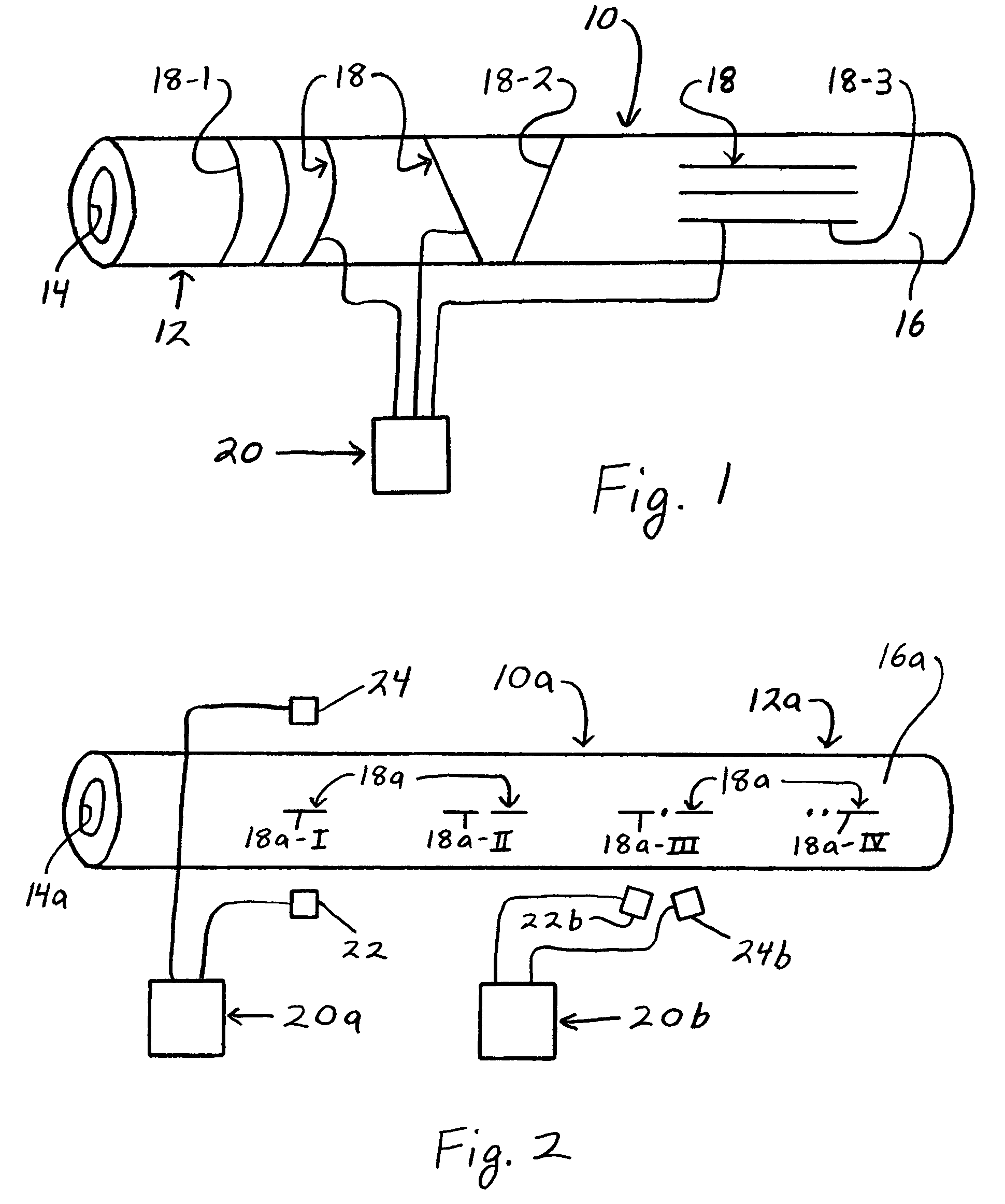
Smart textile vascular graft
The smart textile vascular graft is used with an electrical control unit. The vascular graft includes a tube structure formed of a textile material. At least one active fiber is incorporated in the textile material. The active fiber provides electrical coupling thereof to the electrical control unit to provide for transmission of a control signal between the active fiber and control unit. The active fiber has at least one physical characteristic which is variable and corresponds to the control signal to provide communication between the active fiber and electrical control unit relating to the physical characteristic.
Owner:LIFESHIELD SCI
Ex Vivo Remodeling of Excised Blood Vessels for Vascular Grafts
InactiveUS20100331964A1Increase the diameterIncrease the lengthArtificial cell constructsBlood vesselsMechanical integrityBiomechanics
The present invention provides an ex vivo vascular remodeling methods and system by which an excised, small diameter blood vessel can be harvested and expanded to provide viable vascular grafts, as demonstrated at the physical and molecular levels, and as optimized in vivo. The tissue-engineered vessels generated by the present invention closely resemble native vessels in terms of structure, histologically, including endothelial coverage and intricate structural components such as the internal elastic lamina, viability (as measured with MTT assay and TUNEL analysis), and function (vasoactivity, mechanical and biomechanical properties). Thus, the resulting vascular grafts behave in a manner similar to native arteries in terms of mechanical integrity, and provide clinically relevant patency rates when implanted in vivo. Moreover, the ex vivo methods and system permit the precise control of the mechanical environment involving the excised vessel, while at the same time permitting carefully monitoring of the resulting growth / remodeling, thereby opening new avenues of research regarding the mechanical stimuli responsible for specific aspects of remodeling in vivo.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Preparation method of tissue engineering acellular vascular scaffold
InactiveCN106729979AImprove mechanical propertiesAddressing issues such as aneurysmsTissue regenerationProsthesisCross-linkCell-Extracellular Matrix
The invention discloses a preparation method of a tissue engineering acellular vascular scaffold. Enhanced tissue engineering blood vessels without immunogenicity can be obtained by embedding micron high-molecular polymer scaffold into subcutaneous part or abdominal cavity of a host and performing acellular treatment on the blood vessel scaffold wrapped by utilizing a host immune protection mechanism. The preparation method has the advantages that deficient mechanical performance of the tissue engineering acellular blood vessel can be solved, and the mechanical performance of the original tissue engineering acellular vascular scaffold can be fully improved through a multilayer cross-linked mesh type high-molecular scaffold; the problem of immunogenicity of the tissue engineering blood vessel in allotransplantation can be solved, the scaffold blood vessel formed by migrating cells caused by immunologic mechanism is established in a host animal body, the transplanted immunoreaction can be reduced through acellular treatment, extracellular matrix is fully utilized to provide a good environment for cell regeneration; and different acellular vascular scaffolds can be prepared through designing scaffolds of different shapes and sizes of by utilizing the method, so as to be applied under different blood vessel transplanting conditions.
Owner:NANKAI UNIV
Ex vivo remodeling of excised blood vessels for vascular grafts
InactiveUS20060155164A1Increase the diameterIncrease the lengthArtificial cell constructsBlood vesselsMechanical integrityBiomechanics
The present invention provides an ex vivo vascular remodeling methods and system by which an excised, small diameter blood vessel can be harvested and expanded to provide viable vascular grafts, as demonstrated at the physical and molecular levels, and as optimized in vivo. The tissue-engineered vessels generated by the present invention closely resemble native vessels in terms of structure, histologically, including endothelial coverage and intricate structural components such as the internal elastic lamina, viability (as measured with the MTT assay and TUNEL analysis), and function (vasoactivity, mechanical and biomechanical properties). Thus, the resulting vascular grafts behave in a manner similar to native arteries in terms of mechanical integrity, and provide clinically relevant patency rates when implanted in vivo. Moreover, the ex vivo methods and system permit the precise control of the mechanical environment involving the excised vessel, while at the same time permitting carefully monitoring of the resulting growth / remodeling, thereby opening new avenues of research regarding the mechanical stimuli responsible for specific aspects of remodeling in vivo.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
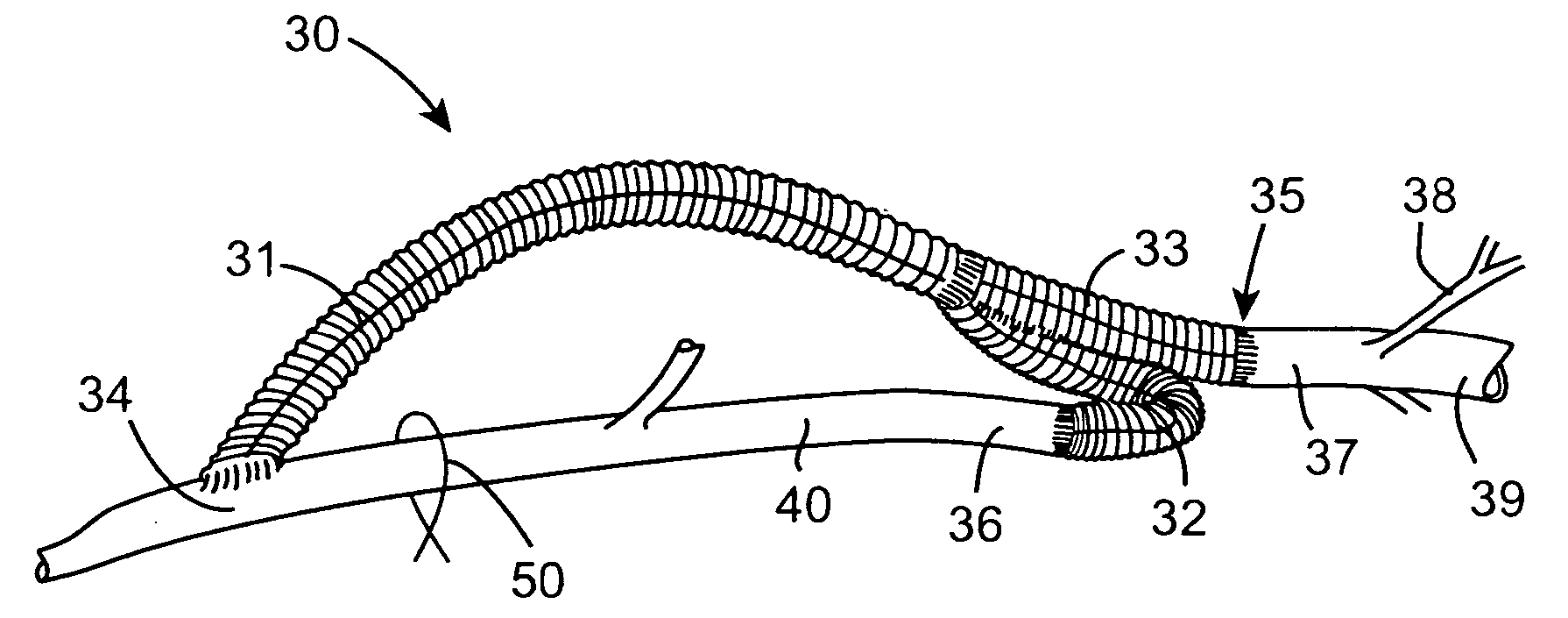
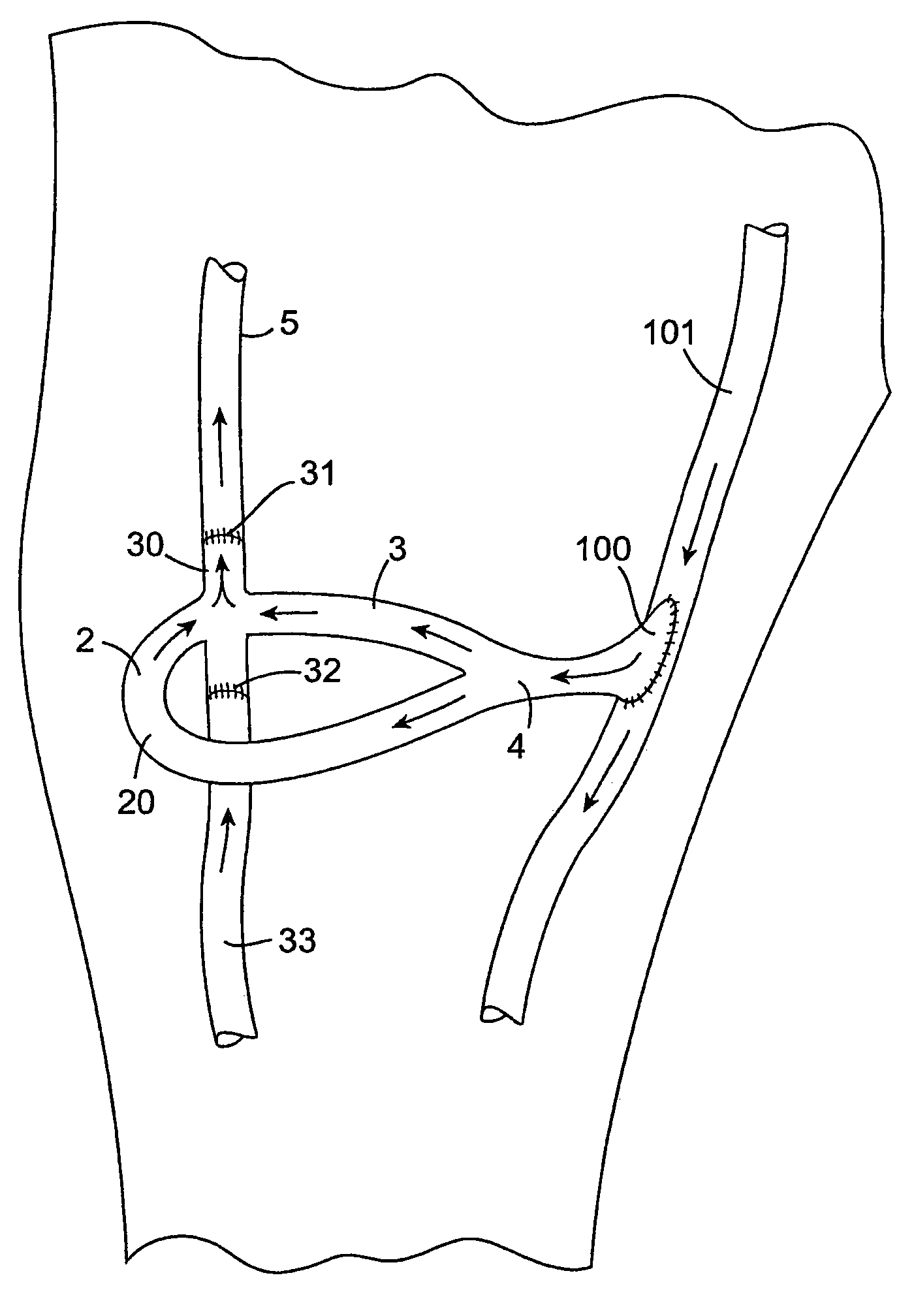
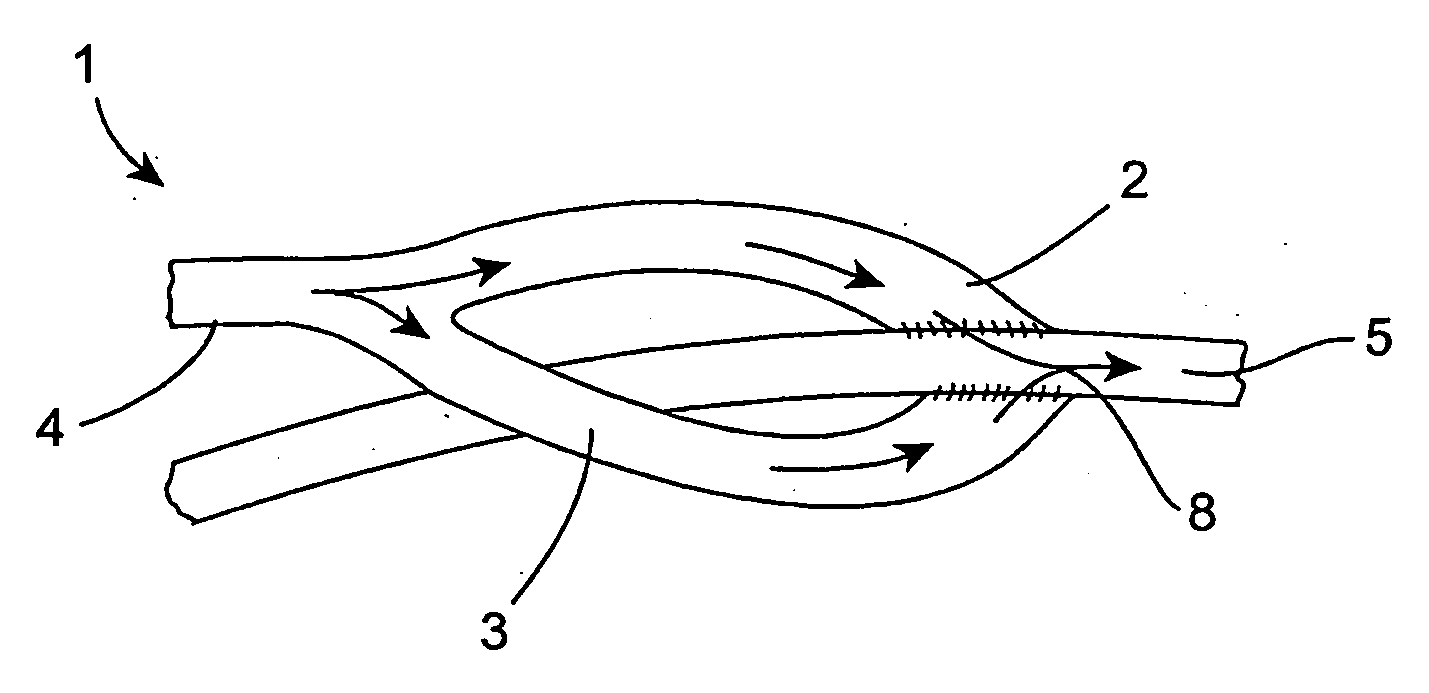
Vascular graft
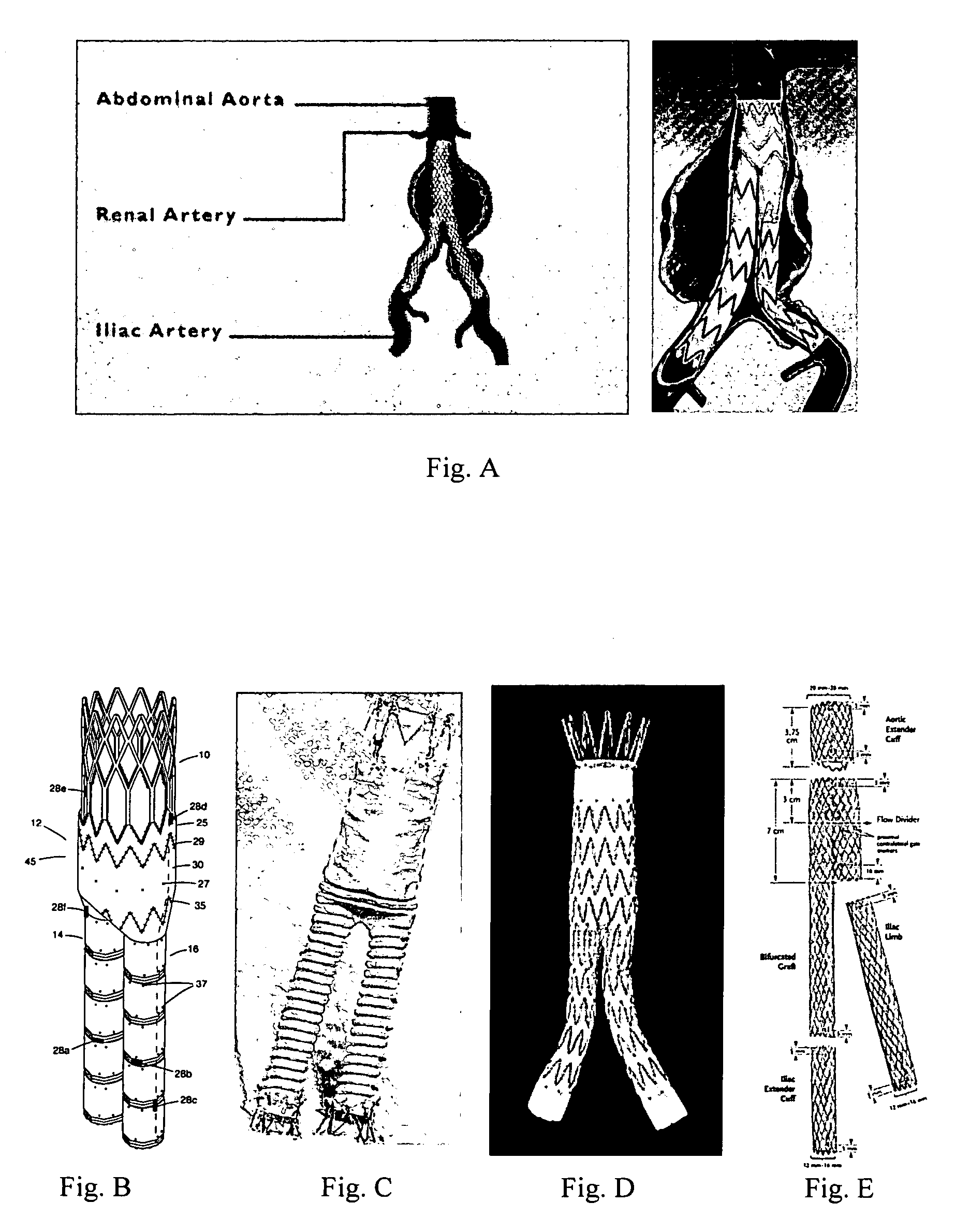
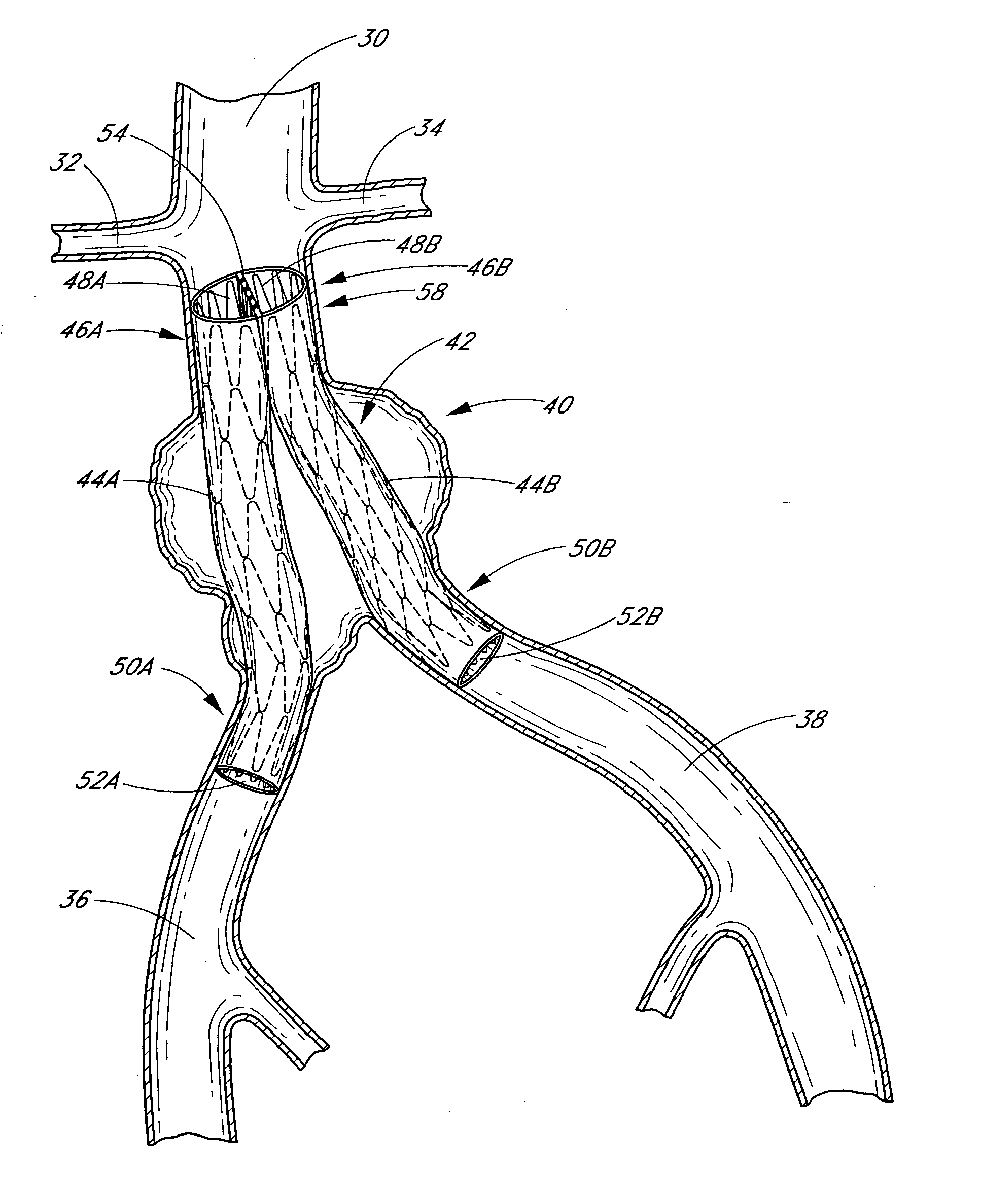
A vascular graft (30) comprises a proximal inlet section (31), a first distal section (32) and a second distal section (33). The first distal section (32) and the second distal section (33) are attached to the proximal inlet section (31) at a Y-shaped bifurcation region. In use the proximal inlet section (31) is attached to a first part (34) of a host artery in an end-to-side anastomosis. A second part (35) of the host artery is cut to form a first section (36) of the host artery on a first side of the cut and a second section (37) of the host artery on a second side of the cut. The first distal section (32) is attached to the first section (36) in an end-to-end anastomosis, and the second distal section (33) is attached to the second section (37) in an end-to-end anastomosis. When implanted the graft (30) directs blood flow from the first part (34) of the host artery through the proximal inlet section (31), into the second distal section (33) and into the second section (37) of the host artery along a single flow path.
Owner:UNIVERSITY OF LIMERICK
Smart textile vascular graft
The smart textile vascular graft is used with an electrical control unit. The vascular graft includes a tube structure formed of a textile material. At least one active fiber is incorporated in the textile material. The active fiber provides electrical coupling thereof to the electrical control unit to provide for transmission of a control signal between the active fiber and control unit. The active fiber has at least one physical characteristic which is variable and corresponds to the control signal to provide communication between the active fiber and electrical control unit relating to the physical characteristic.
Owner:LIFESHIELD SCI
Vascular graft
InactiveUS7651526B2Minimize the possibilityImprove impactStentsBlood vesselsMedicinePercent Diameter Stenosis
A vascular graft includes a proximal section, integral with two branches which terminate in a distal end-to-end section. The end-to-end section is attached to a host artery at end-to-end anastomoses. Flow of blood from the proximal section to the host artery occurs with a self-correcting flow pattern at the opposing junctions, avoiding arterial bed impingement and associated risk of restenosis.
Owner:UNIVERSITY OF LIMERICK
Vascular graft
InactiveUS20050004664A1Marked and distinct improvement in performanceMarked and distinct improvement in and manufacturing characteristicBlood vesselsVinyl etherTetrafluoroethylene
Disclosed are vascular grafts including an expanded copolymer of tetrafluoroethylene (TFE) and perfluoropropylene vinyl ether (PPVE). In certain embodiments, the copolymer includes between about 0.01% and about 1.5% PPVE. Vascular grafts exhibit superior performance properties, e.g., radial strength, and suture strength, and manufacturing properties, e.g., sinter time. Methods of forming vascular grafts from the copolymer also are described.
Owner:ATRIUM MEDICAL
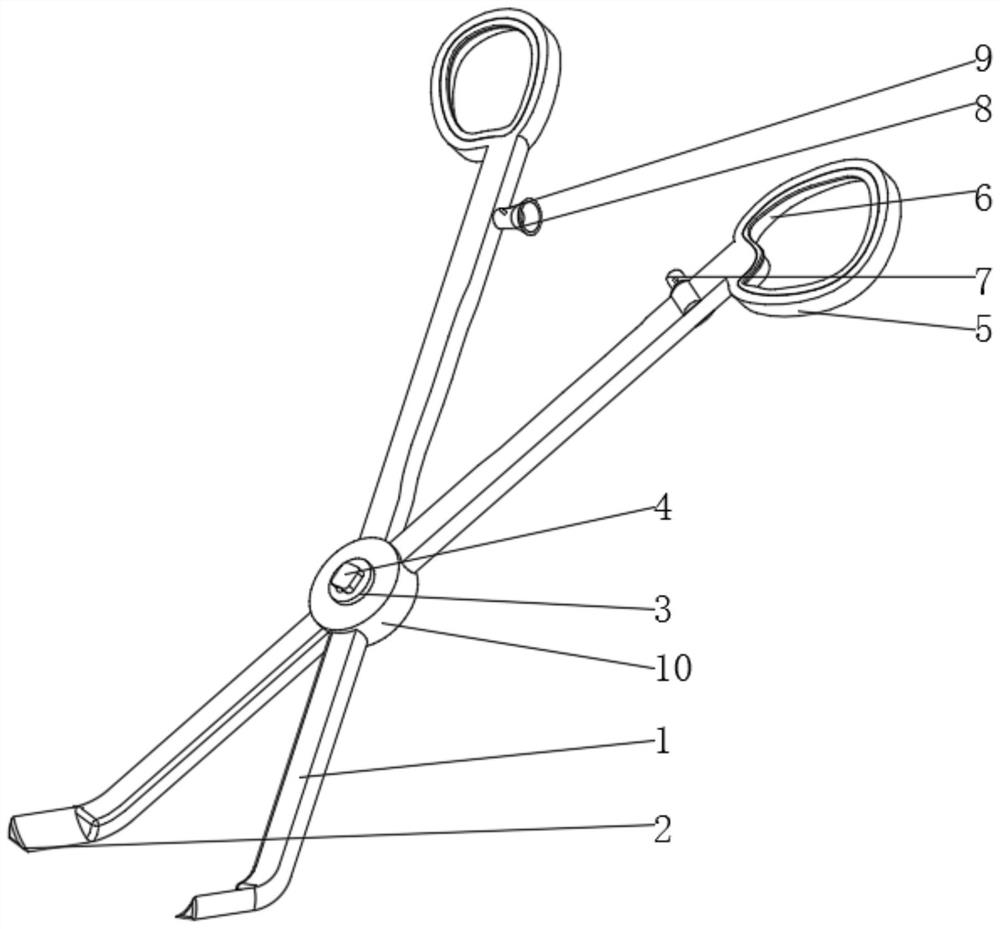
Vascular transplantation equipment for cardiovascular surgery
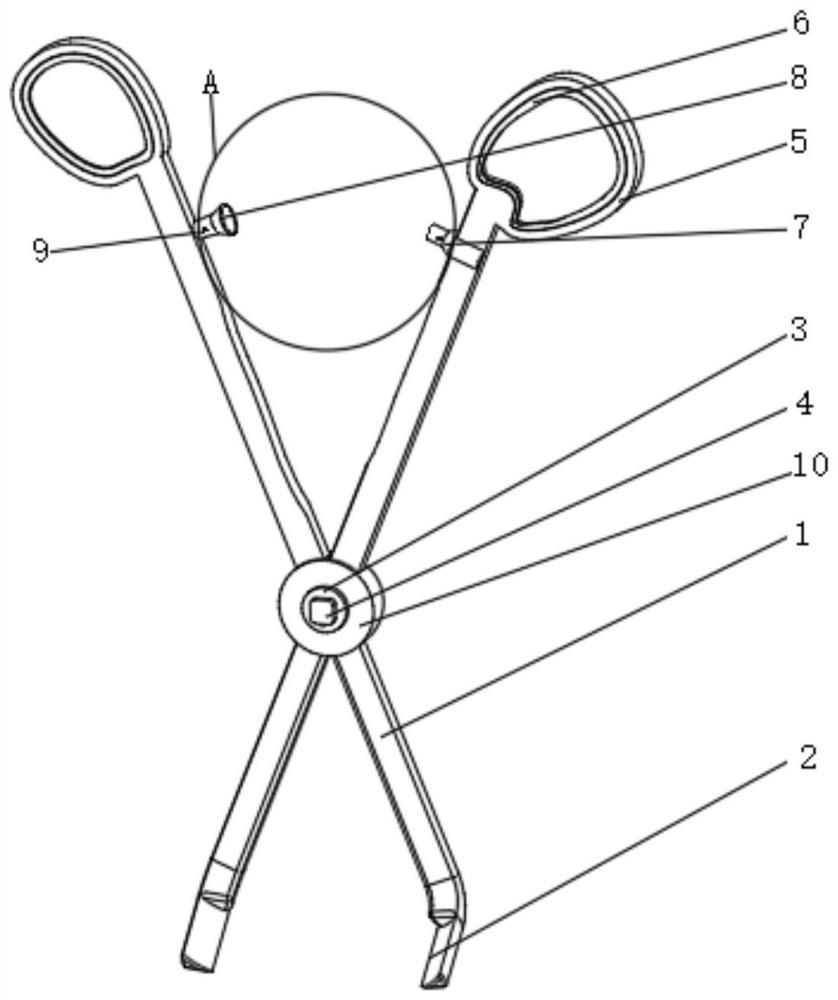
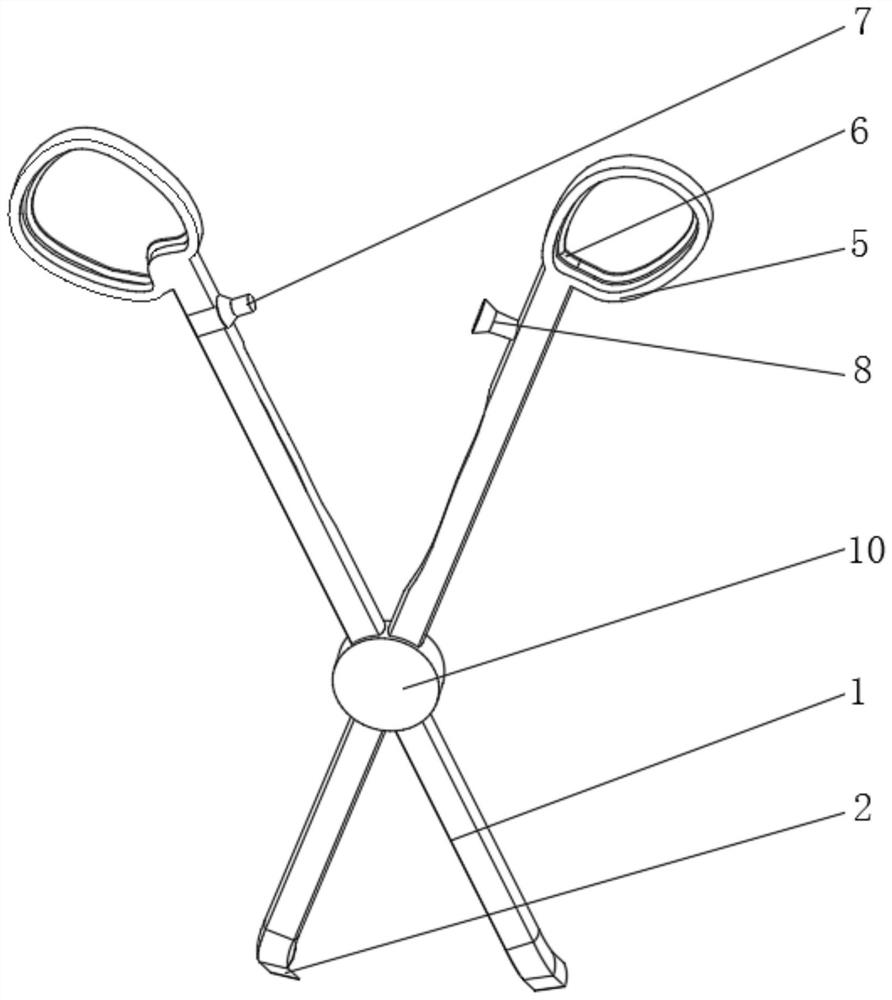
ActiveCN112022287AImprove cutting effectTimely and effective real-time transmissionDiagnosticsSurgical scissorsRubber ringVascular grafting
The invention relates to the technical field of medical instruments, and discloses vascular transplantation equipment for cardiovascular surgery. The equipment comprises two handle rods, cutter blocksare fixedly mounted at the bottoms of the two handle rods, a fixer is fixedly mounted at the junction of the two handle rods, a rotating disc is fixedly mounted on the front surface of the fixer, anda rotating disc is fixedly mounted on the back surface of the fixer. A miniature camera is movably clamped to the front face of the rotating disc, holding rings are fixedly mounted at the tops of thetwo handle rods, and rubber rings are fixedly mounted on the front faces of the interiors of the two holding rings. According to the vascular transplantation equipment for the cardiovascular surgery,the purposes of easy observation and good shearing effect are achieved by arranging the knife block, the handle rods, the miniature camera and the like, the included angle between the knife block andthe handle rod is 60 degrees, so that the situation of obviously obstructing the view during working is avoided, the knife block is of an obvious triangular structure, and the shearing effect is good; and meanwhile, the miniature camera is matched, so that the operation picture and the internal condition can be timely and effectively transmitted in real time.
Owner:广州极诣科技有限公司
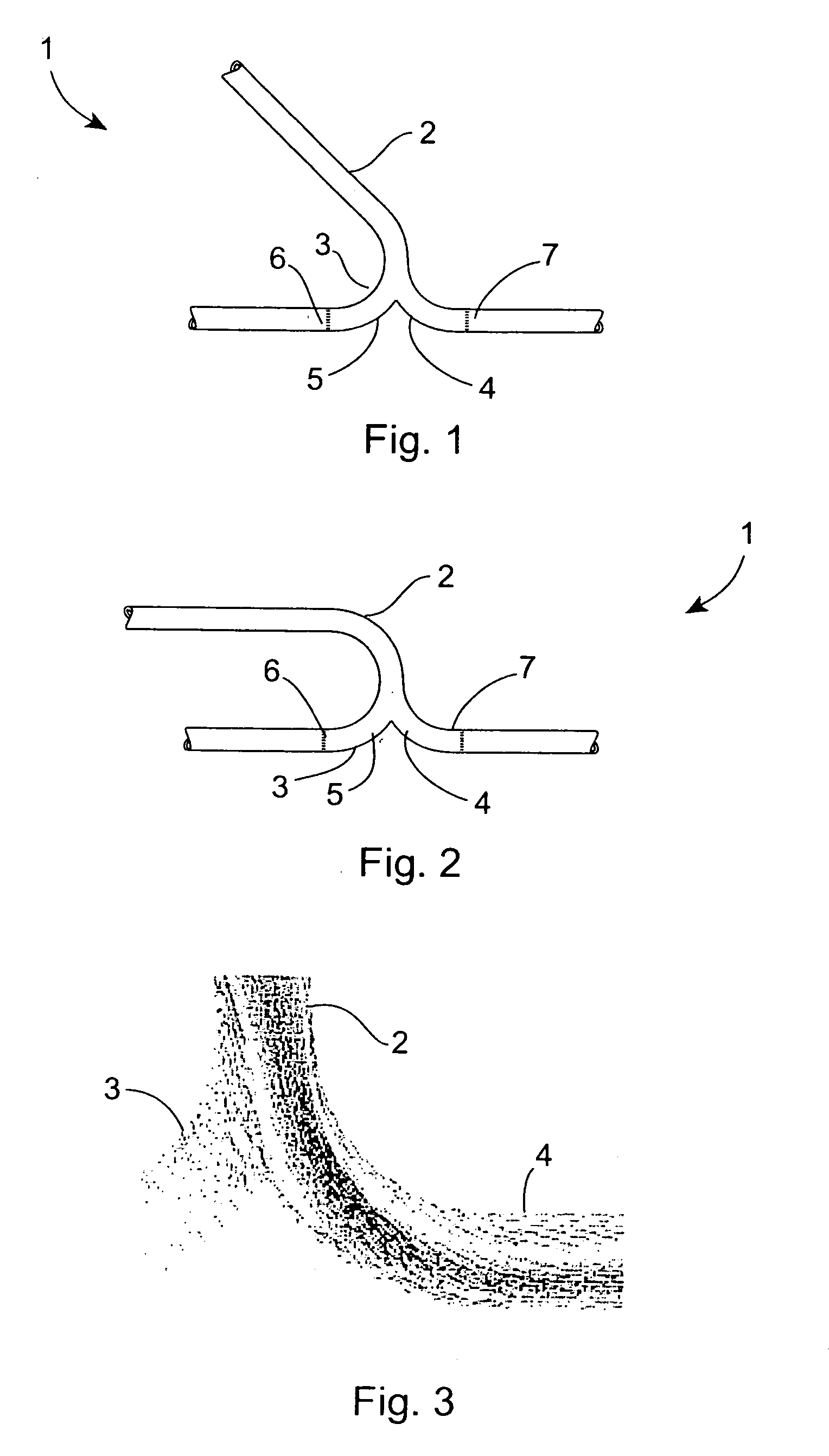
Vascular graft
InactiveUS20060116753A1Minimize the possibilityImprove impactStentsBlood vesselsPercent Diameter StenosisVascular grafting
A vascular graft (20) comprises a proximal section (4), integral with two branches (2, 3) which terminate in a distal end-to-end section (30). The end-to-end section (30) is attached to a host artery (5) at end-to-end anastomoses (31, 32). Flow of blood from the proximal section (4) to the host artery (5) occurs with a self-correcting flow pattern at the opposing junctions, avoiding arterial bed impingement and associated risk of restenosis.
Owner:UNIVERSITY OF LIMERICK
Compositions and methods for ex vivo preservation of blood vessels for vascular grafts using inhibitors of tumor necrosis factor-alpha
InactiveUS20050201989A1Improve survivabilityAvoid damageBiocideDead animal preservationBlood levelTumor necrosis factor alpha
The present invention relates to ex vivo methods for preserving / maintaining blood vessels that are to be used as vascular grafts by inhibiting TNF-α. The present invention also relates to compositions comprising an inhibitor of a TNF-α for use in the methods of the invention, which compositions are free of active TGF-β or does not contain active TGF-β in an amount greater than endogenous blood levels.
Owner:ST CAMILLUS MEDICAL
Medical Device for Anastomosis
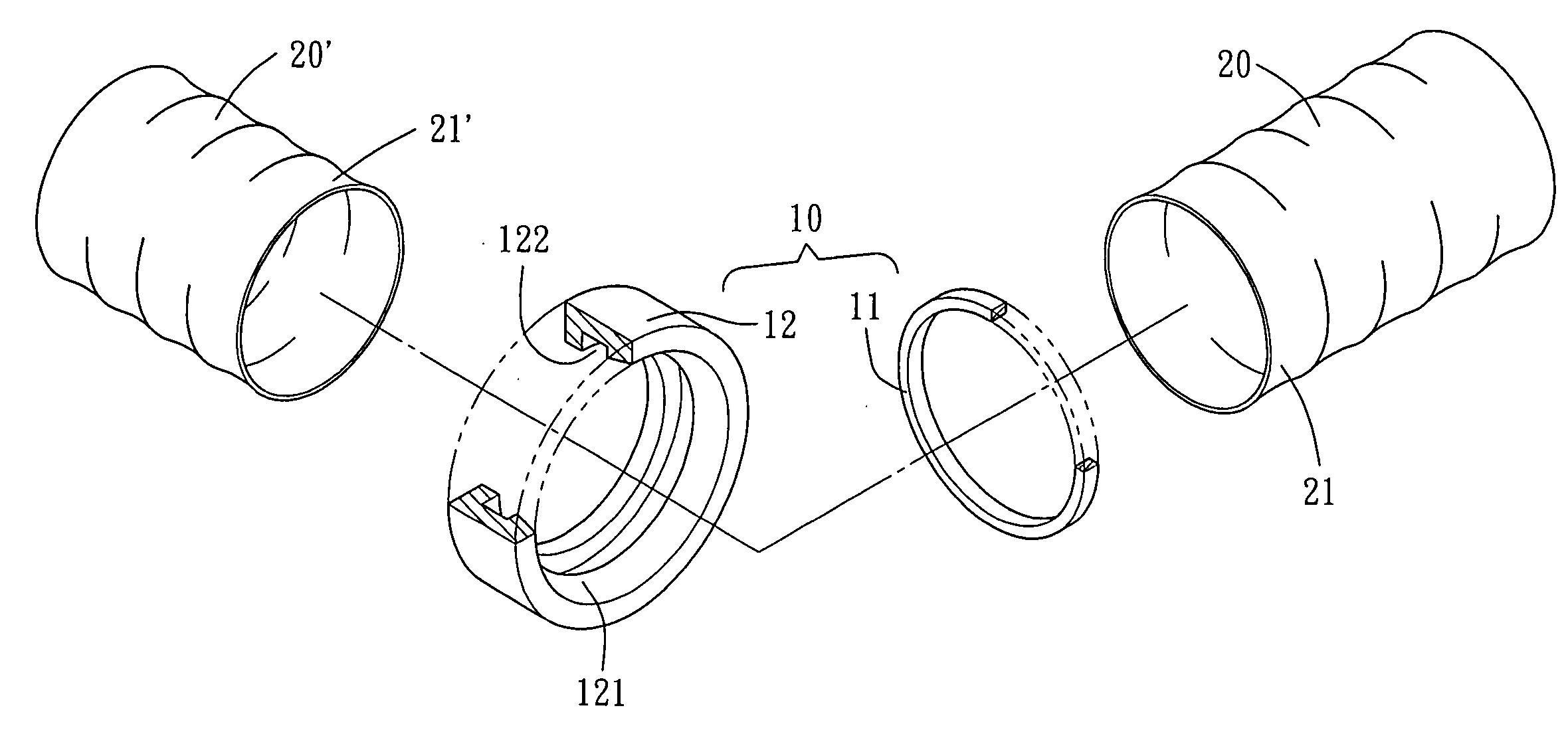
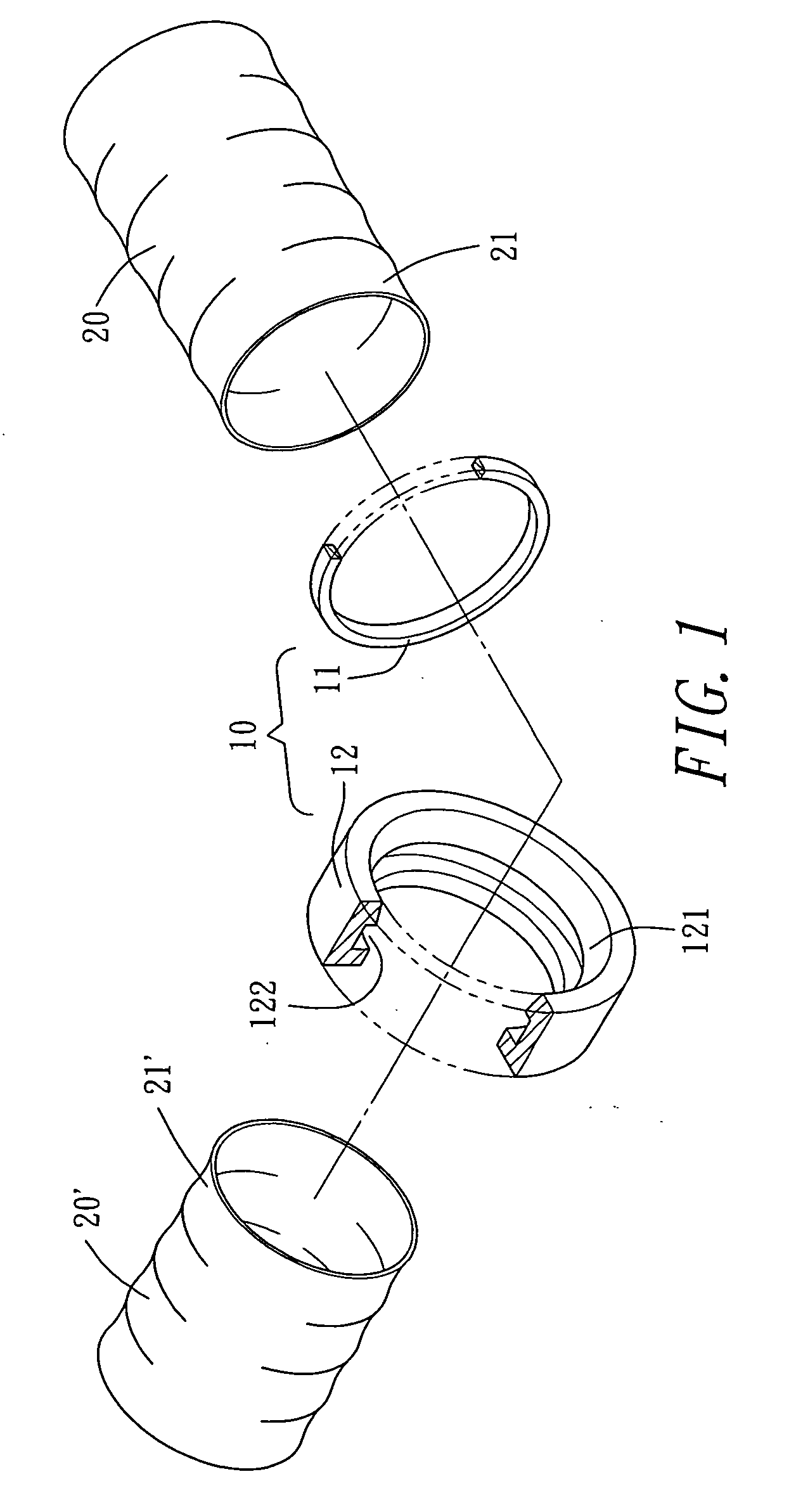
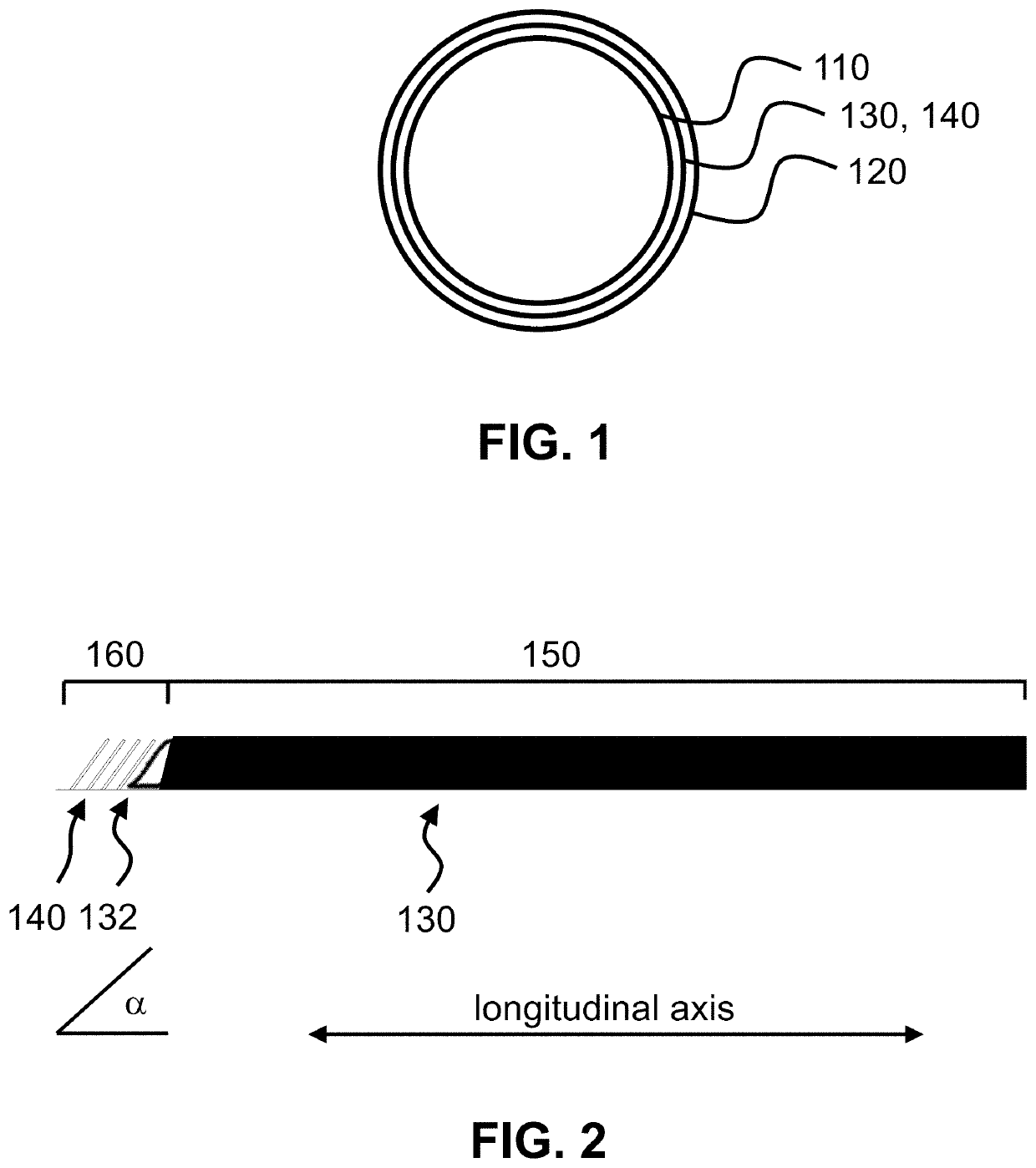
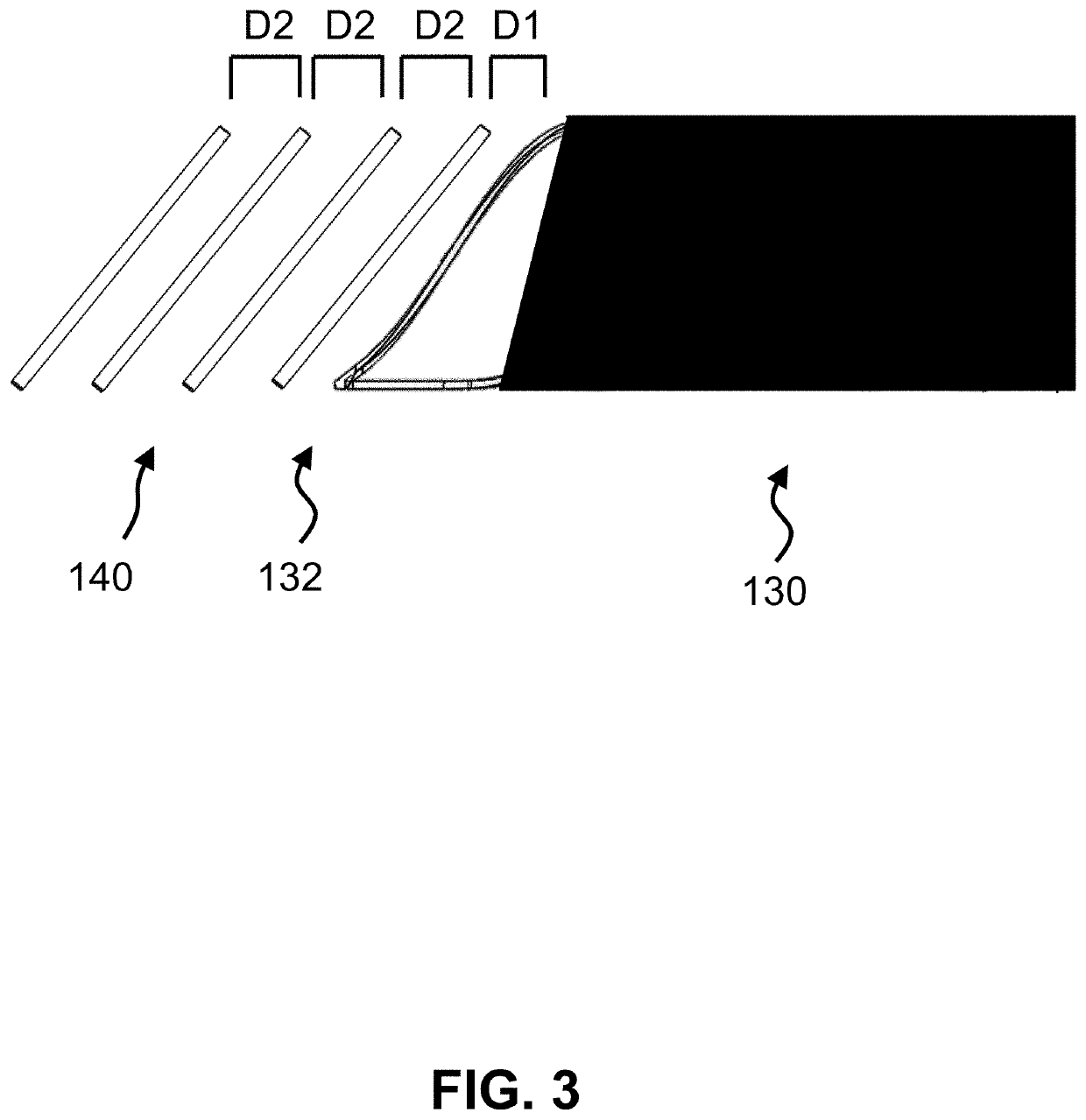
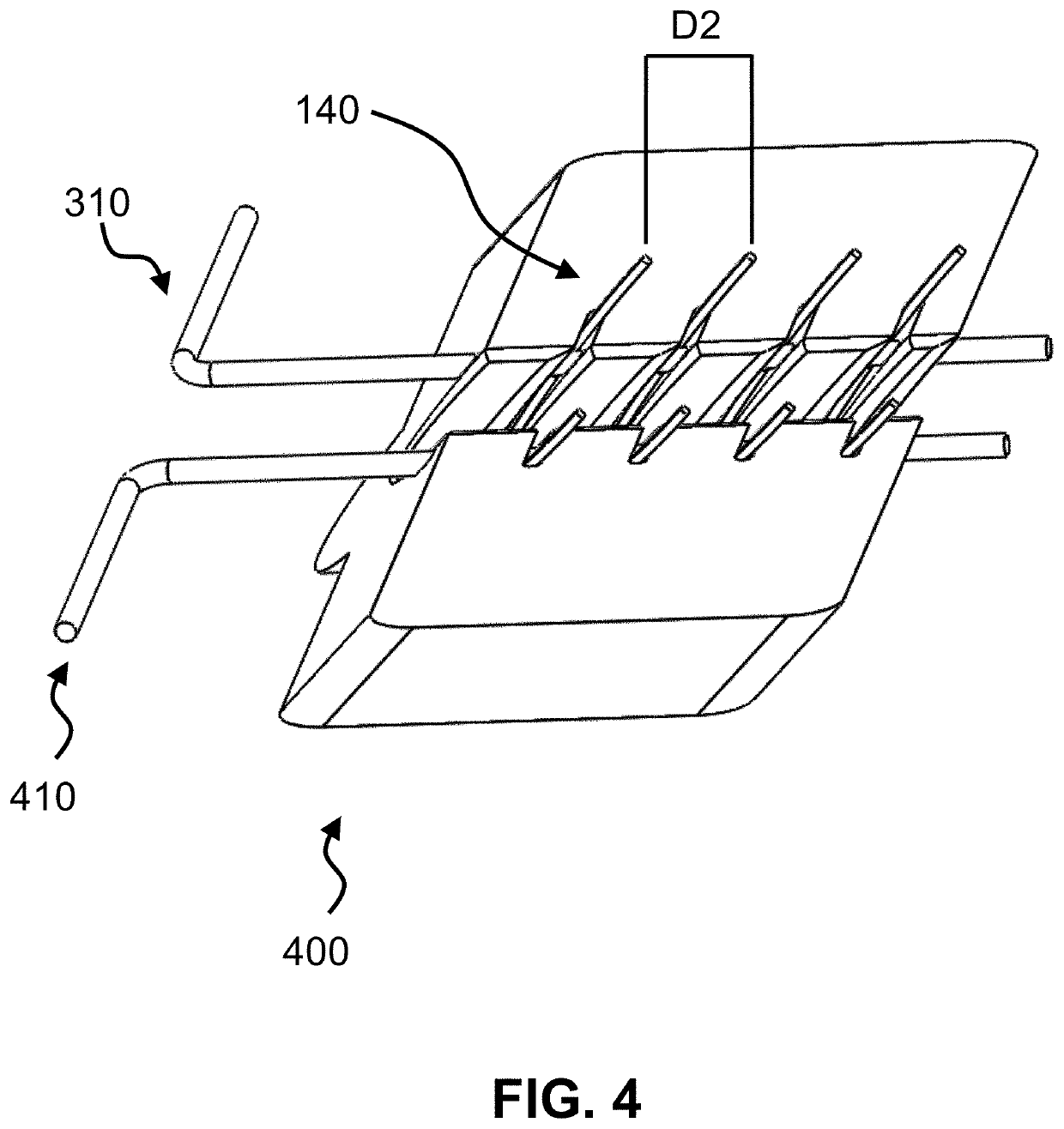
ActiveUS20210267599A1Sufficient kink resistanceLarge graft ostiumStentsSurgeryAnastomosis couplerVascular grafting
A medical device for an anastomosis is provided. The medical device distinguishes an inner tubular layer, an outer tubular layer, and a support element defining a longitudinal axis. It further distinguishes two or more independent C-rings distributed and positioned at an acute orientation angle relative to the longitudinal axis of the support element at one end of the support element. The support element and the two or more C-rings are embedded in between the inner and the outer tubular layers. The types of applications one could envision are e.g. a proximal anastomosis, distal anastomosis, or side-to-side anastomoses, in a customized pre-fabricated graft. Embodiments of the invention could also be incorporated into an anastomotic connector device design. Embodiments of the invention could further be envisioned as vascular grafts applications such as Coronary Artery Bypass Graft (CABG), dialysis access grafts and peripheral vascular applications.
Owner:XELTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com