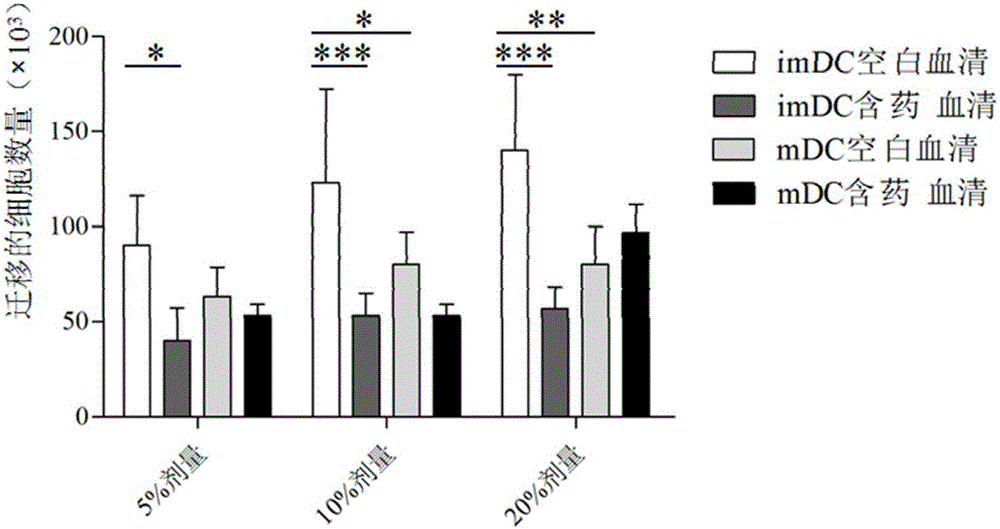
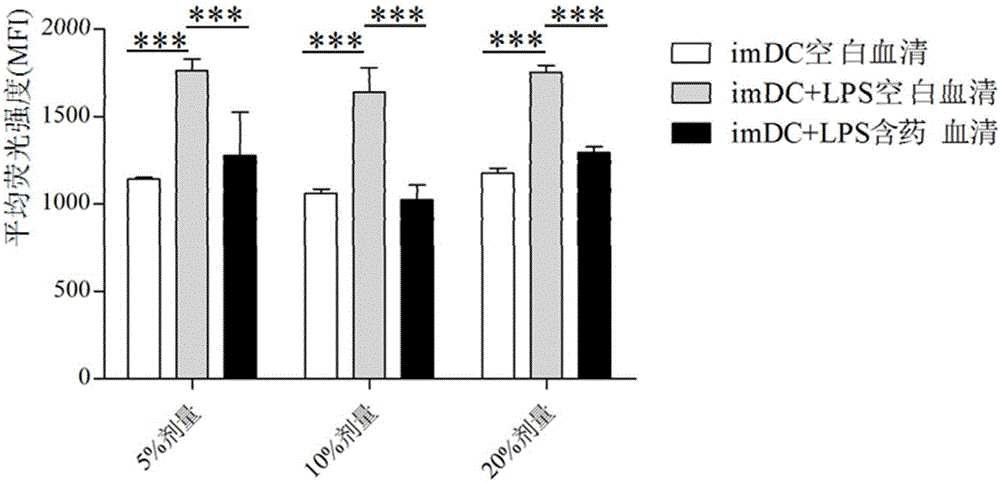
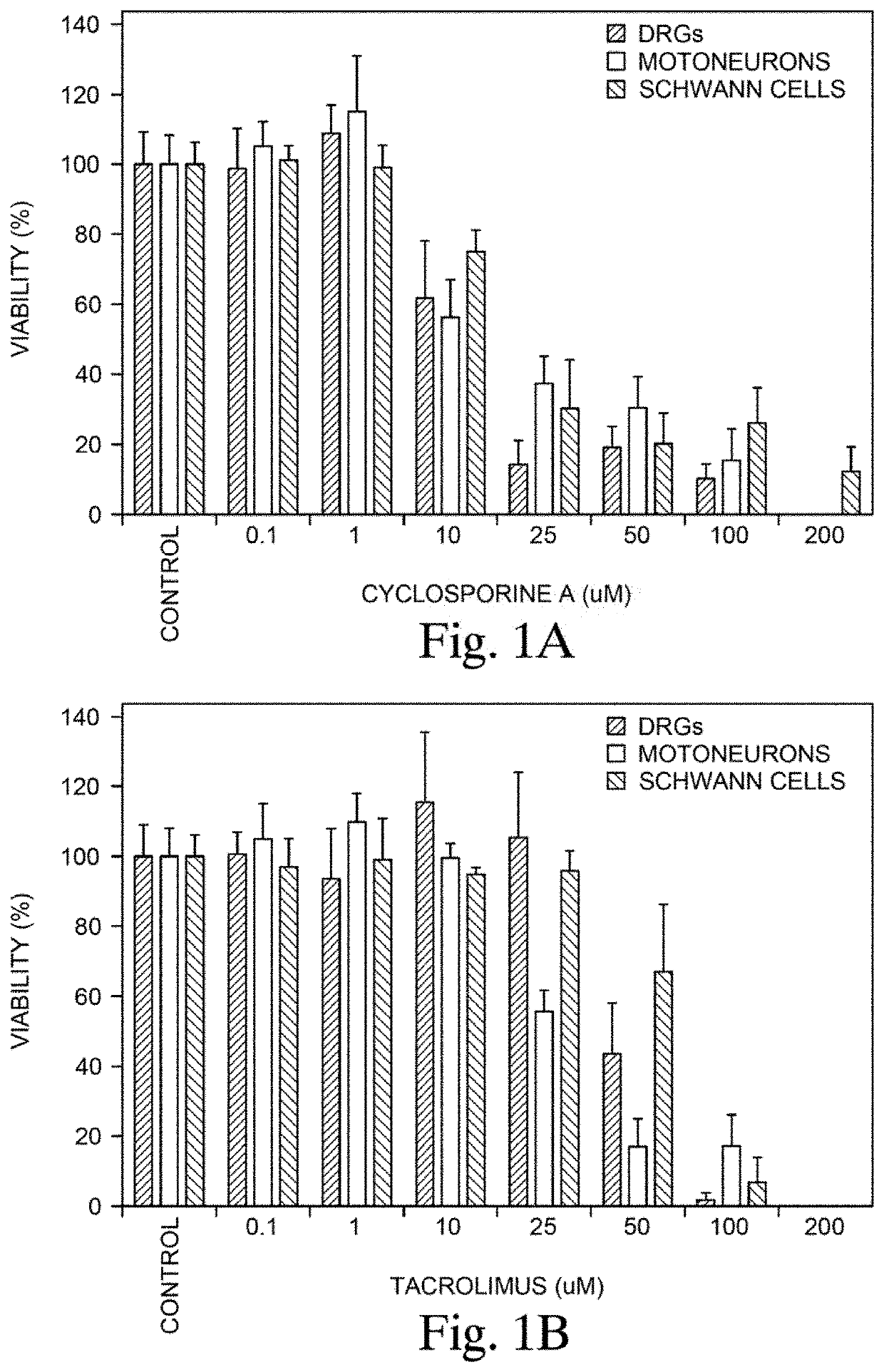
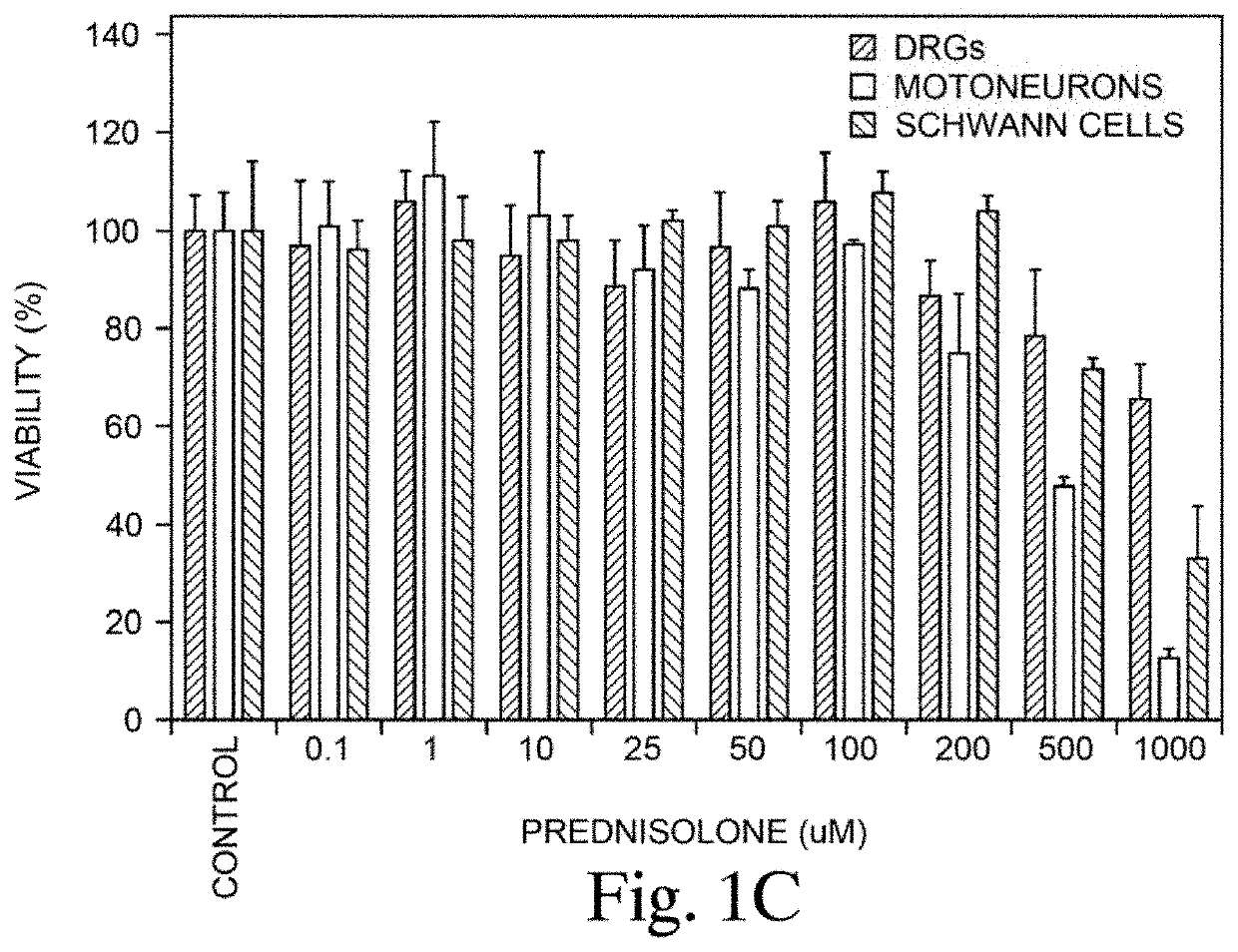
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "Allotransplantation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Allotransplant (allo- meaning "other" in Greek) is the transplantation of cells, tissues, or organs to a recipient from a genetically non-identical donor of the same species. The transplant is called an allograft, allogeneic transplant, or homograft. Most human tissue and organ transplants are allografts.
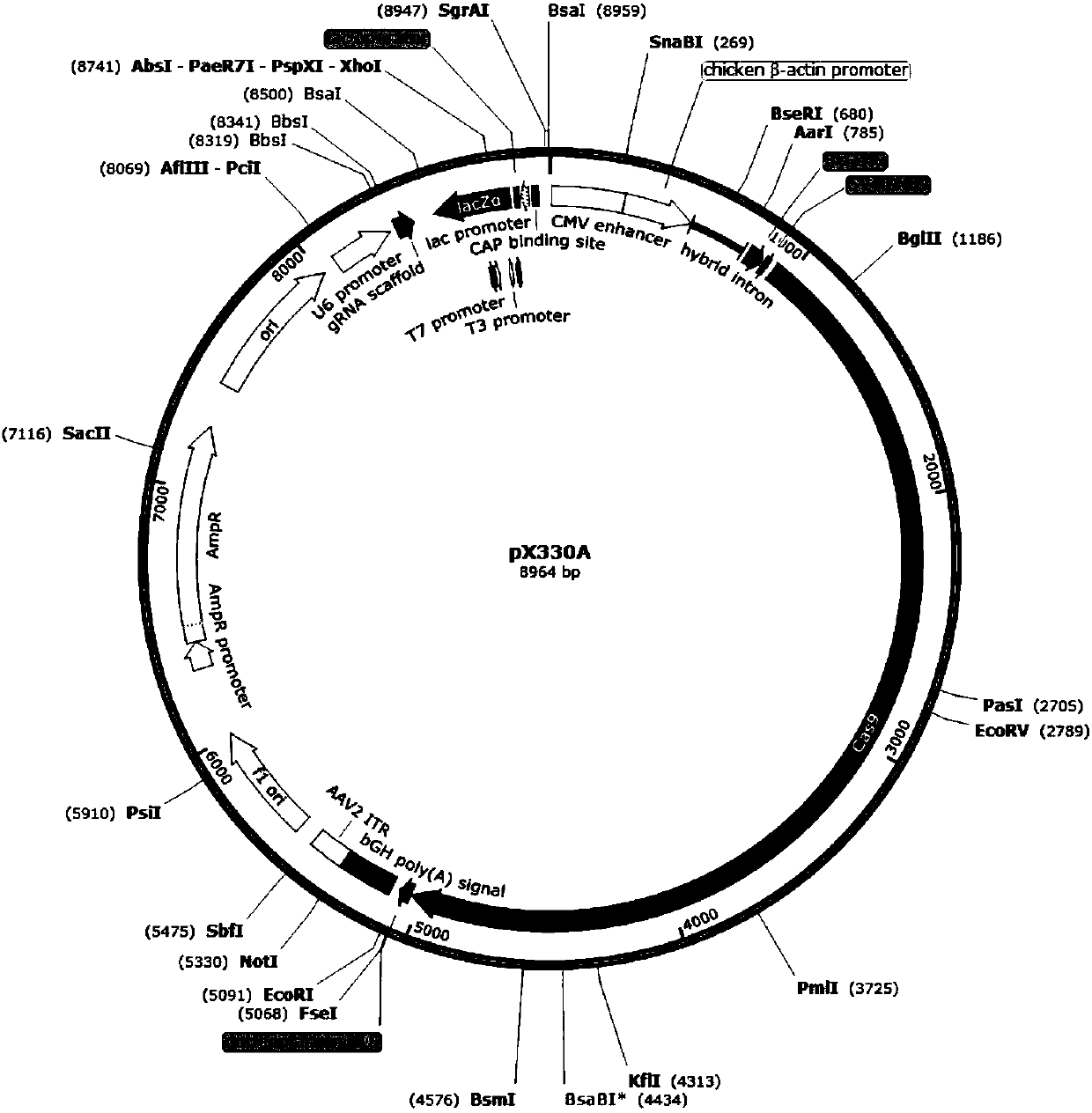
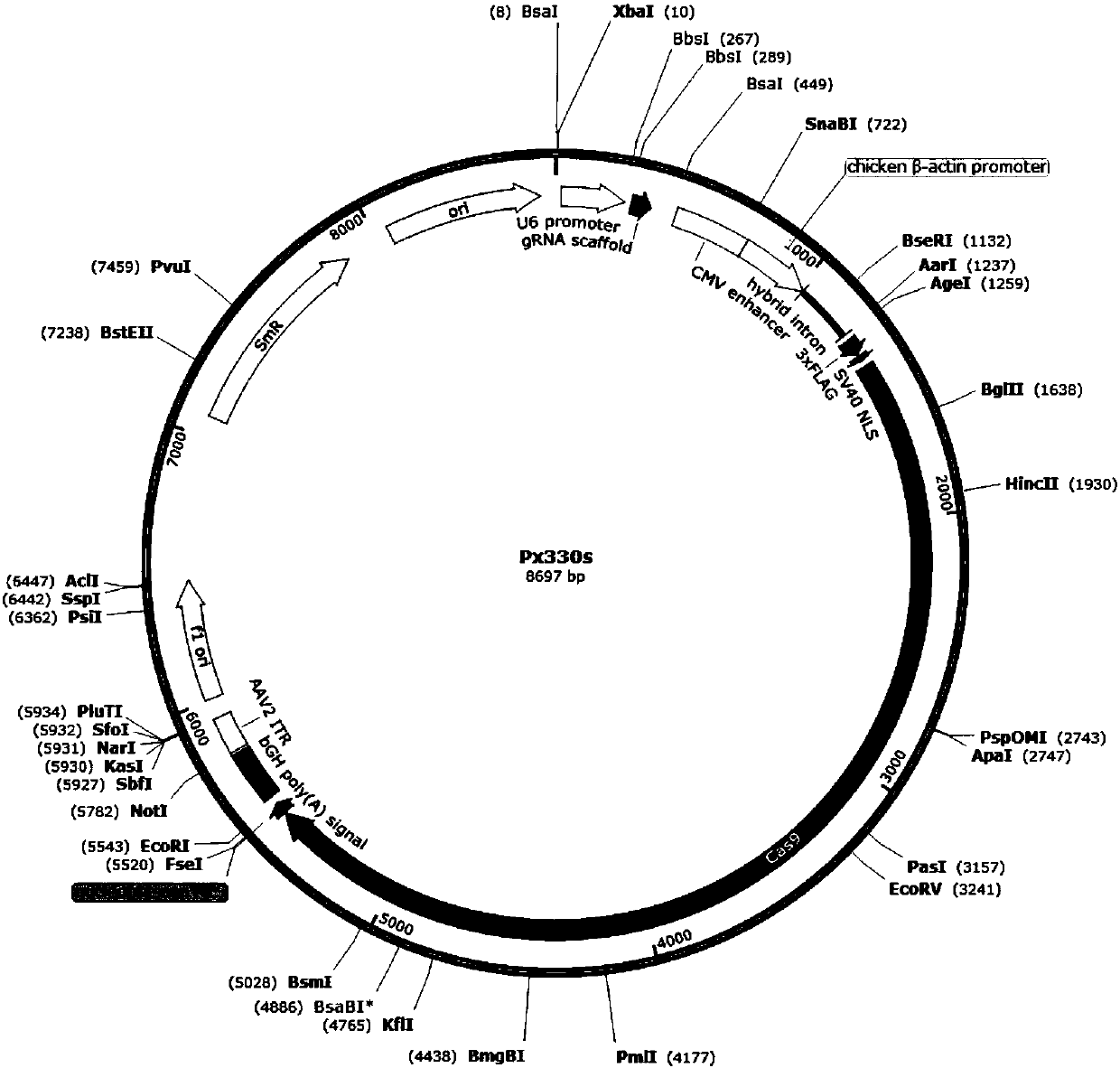
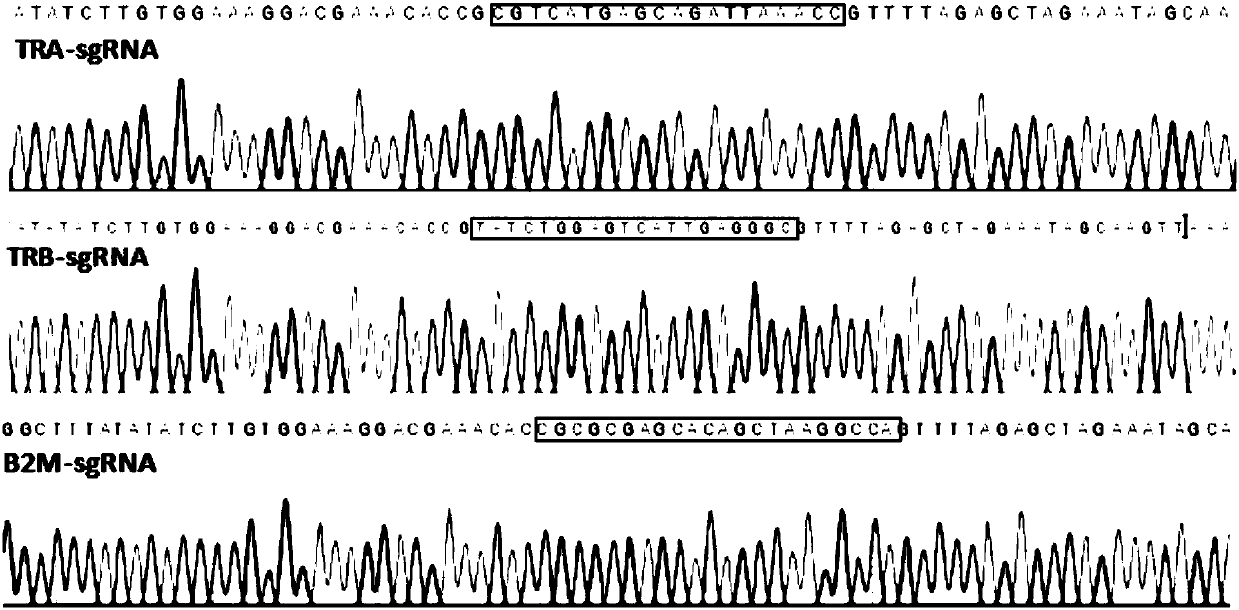
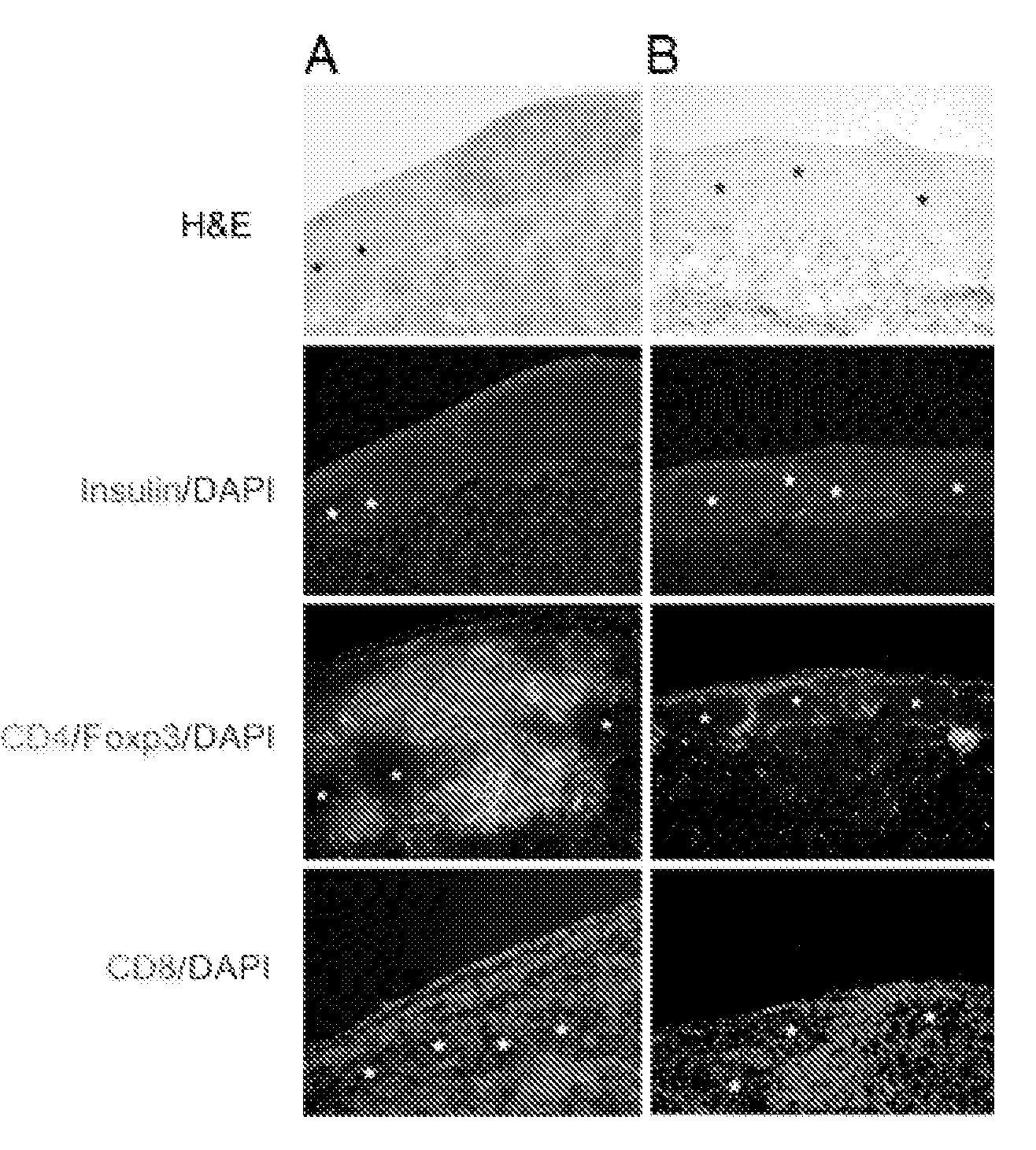
Method for preparing T cells with knockout of double genes TCR and HLA
ActiveCN107630006AImprove securityHas zero off-target effectsVector-based foreign material introductionForeign genetic material cellsAllotransplantationT cell
The invention discloses a method for preparing T cells with knockout of double genes TCR and HLA. The method comprises the following steps: co-transfecting T cells by plasmids of CRISPR / Cas9-HLA and CRISPR / CAs9-TCR, and carrying out knockout of double genes TCR and HLA to obtain the T cells with knockout of double genes TCR and HLA. The method can be used for allotransplantation without causing immunological rejection.
Owner:上海兴瑞一达生物科技有限公司
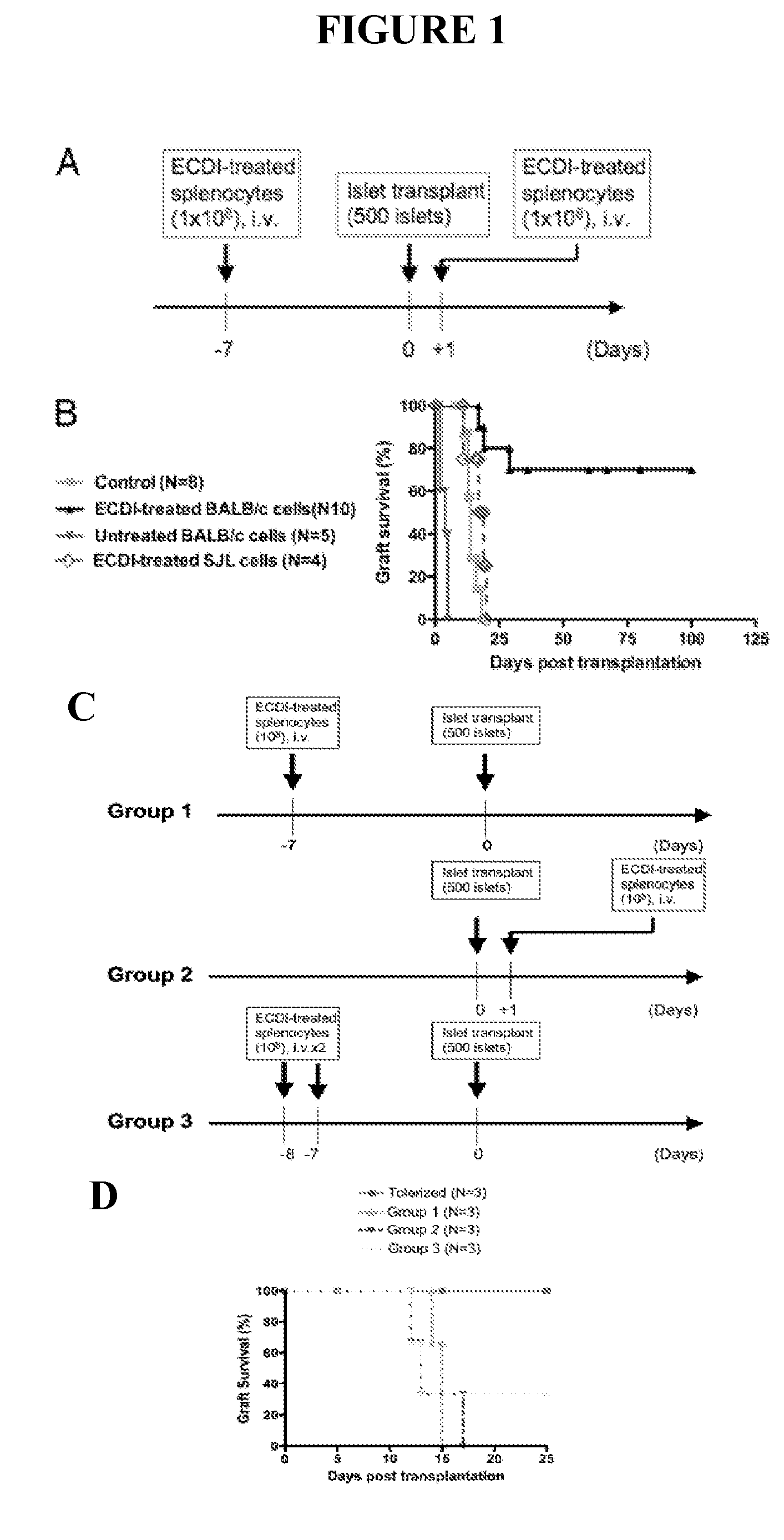
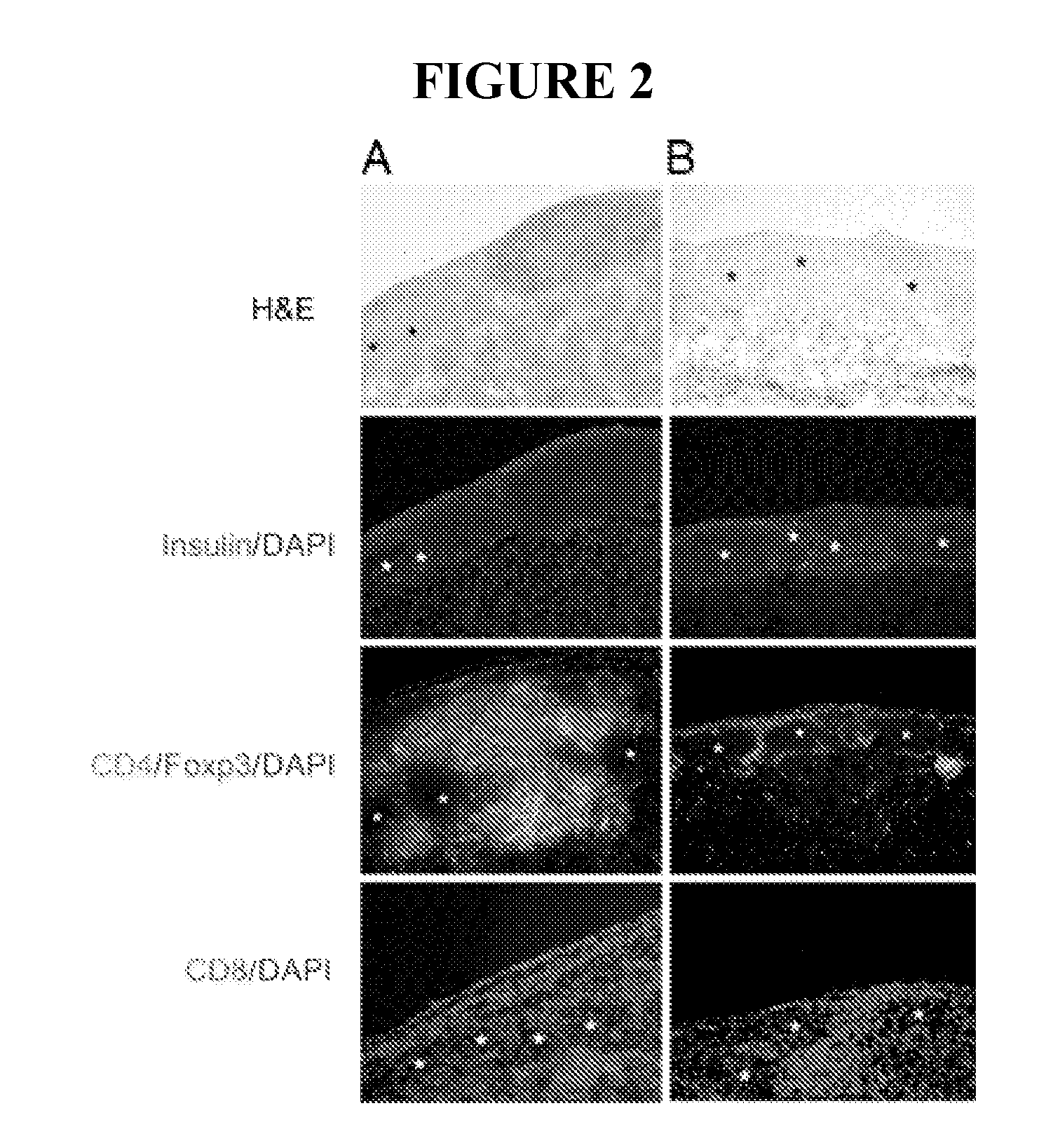
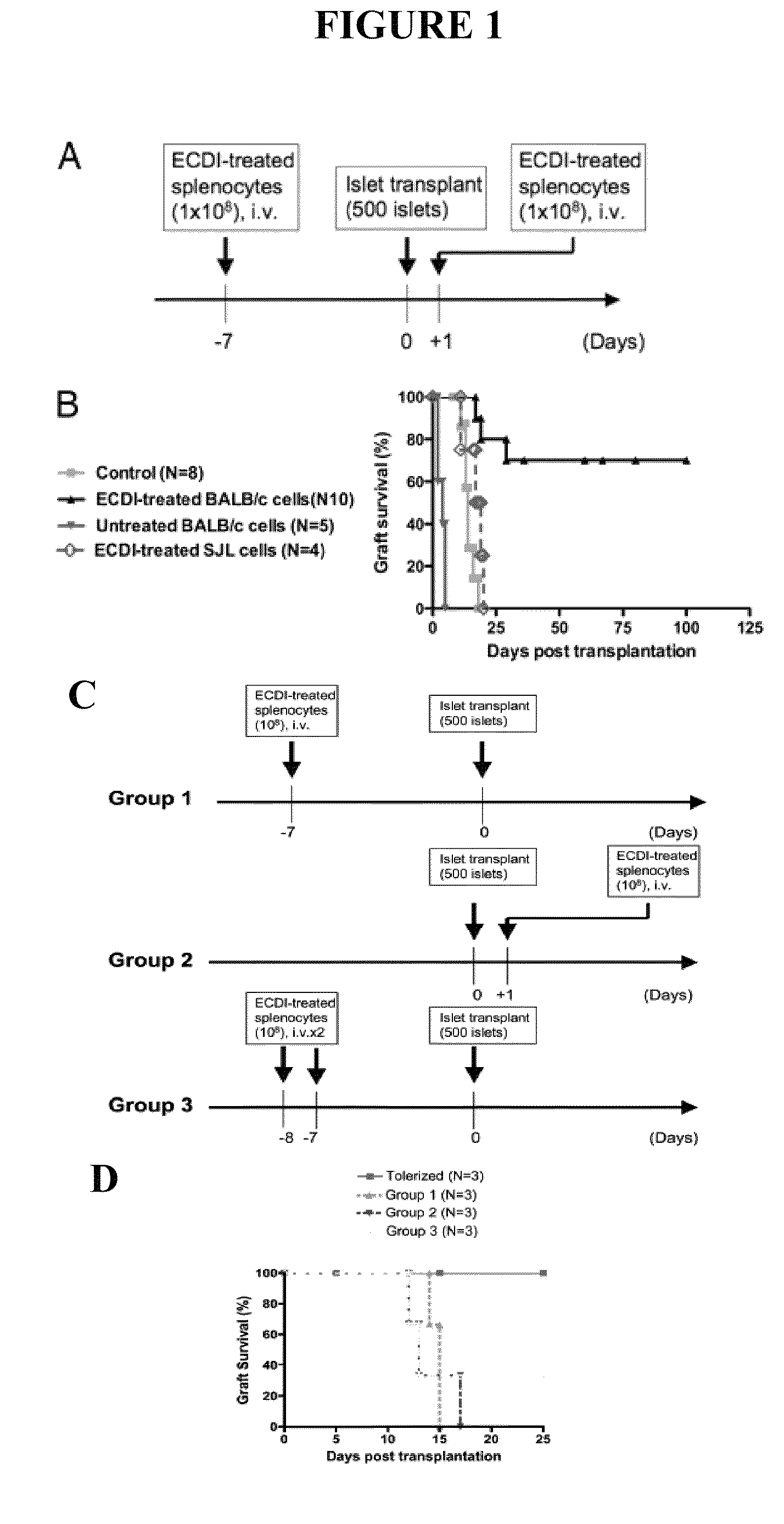
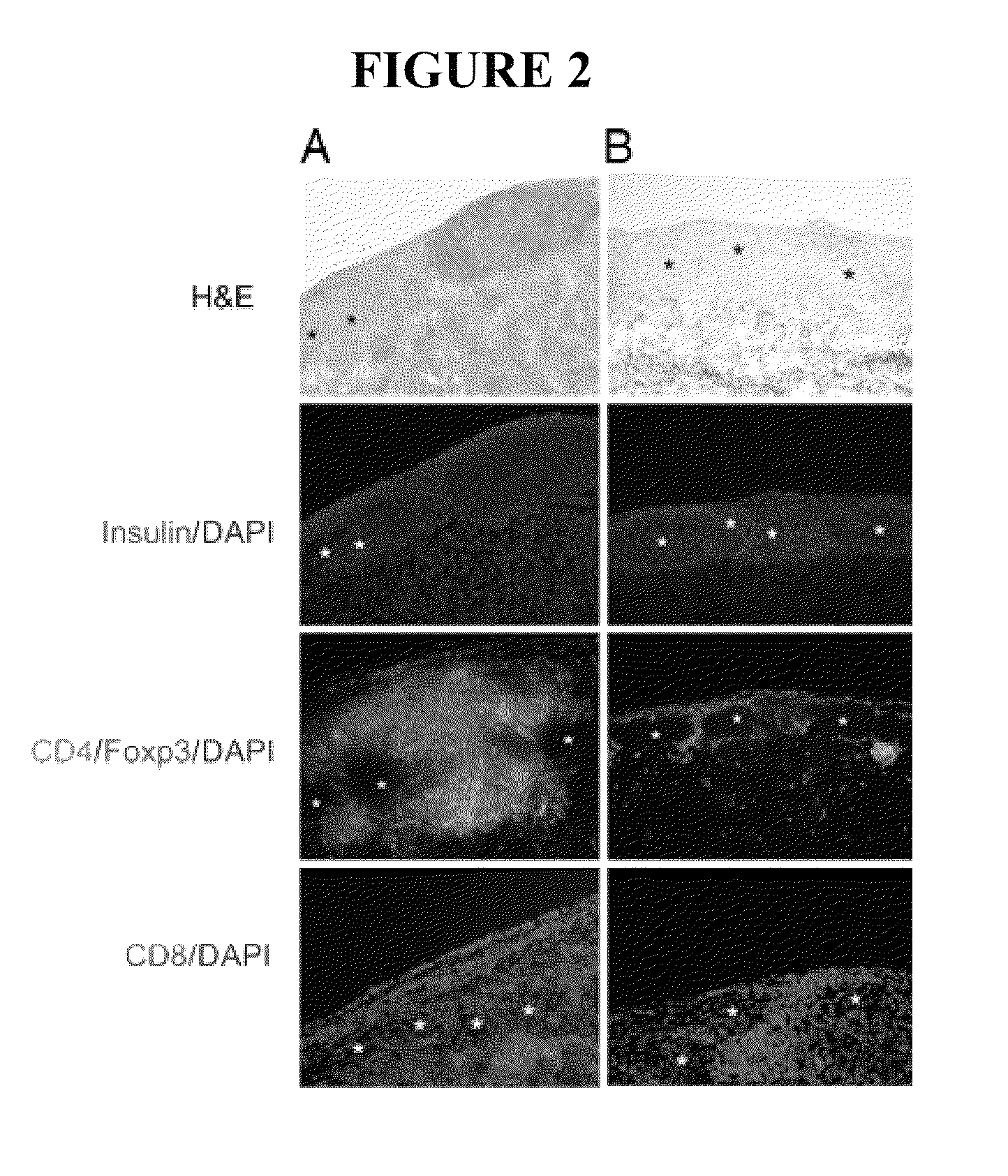
Use of ecdi-fixed cell tolerance as a method for preventing allograft rejection
Owner:NORTHWESTERN UNIV
Novel biological implant compositions, implants and methods
The present application is directed to the field of implants comprising processed hard or soft biological tissues for use in implantation in humans. The molded biological tissue implants of the present application are preferably made from allograft soft tissue sources and demineralized bone matrix. The present application provides biological implants exhibiting advantageous properties of absorption, expansion, resiliency and shape retention. The properties of the biological implants produced by the methods of the present application are leveraged in the creation and development of novel biological implant constructs and of novel methods of treatment and surgical technique.
Owner:RTI BIOLOGICS INC
Compositions and methods for modulation of suppressor t cell activation
InactiveUS20100061984A1Relieve symptomsIncreased acetylation levelsCompounds screening/testingAntibacterial agentsAntigenImmunologic disorders
Methods of treating autoimmune disorders, coronary artery disease, allergy symptoms, allograft rejection sepsis / toxic shock are disclosed. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3 in combination with a T suppressor stimulus and / or an antigen. Some methods comprise administering one or more regulatory compositions to activate the T suppressor cells by increasing the acetylation level and / or protein level of FOXP3. Some methods comprise administering soluble GITR or antibodies that bind to GITR ligand. Methods of treating cancer, infectious diseases, and immune deficiency are also disclosed as are vaccination methods. The methods comprise administering one or more regulatory compositions to inactivate the T suppressor cells by reducing the acetylation level and / or protein level of FOXP3. Improved vaccines and vaccination methods are disclosed. Methods of identifying compounds that are useful to modulate acetylation level and / or protein level of FOXP3 and treat diseases are disclosed.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Composition for autotransplantation or allotransplantation using dental pulp stem cell, and use of the composition
The object is to provide a novel use application of a dental pulp stem cell collected from a deciduous tooth or a permanent tooth. Disclosed is a composition for autotransplantation or allotransplantation, which is characterized by comprising a dental pulp stem cell collected from a deciduous tooth or a permanent tooth.
Owner:NAGOYA UNIVERSITY
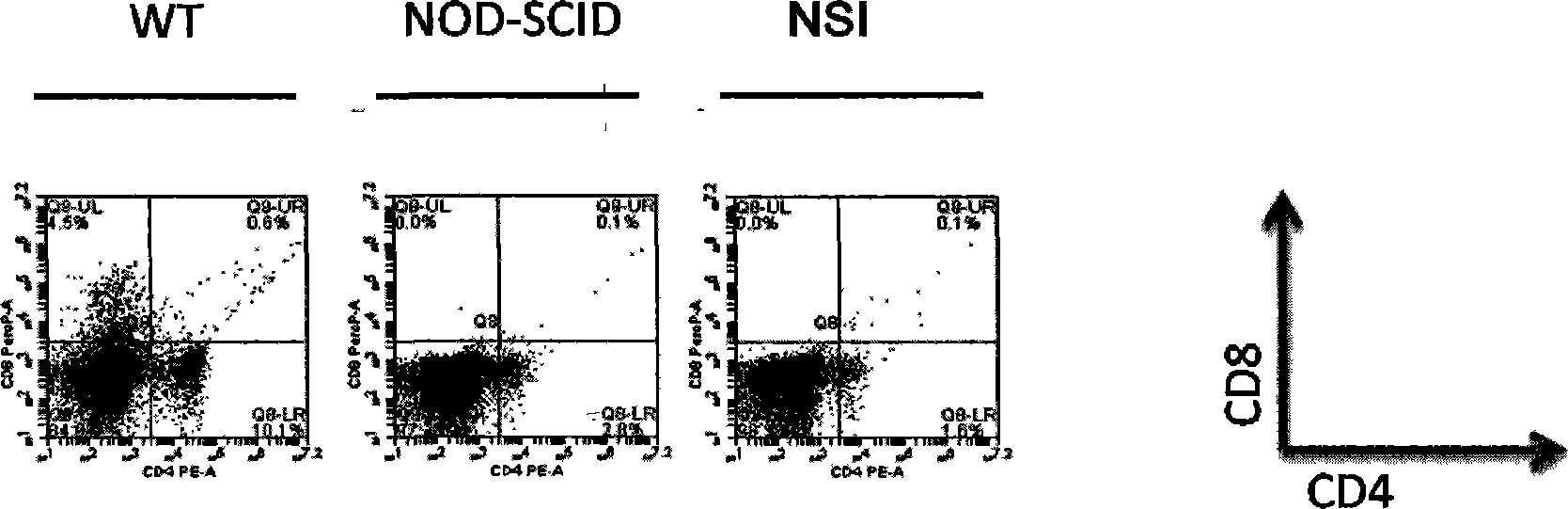
Method for establishing immunodeficiency mouse model
ActiveCN103409468AShorten the timeStrain background pureMicroinjection basedAnimal husbandryReverse transcriptaseImmunodeficient mouse model
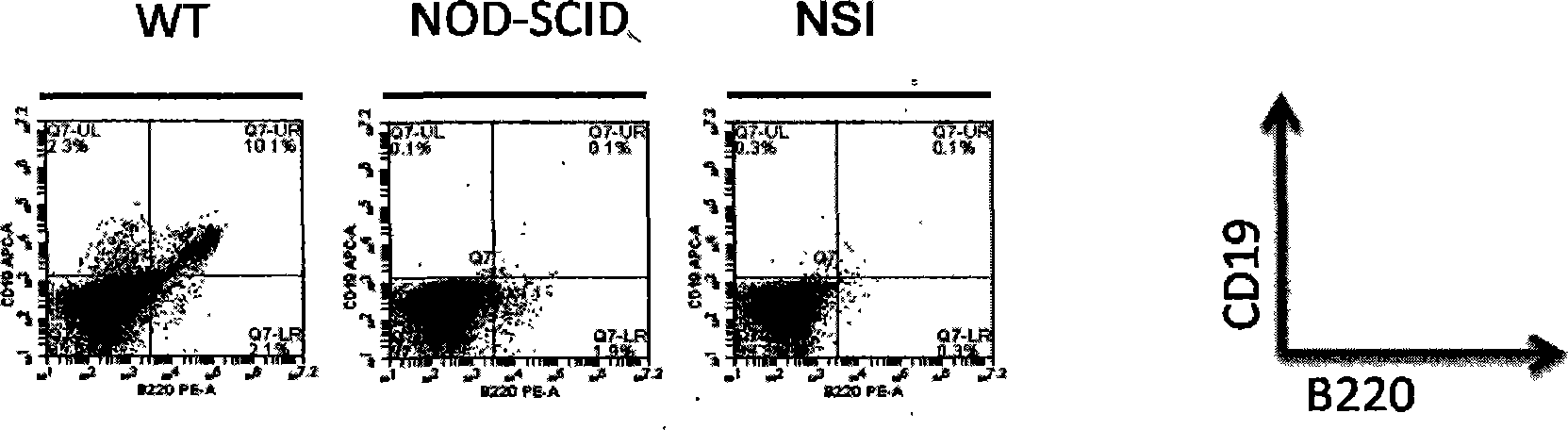
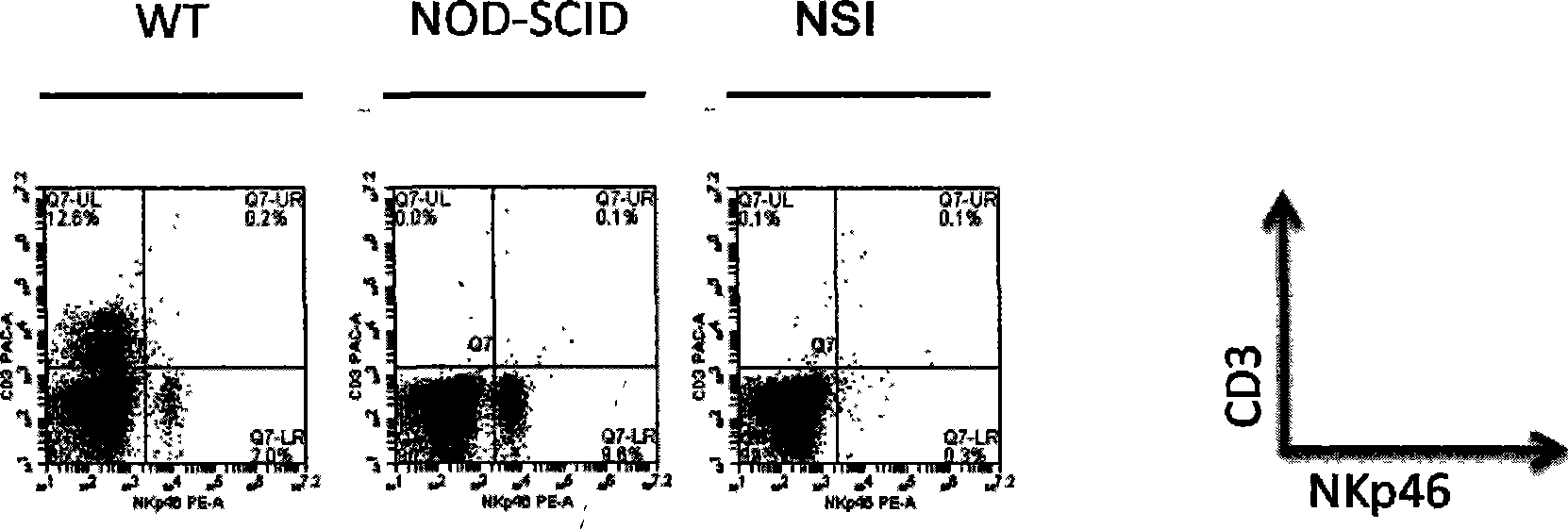
The invention discloses a method for establishing an immunodeficiency mouse model. The method comprises the following steps of: (1) constructing DNA (deoxyribonucleic acid) which enables functions of an immune related gene in a mouse cell to be lost; (2) purifying mRNA (messenger ribonucleic acid), and cryopreserving in a super-purified water of RNAase free; (4) feeding a pregnant female mouse and a pseudopregnant female mouse under an SPF (specific pathogen free) level feeding condition; (5) taking a fallopian tube from the abdominal cavity of the pregnant female mouse in the step (4), and collecting fertilized ovum; (6) injecting the mRNA obtained in the step (3) into the pronucleus of the fertilized ovum in the step (5) by using a microinjection method; (7) transplanting the fertilized ovum obtained in the step (6) into the body of the pseudopregnant female mouse obtained in the step (4), and enabling the pseudopregnant female mouse to culture the second generation; (8) establishing a stable deficient mouse strain. The method has the advantages that a mouse model which completely does not have immunological rejection to allotransplantation can be obtained for testing.
Owner:湖南昭泰生物医药有限公司
Method for non-autologous cartilage regeneration
InactiveUS20100209397A1Reduce riskPoor mechanical propertyBiocideSkeletal disorderCell membraneAllotransplantation
The present invention relates in general to the field of tissue engineering, and in particular to methods for repair and regeneration of diseased and injured bone and cartilage tissue by allotransplantation or xenotransplantation of neonatal mandibular condyle-derived chondrocytes. The composition may comprise mandibular condyle-derived chondrocytes in the form of a chondrocyte film, per se, or in combination with a scaffold.
Owner:CARTICURE
Growth arrest specific gene 6 peptides, antibodies, compositions, methods and uses
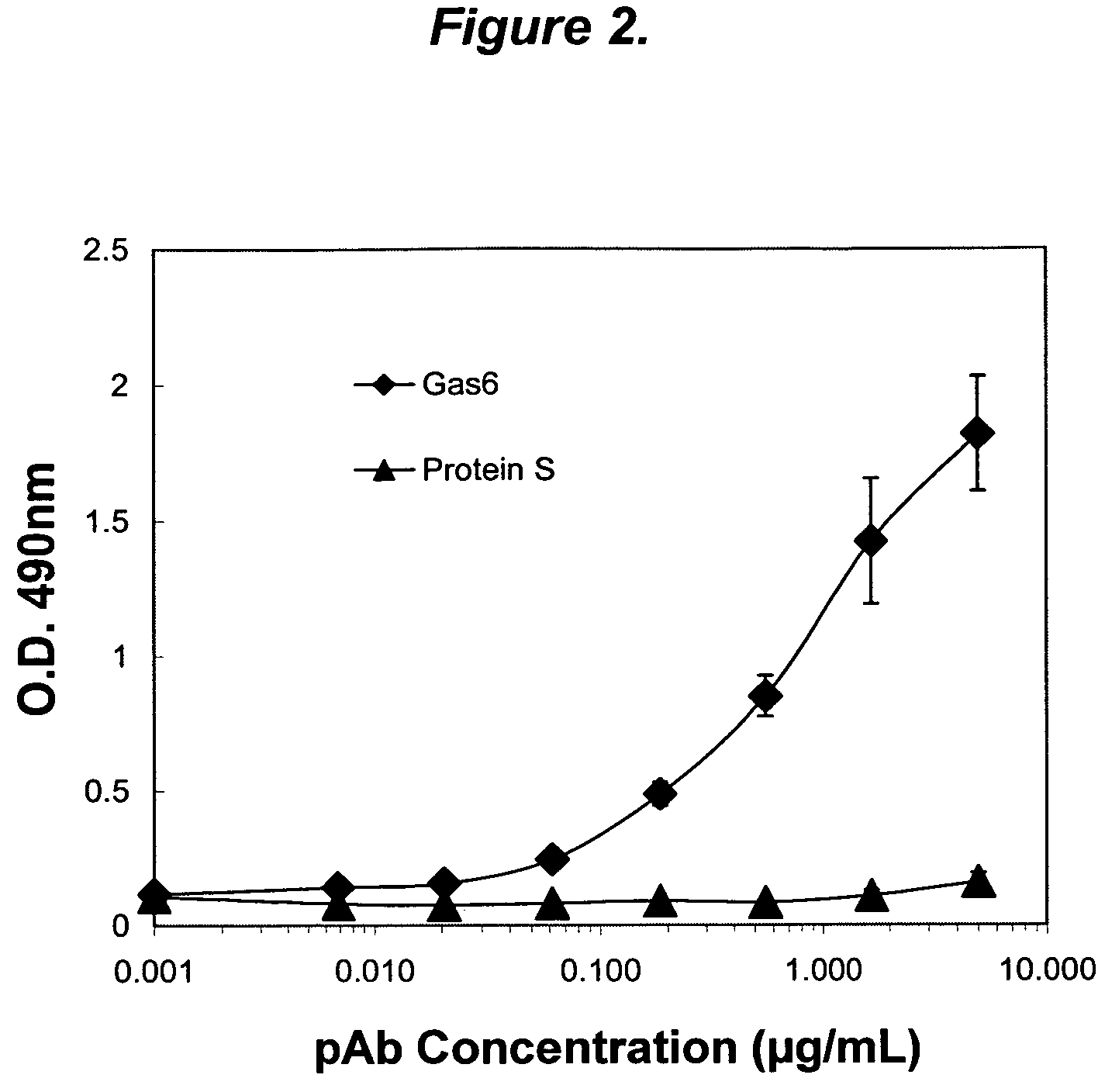
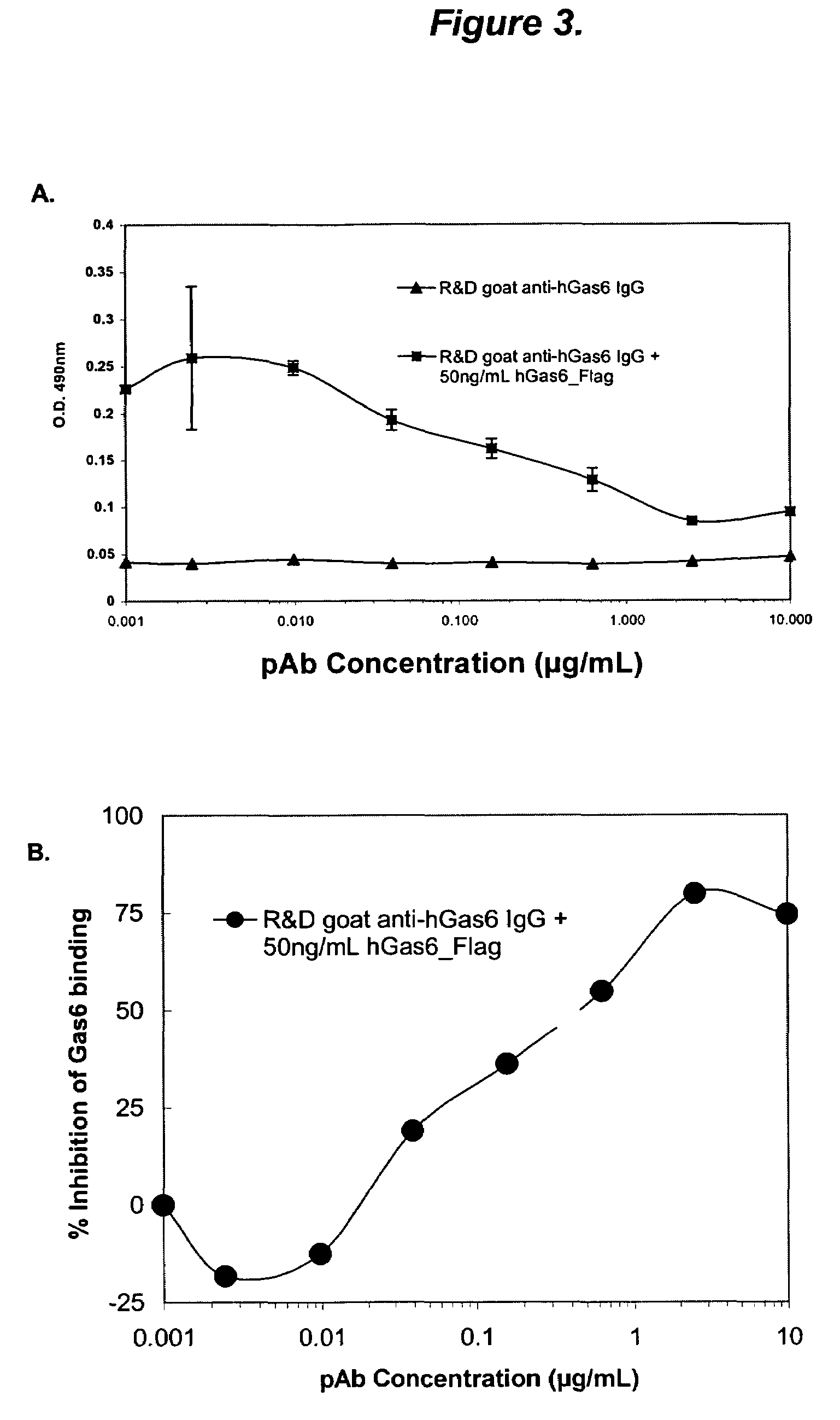
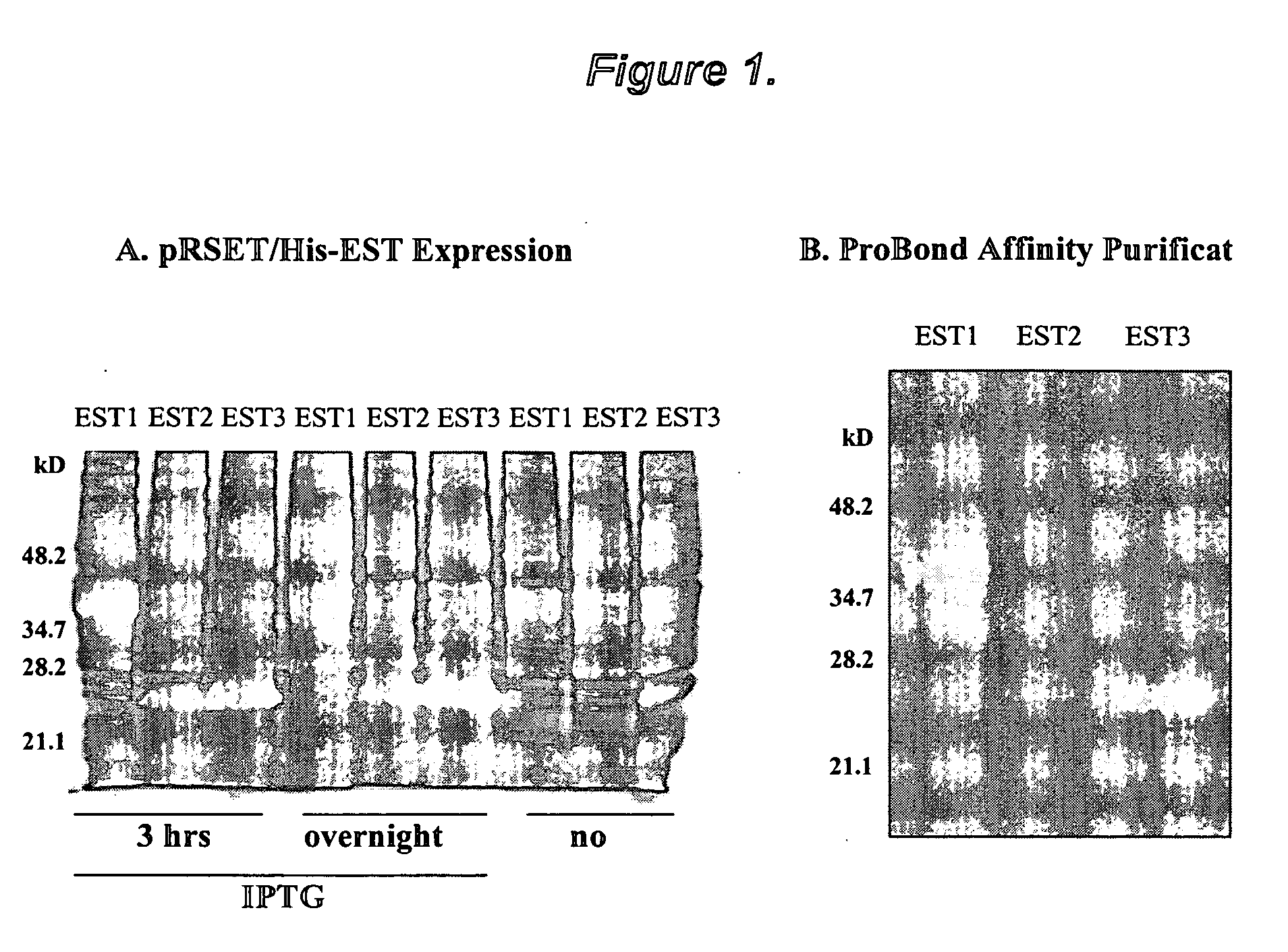
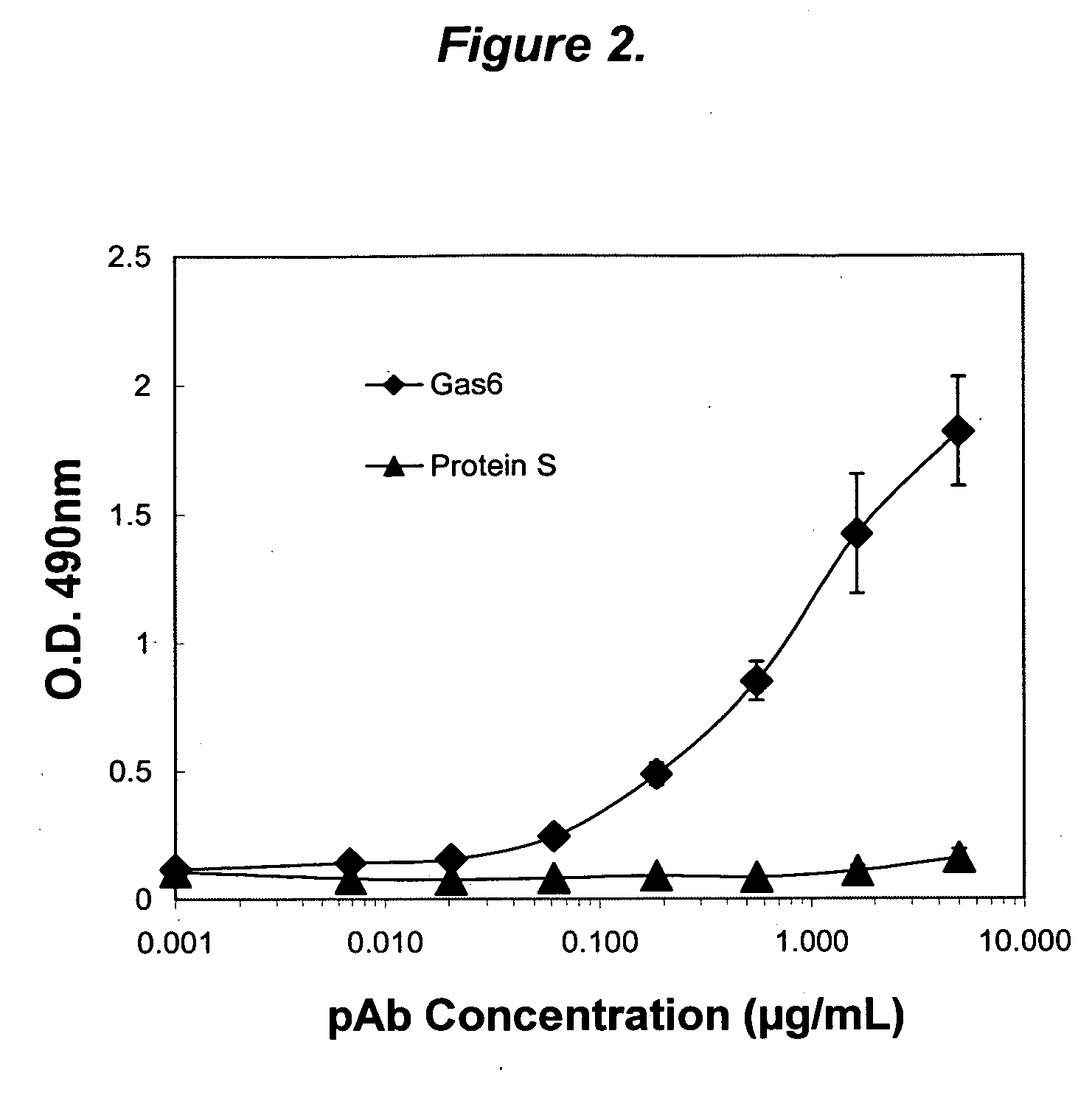
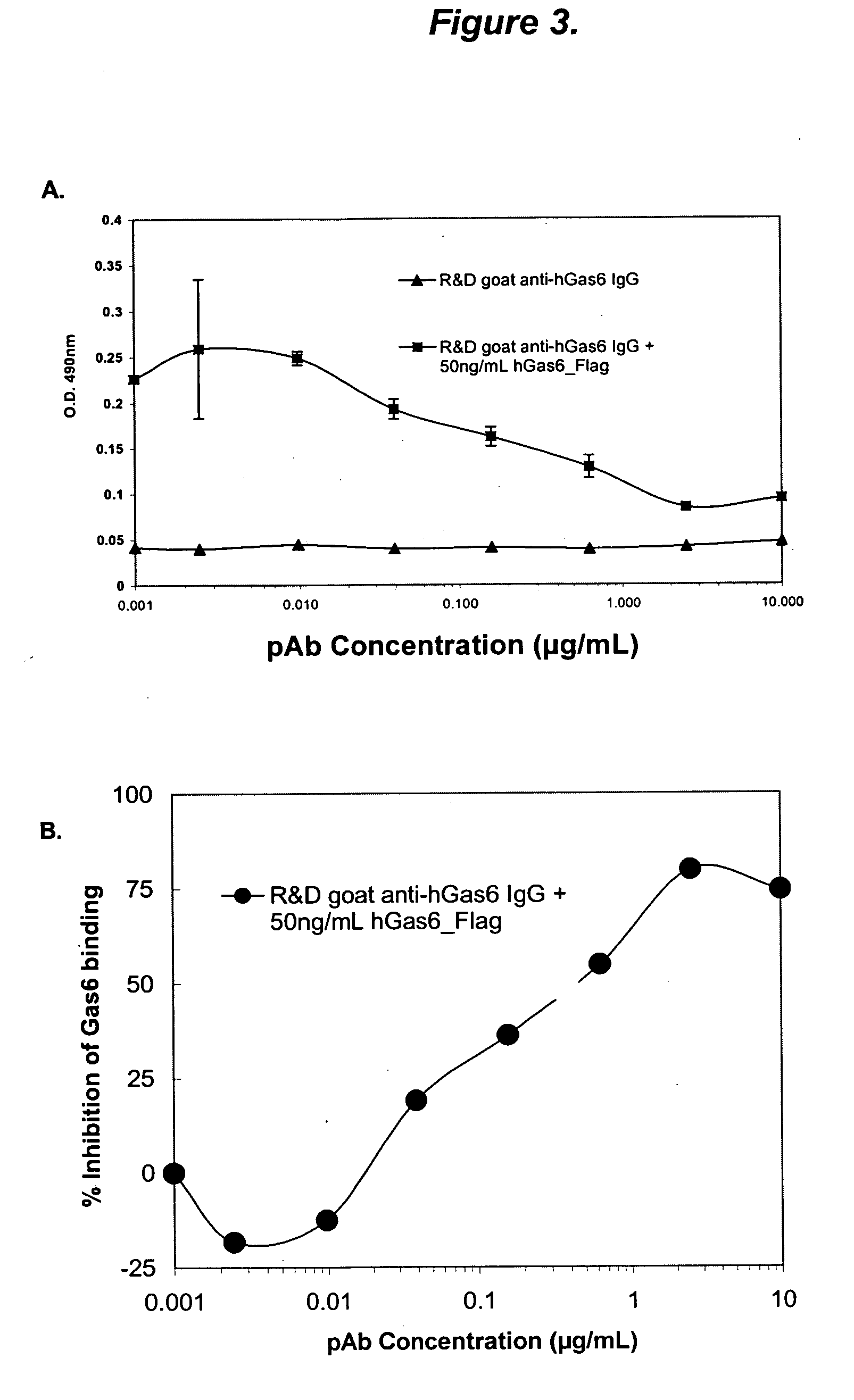
The present invention provides novel proteins and peptides from the receptor binding region of human Growth Arrest Specific Gene 6 (Gas6) and antibodies, including specified portions or variants, specific for at least one such Gas6 peptide or fragment thereof. The aforesaid peptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-Gas6 antibodies. The invention also provides for the nucleic acids encoding such peptides and anti-Gas6 antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, including therapeutic formulations, administration and devices. Fifteen novel peptide sequences from the Gas6 G domain that are implicated in Gas6 interactions with its receptors are identified, isolated, and synthesized so as to allow generation of anti-Gas6 antibodies. The peptide sequences include three ESTs that encompass regions predicted to contribute to receptor binding or that can raise anti-Gas6 antibodies. This invention provides for such antibodies to be used in modulating or treating at least one Gas6-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, thromboembolic disease, ischemic disease, venous thromboembolism, arterial or venous thrombosis, pulmonary embolism, restenosis, diabetic angiopathy and allograft atherosclerosis.
Owner:CENTOCOR ORTHO BIOTECH
Use of ECDI-fixed cell tolerance as a method for preventing allograft rejection
Owner:NORTHWESTERN UNIV
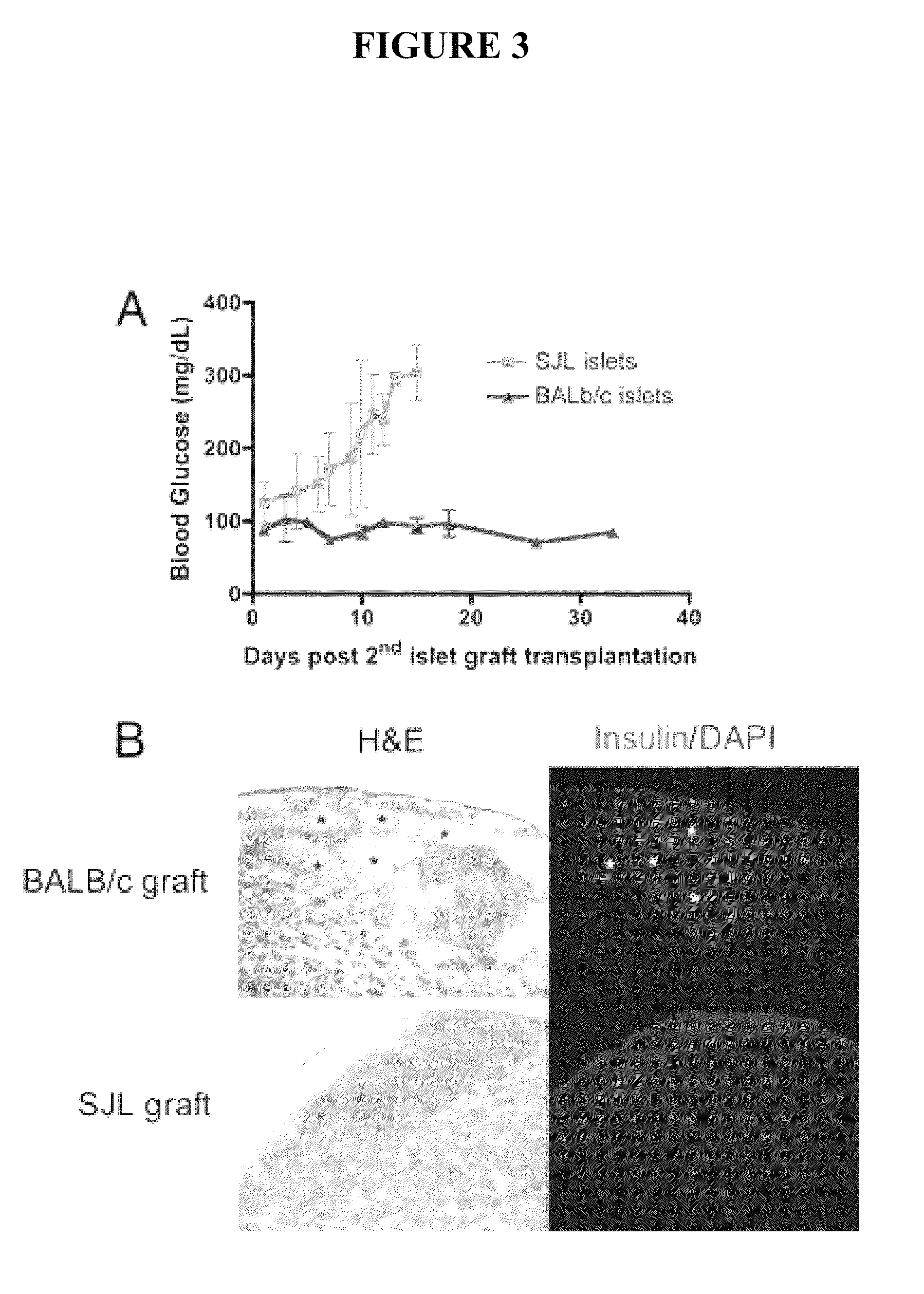
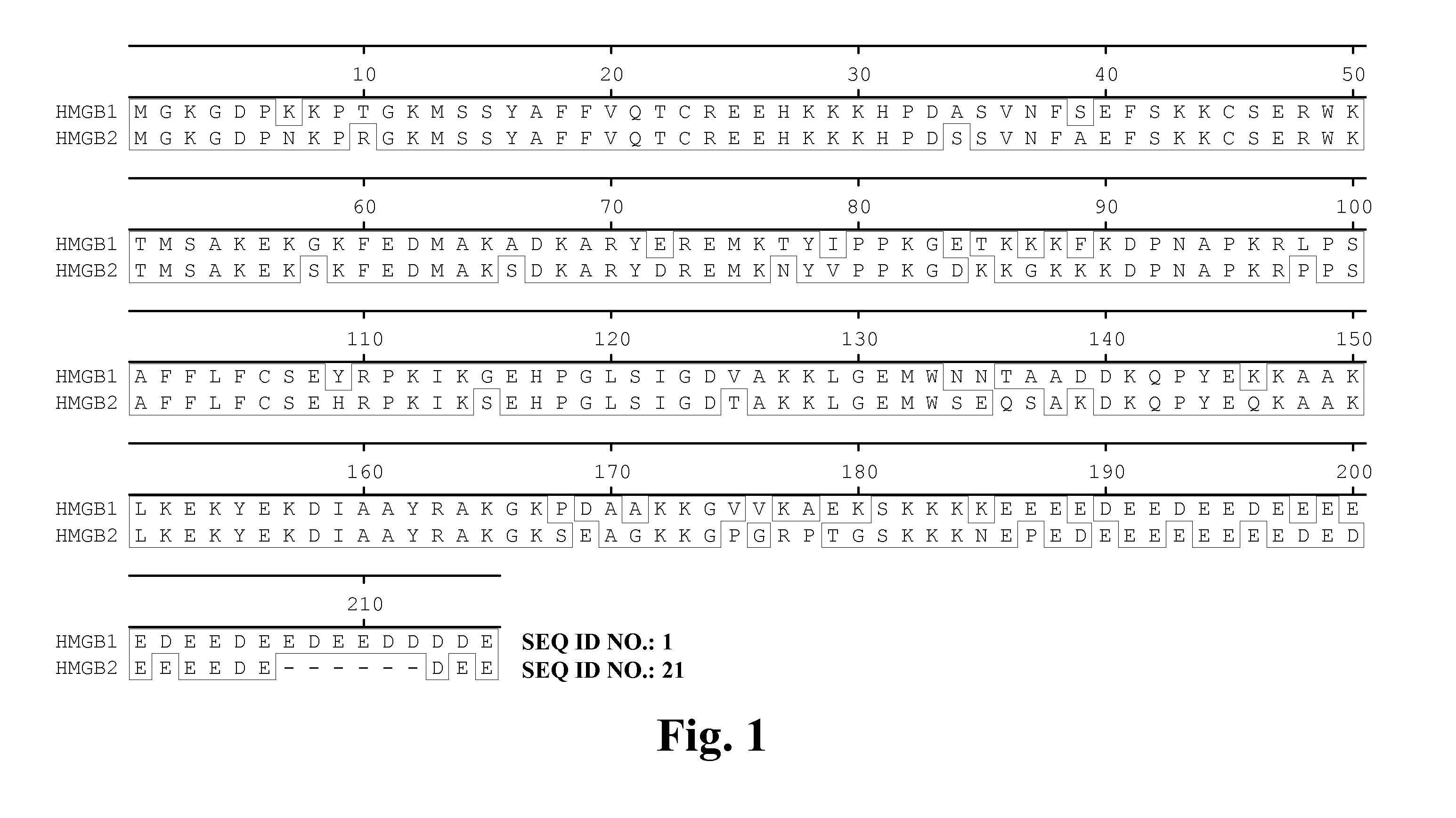
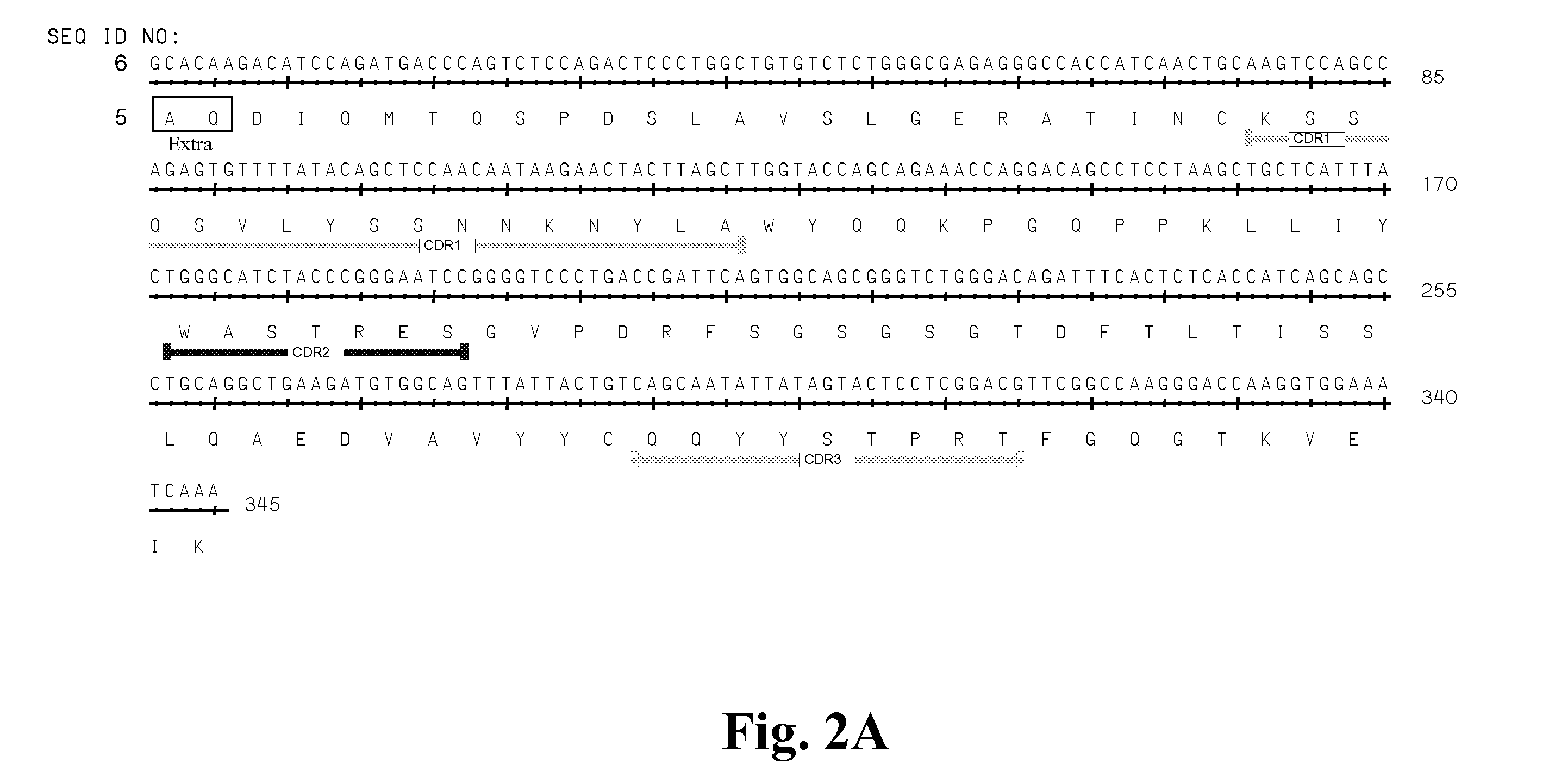
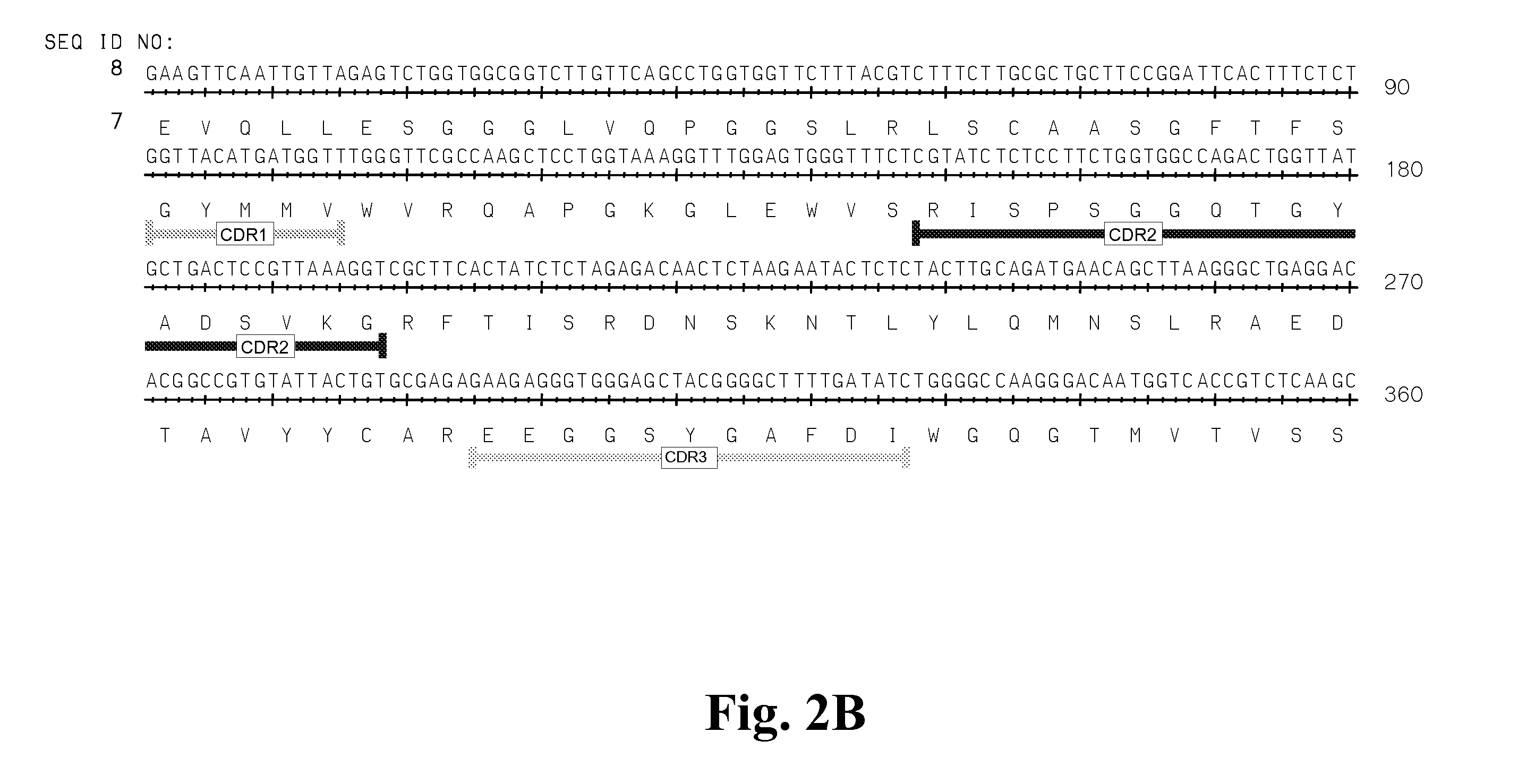
High Affinity Antibodies Against HMGB1 and Methods Of Use Thereof
InactiveUS20100061987A1Reduce bone loss and/or cartilage damageAntibacterial agentsAntipyreticAntiendomysial antibodiesAllograft rejection
Owner:MEDIMMUNE LLC
Growth arrest specific gene 6 peptides, antibodies, compositions, methods and uses
The present invention provides novel proteins and peptides from the receptor binding region of human Growth Arrest Specific Gene 6 (Gas6) and antibodies, including specified portions or variants, specific for at least one such Gas6 peptide or fragment thereof. The aforesaid peptides can be used to generate human, primate, rodent, mammalian, chimeric, humanized and / or CDR-grafted anti-Gas6 antibodies. The invention also provides for the nucleic acids encoding such peptides and anti-Gas6 antibodies, complementary nucleic acids, vectors, host cells, and methods of making and using thereof, including therapeutic formulations, administration and devices. Fifteen novel peptide sequences from the Gas6 G domain that are implicated in Gas6 interactions with its receptors are identified, isolated, and synthesized so as to allow generation of anti-Gas6 antibodies. The peptide sequences include three ESTs that encompass regions predicted to contribute to receptor binding or that can raise anti-Gas6 antibodies. This invention provides for such antibodies to be used in modulating or treating at least one Gas6-related disease in a cell, tissue, organ, animal, or patient. Such diseases may include, but are not limited to, thromboembolic disease, ischemic disease, venous thromboembolism, arterial or venous thrombosis, pulmonary embolism, restenosis, diabetic angiopathy and allograft atherosclerosis.
Owner:CENTOCOR ORTHO BIOTECH
Substituted fused tricyclic compounds, compositions and medicinal applications thereof
The present invention relates to substituted fused tricyclic compounds of formula (I) or (Ia), their tautomers, polymorphs, stereoisomers, prodrugs, solvates, co-crystals, pharmaceutically acceptable salts, pharmaceutical compositions containing them and methods of treating conditions and diseases that are mediated by JAK activity. The compounds of the present invention are useful in the treatment, prevention or suppression of diseases and disorders mediated by JAK activity. Such conditions include, but not limited to, arthritis, Alzheimer's disease, autoimmune thyroid disorders, cancer, diabetes, leukemia, T-cell prolymphocytic leukemia, lymphoma, myleoproliferation disorders, lupus, multiple myeloma, multiple sclerosis, osteoarthritis, sepsis, psoriatic arthritis, prostate cancer, T-cell autoimmune disease, inflammatory diseases, chronic and acute allograft transplant rejection, bone marrow transplant, stroke, asthma, chronic obstructive pulmonary disease, allergy, bronchitis, viral diseases, or Type I diabetes, complications from diabetes, rheumatoid arthritis, asthma, Crohn's disease, dry eye, uveitis, inflammatory bowel disease, organ transplant rejection, psoriasis and ulcerative colitis. The present disclosure also relates to process for the preparation of such compounds, and to pharmaceutical compositions containing them.
Owner:IMPETIS BIOSCI LTD
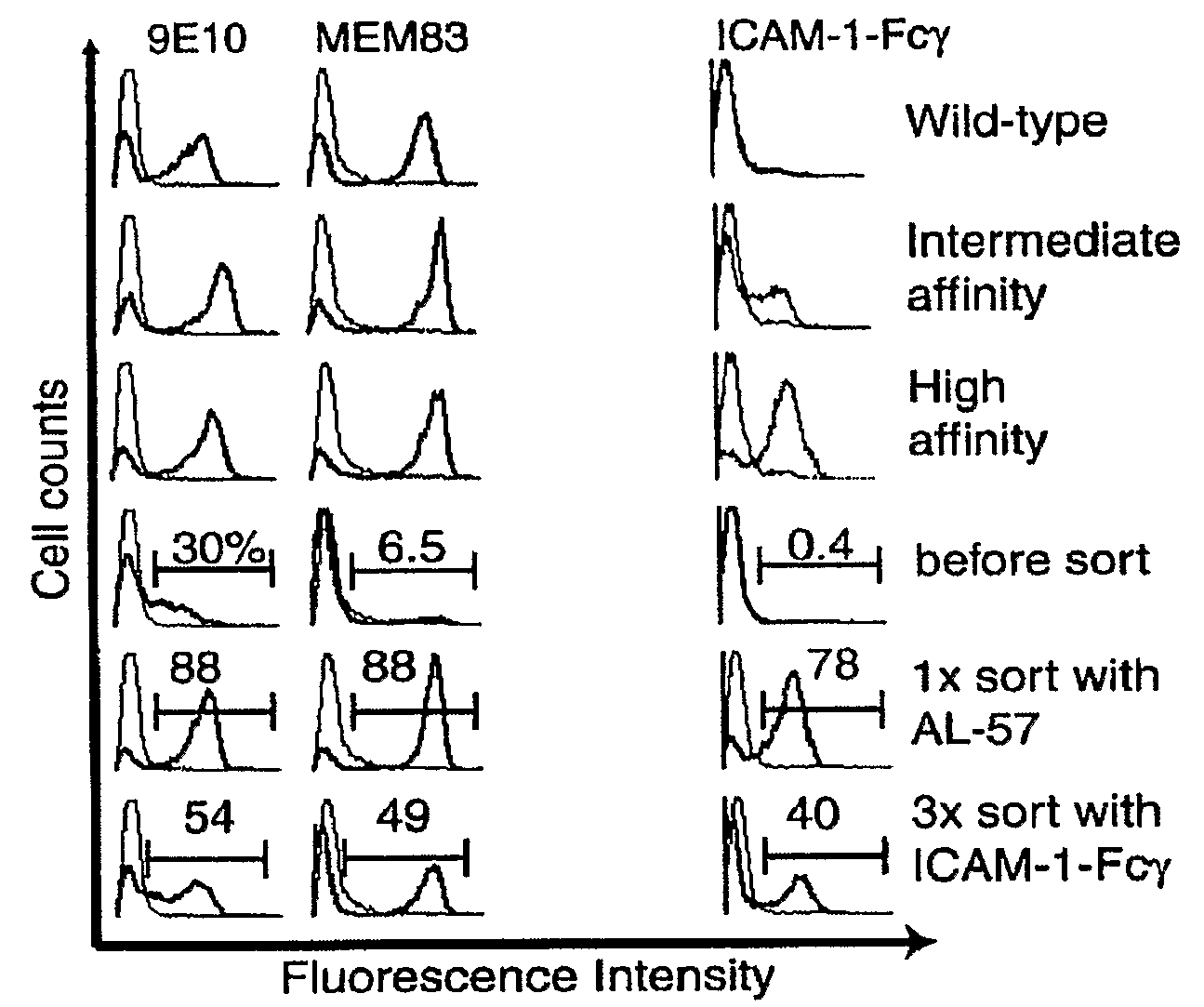
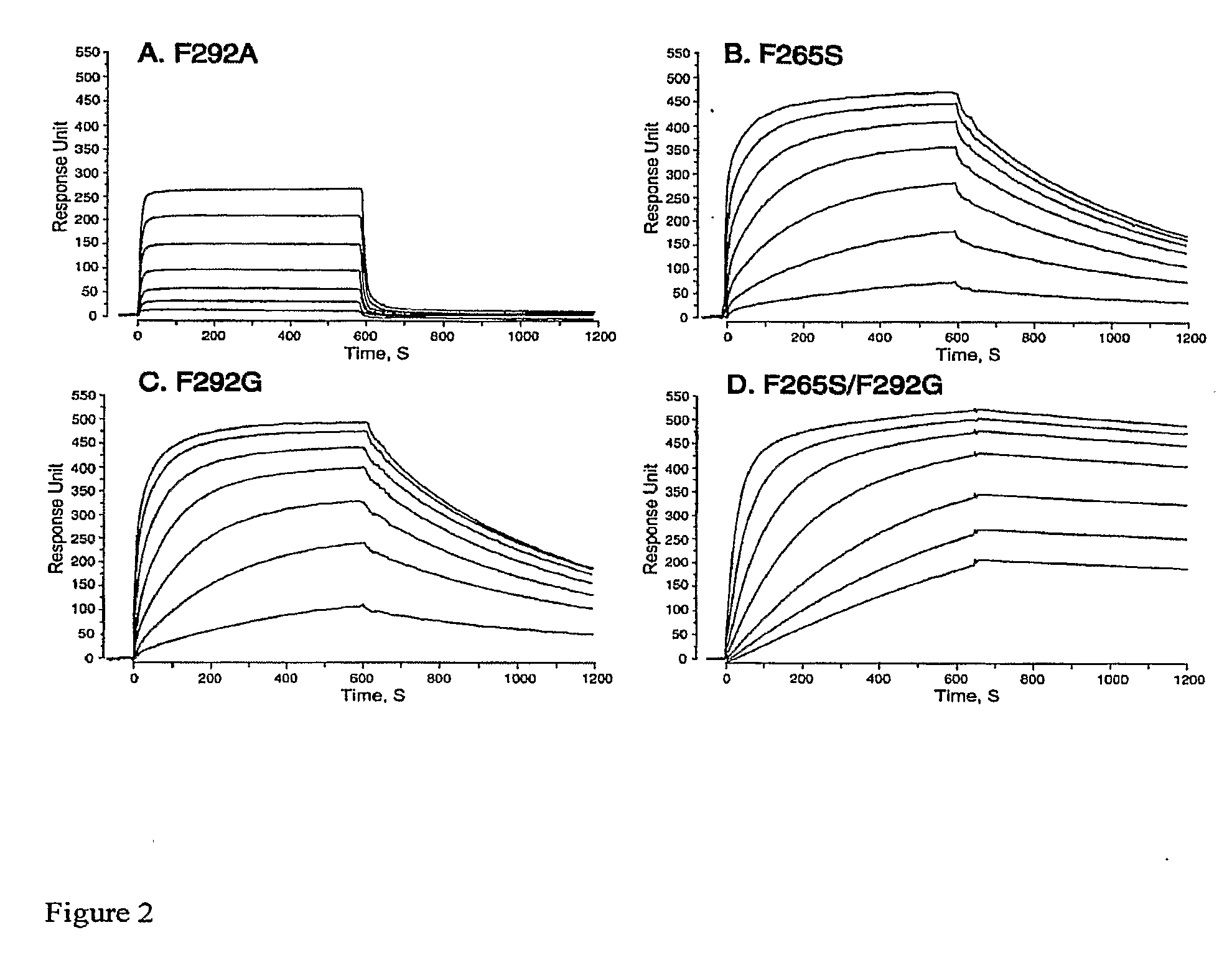
Integrin Alpha L I Domain Mutants with Increased Binding Affinity
The present invention provides an isolated polypeptide capable of binding to aCAM-1, comprising the integrin (XL I domain or biologically active portion thereof, wherein one or more residues is substituted, wherein the substituted polypeptide binds ICAM-I at a higher affinity than wild type integrin CCL protein. The invention provides a method for inhibiting ICAM-I and a pharmaceutical composition comprising an integrin (XL I domain polypeptide or biologically active portion of the polypeptides. The invention also provides a method of treating or preventing an LFA-I mediated ICAM-1 associated disease such as inflammation, artherosclerosis, allograft rejection, diabetes, T-cell mediated sensitization reaction, psoriasis, HIV infection, or rheumatoid arthritis.
Owner:IMMUNE DISEASE INST INC
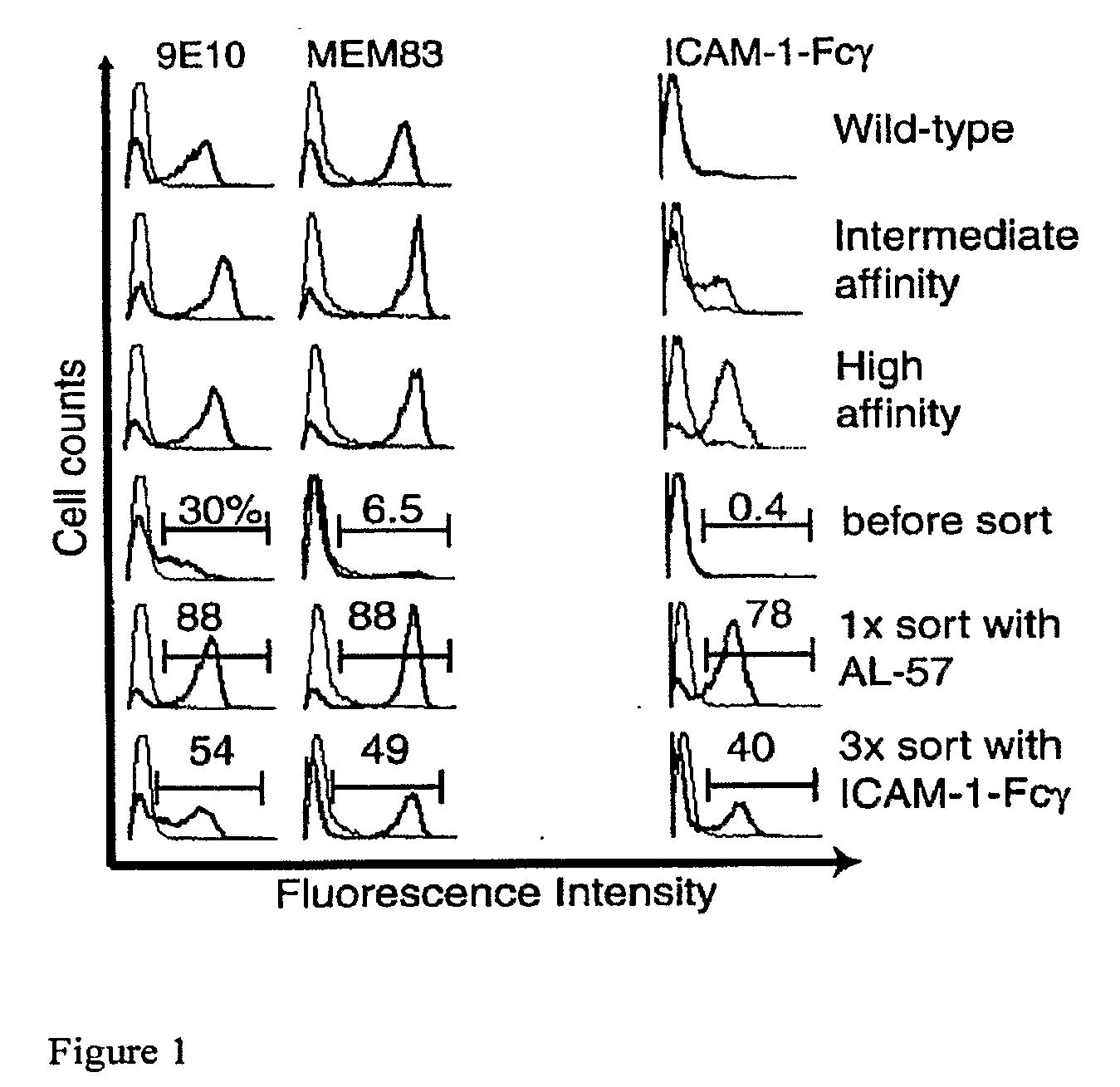
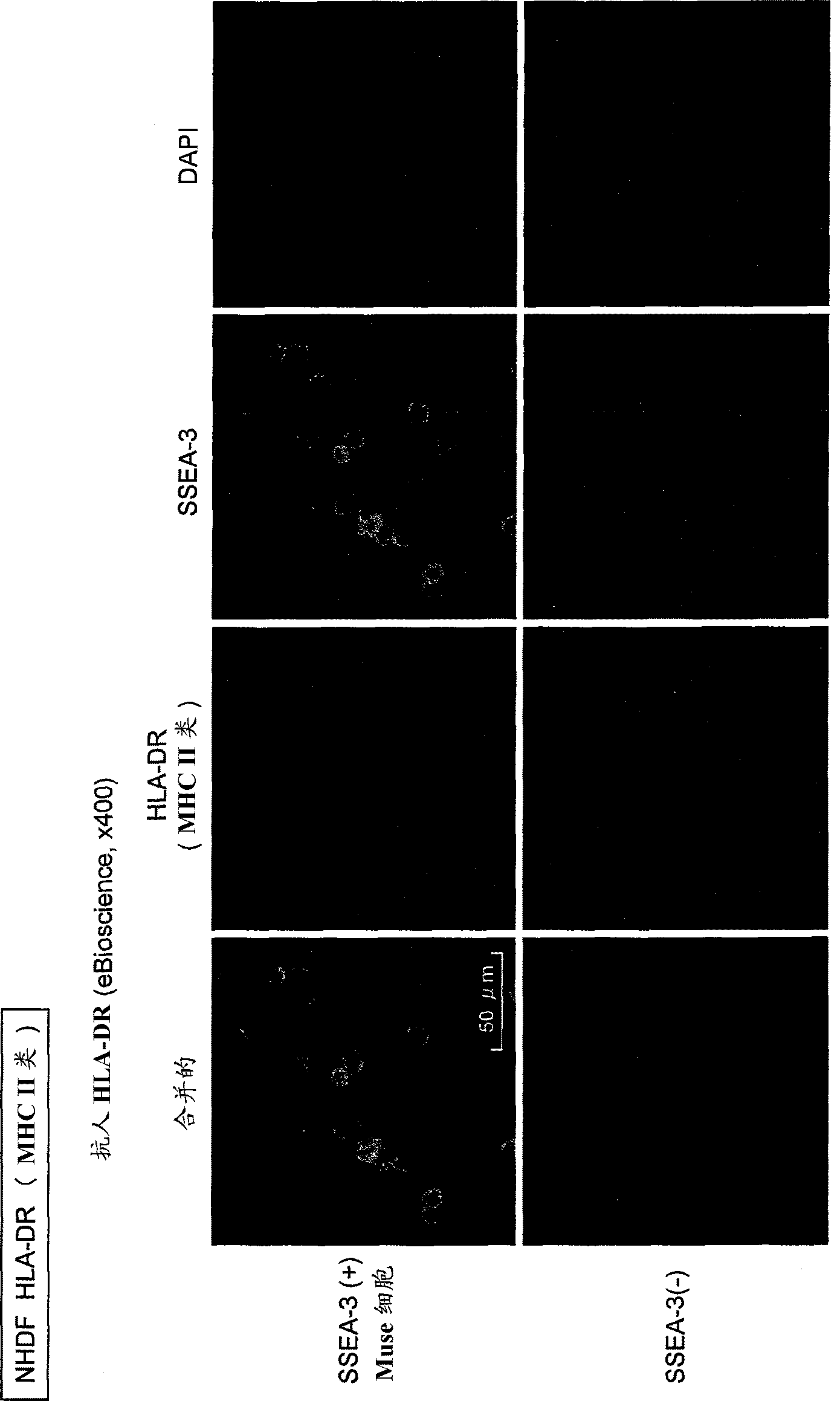
Composition for allotransplantation cell therapy, said composition containing SSEA-3 positive pluripotent stem cell capable of being isolated from body tissue
The purpose of the present invention is to provide a composition for allotransplantation cell therapy, said composition containing a pluripotent stem cell. A composition for allotransplantation cell therapy, said composition containing a SSEA-3 positive pluripotent stem cell which is capable of being isolated from body tissue and which does not express HLA class II antigens.
Owner:克里奥公司
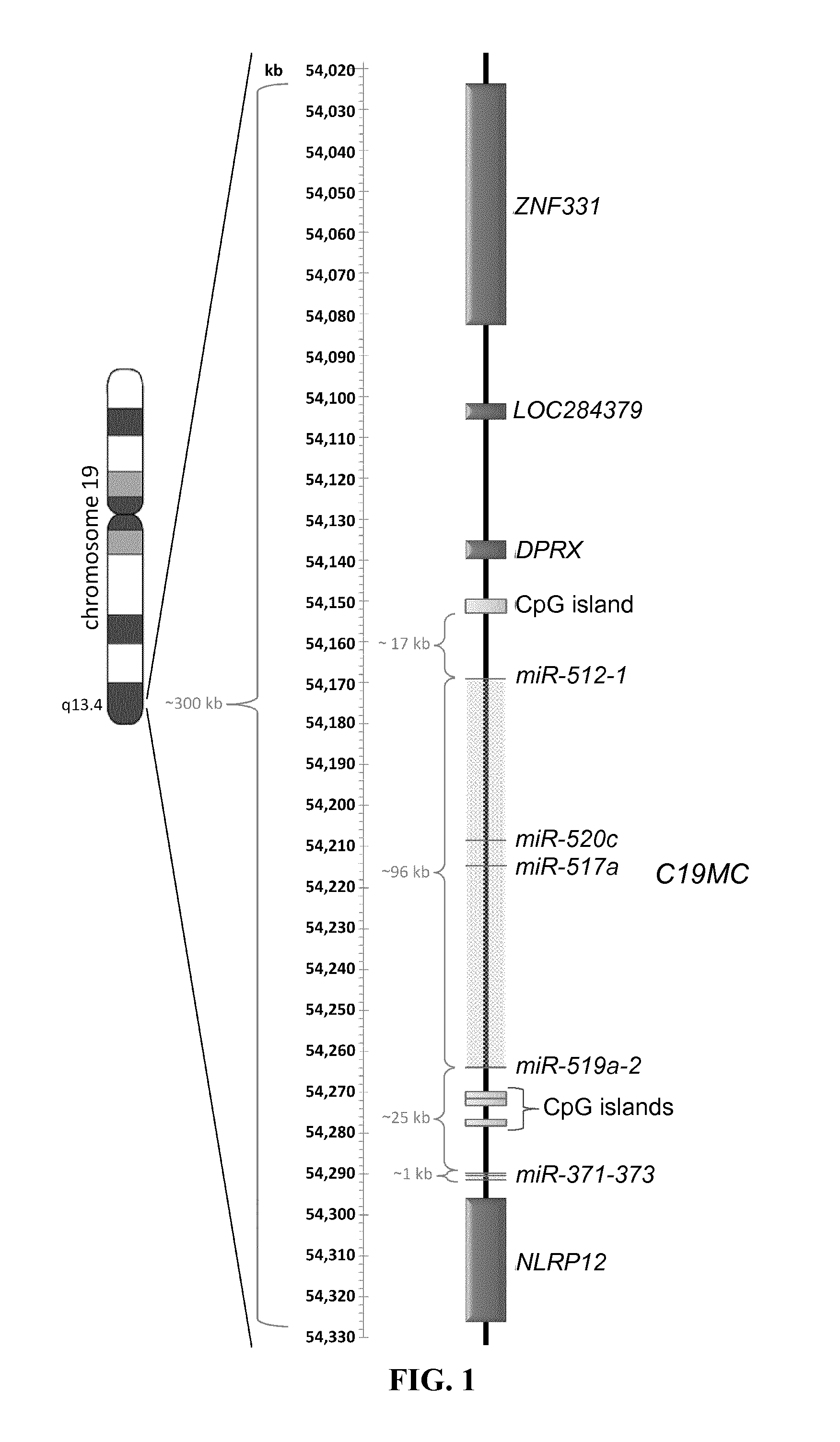
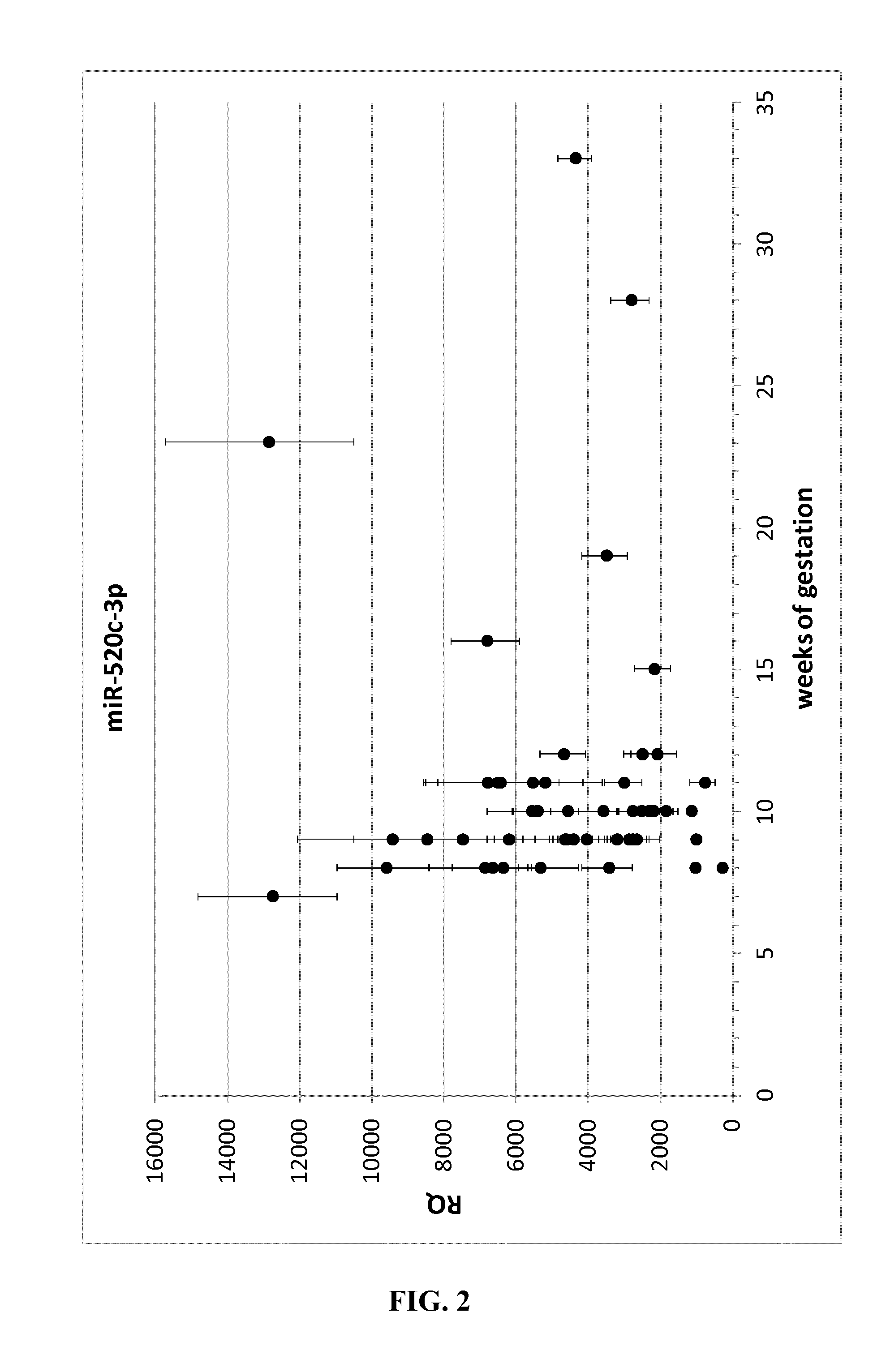
Expression of mirnas in placental tissue
InactiveUS20140294840A1Avoid problemsReduce usageOrganic active ingredientsSenses disorderPregnancyTolerability
Provided are human miRNAs associated with the generation of immunological tolerance during pregnancy as well as fragments, derivatives and variants thereof for use in immunomodulation. Said miRNAs may be used in diagnosis and treatment of disorders associated with a deregulated immune response, autoimmune disorders, pregnancy associated diseases, failure or problems of placentation and complications resulting from allotransplantations. In addition, new pharmaceutical and diagnostic compositions for use in diagnosis and therapy of said disorders are described.
Owner:BULLERDIEK JORN DR
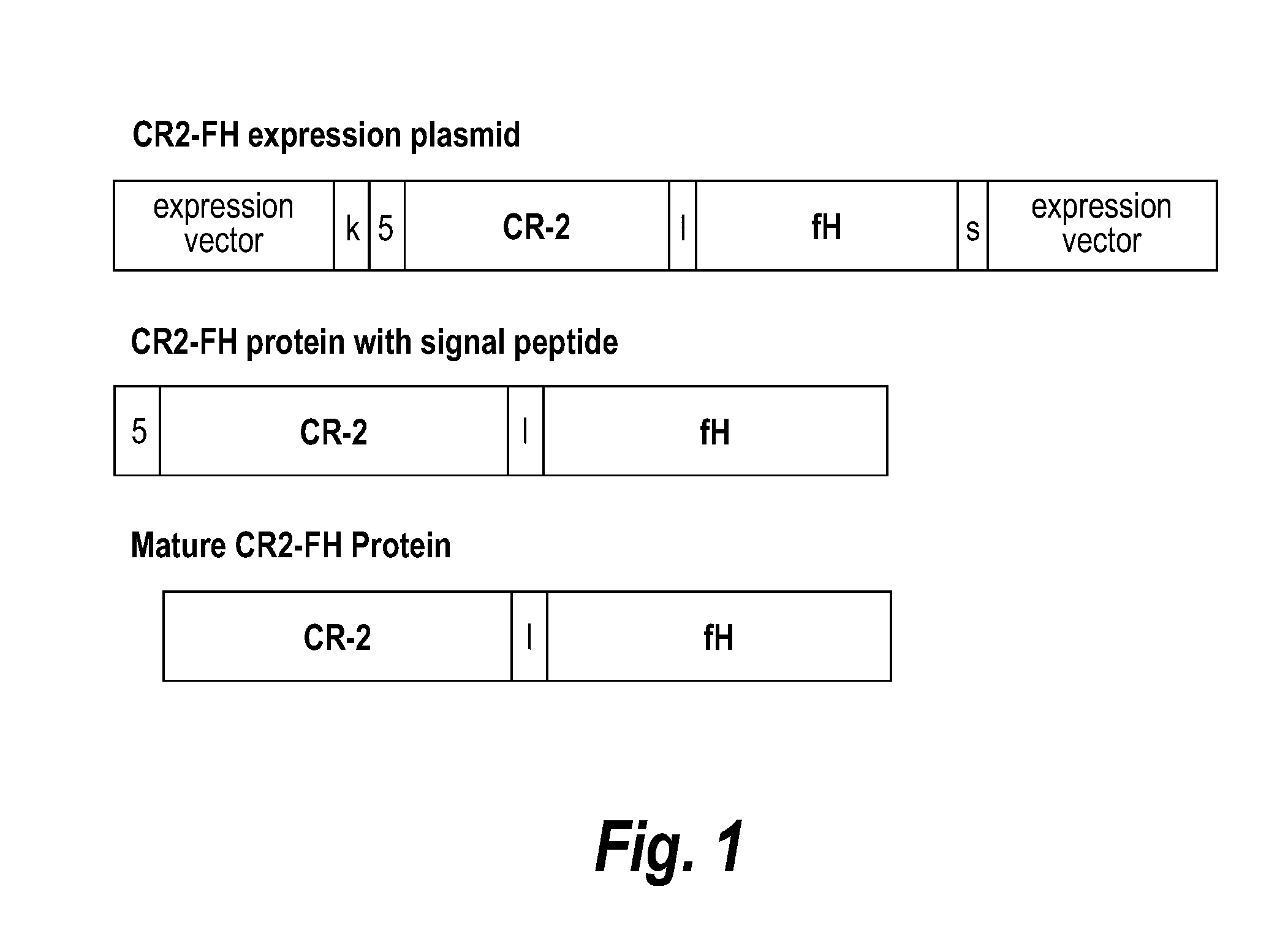
Treatment of graft rejection by administering a complement inhibitor to an organ prior to transplant
InactiveUS20160184391A1Easily penetrate organMinimize impactPeptide/protein ingredientsAntibody mimetics/scaffoldsIMMUNE SUPPRESSANTSAntiendomysial antibodies
Methods of prolonging survival of a transplanted organ, as well as methods of preventing or attenuating rejection of a transplanted organ are provided. These methods involve contacting the organ with an inhibitor of complement activity (e.g., a complement inhibitor that has a maximum molecular weight of 70 kDa and / or a half-life shorter than 10 days, such as a CR2-FH fusion protein or a single chain anti-C5 antibody), prior to transplantation The methods also include administering to the allotransplant recipient an inhibitor of complement activity together with one or more immunosuppressants. A pretreatment with an alternative complement inhibitor was found to be effective in improving graft survival and decreasing ischemia-reperfusion injury in animal.
Owner:ALEXION PHARMA INC
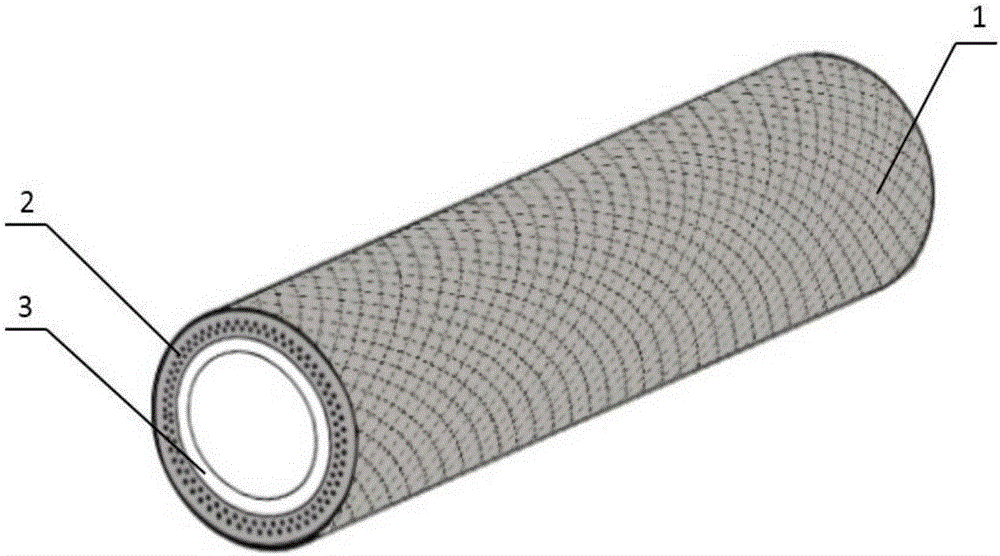
Preparation method of tissue engineering acellular vascular scaffold
InactiveCN106729979AImprove mechanical propertiesAddressing issues such as aneurysmsTissue regenerationProsthesisCross-linkCell-Extracellular Matrix
The invention discloses a preparation method of a tissue engineering acellular vascular scaffold. Enhanced tissue engineering blood vessels without immunogenicity can be obtained by embedding micron high-molecular polymer scaffold into subcutaneous part or abdominal cavity of a host and performing acellular treatment on the blood vessel scaffold wrapped by utilizing a host immune protection mechanism. The preparation method has the advantages that deficient mechanical performance of the tissue engineering acellular blood vessel can be solved, and the mechanical performance of the original tissue engineering acellular vascular scaffold can be fully improved through a multilayer cross-linked mesh type high-molecular scaffold; the problem of immunogenicity of the tissue engineering blood vessel in allotransplantation can be solved, the scaffold blood vessel formed by migrating cells caused by immunologic mechanism is established in a host animal body, the transplanted immunoreaction can be reduced through acellular treatment, extracellular matrix is fully utilized to provide a good environment for cell regeneration; and different acellular vascular scaffolds can be prepared through designing scaffolds of different shapes and sizes of by utilizing the method, so as to be applied under different blood vessel transplanting conditions.
Owner:NANKAI UNIV
Methods of preparation and composition of peptide constructs useful for treatment of autoimmune and transplant related host versus graft conditions
InactiveUS7256254B2Effectively eliminate set and subsetTreating or preventing inappropriate autoimmune responsePeptide/protein ingredientsPeptide sourcesCardiac myosinImmunologic disorders
Peptide constructs including a first peptide segment which includes an amino acid sequence associated with autoimmune disease, asthma, allergy or xeno- or allograft transplantation rejections bonded directly or via a linker or spacer to a second peptide which binds to T cells and which will redirect the immune response from a harmful Th1 response to a less harmful Th2 response, or which will bind to T cells to initiate, but not complete, an immune response causing the T cells to which the first peptide binds, to undergo anergy and apoptosis, are useful in treating autoimmune conditions. For instance, the peptide construct NGQEEKAGVVSTGLIGGGDSAFDVLSFTAEEKAGVYK (SEQ ID NO:14) wherein Th2 stimulating Peptide G (SEQ ID NO:15) is covalently linked, via spacer GGG, to cardiac myosin molecule My1 (SEQ ID NO:16), can be used for treatment or prevention of myocarditis.
Owner:CEL SCI CORP
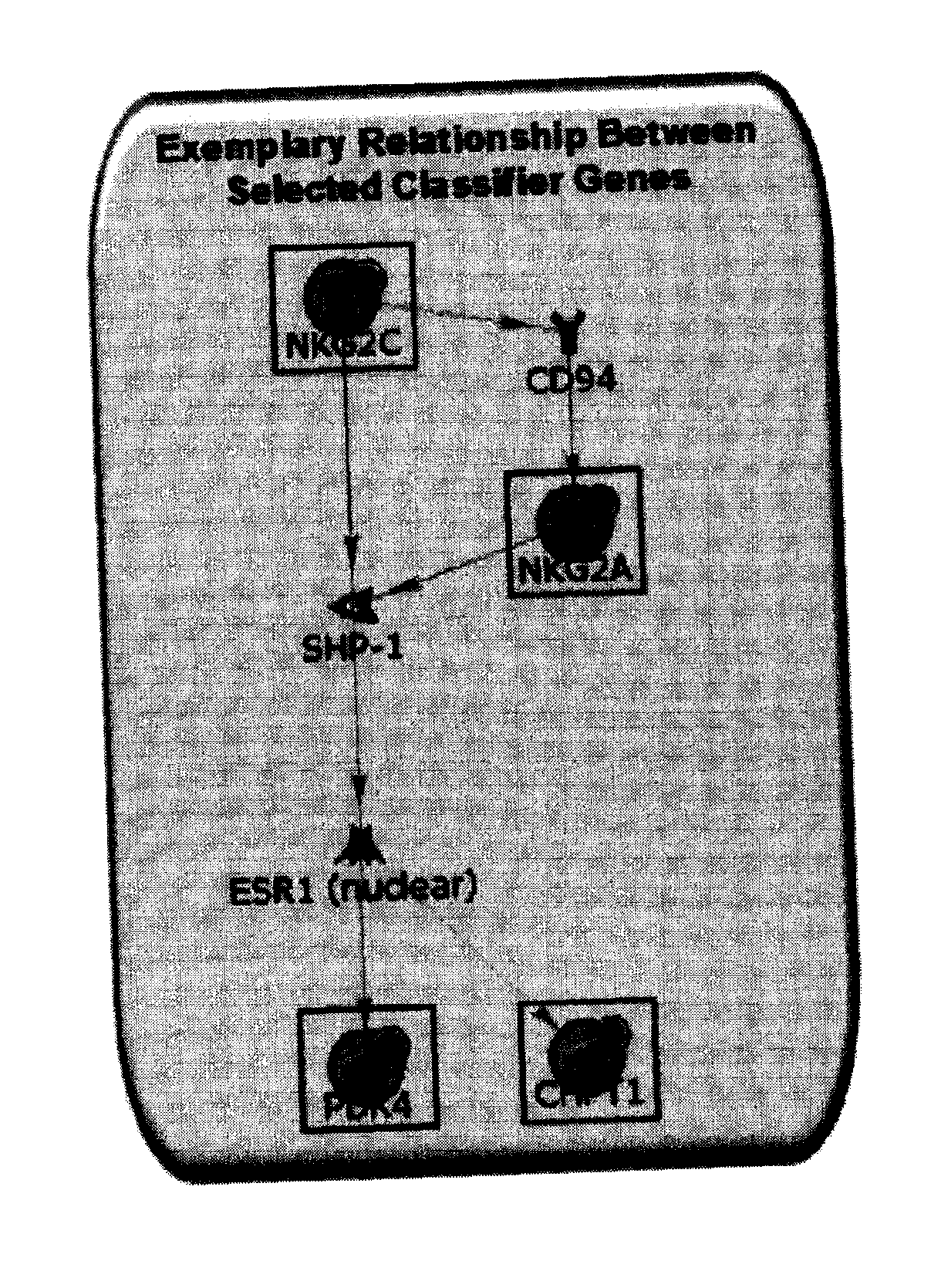
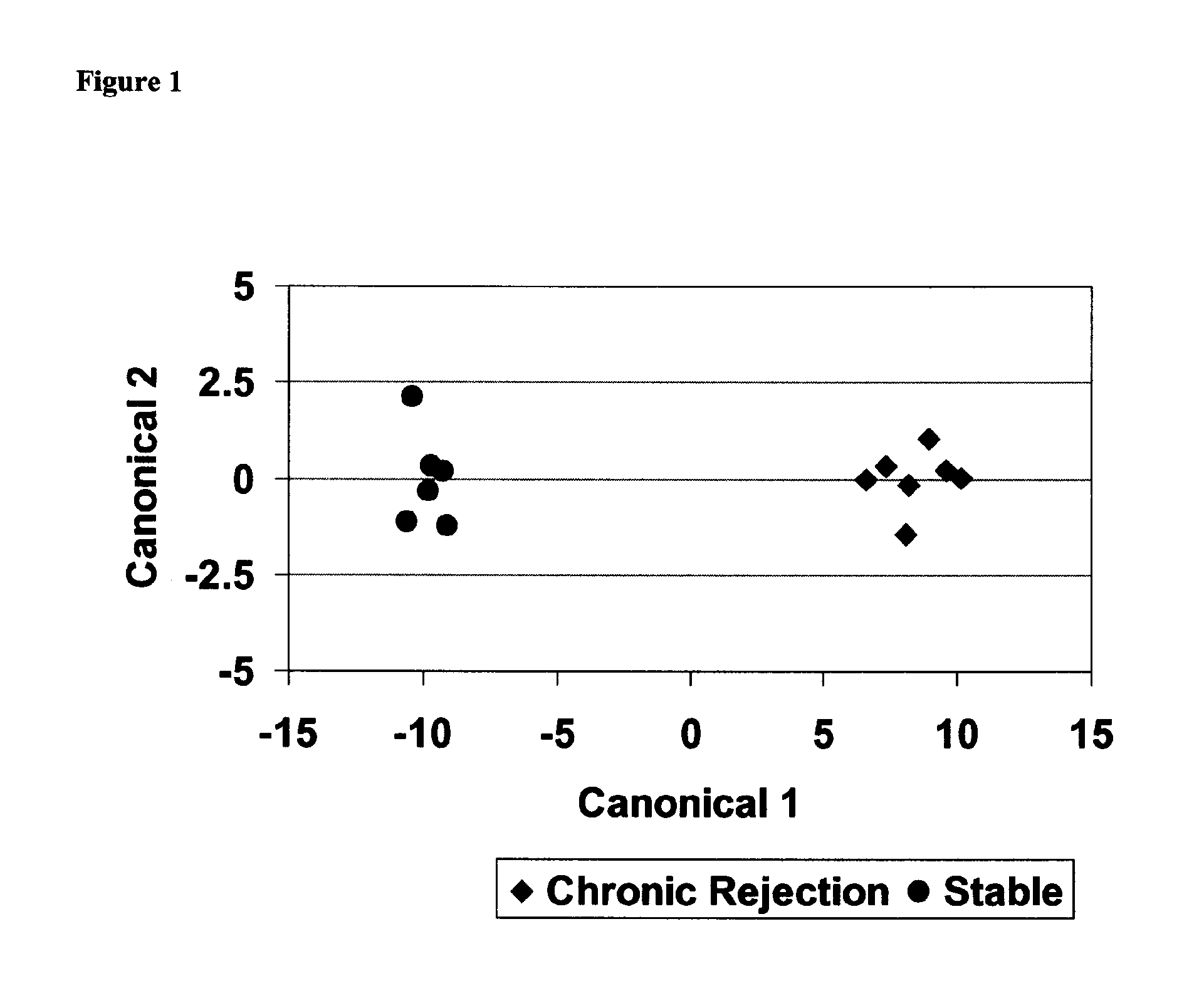
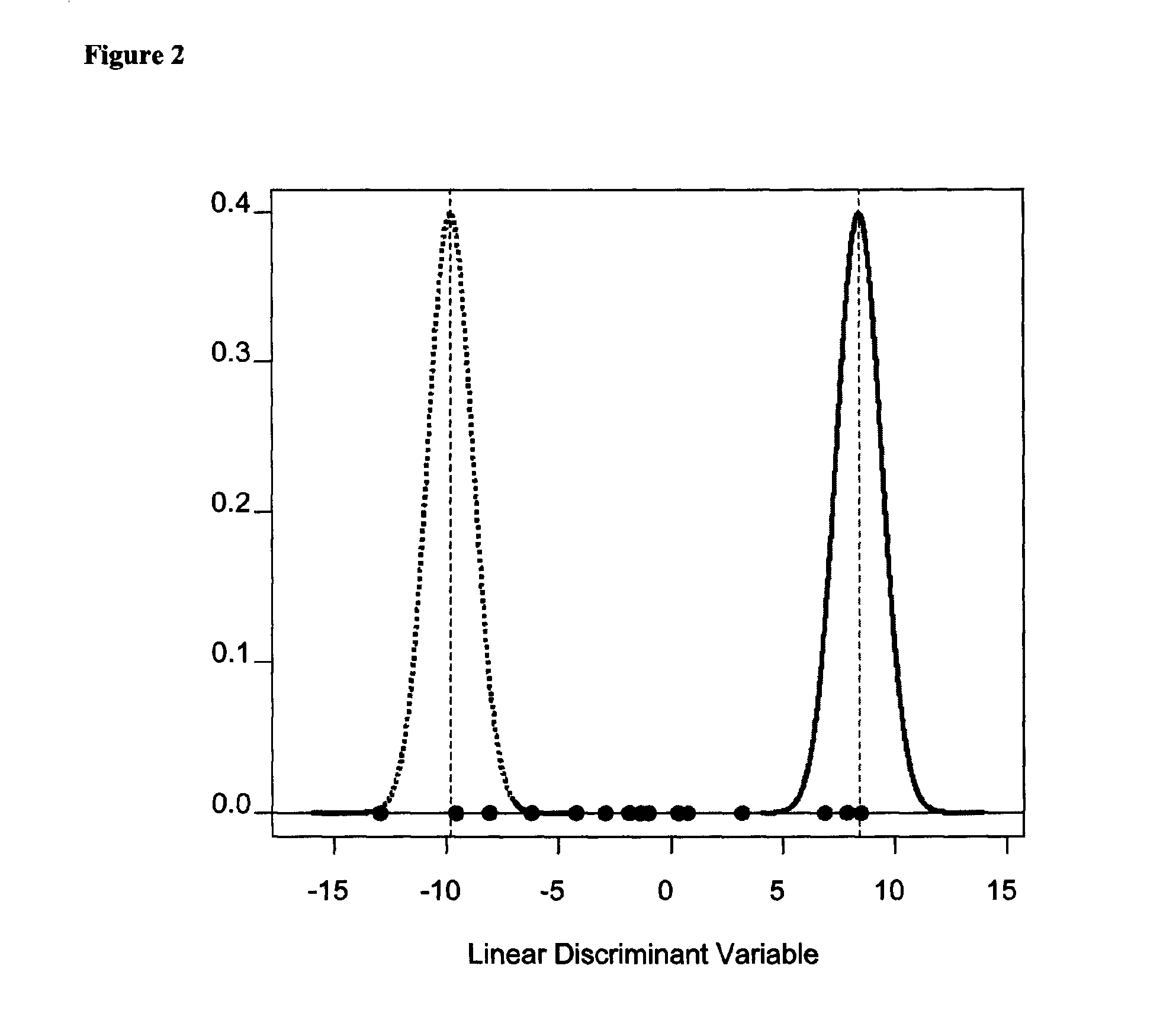
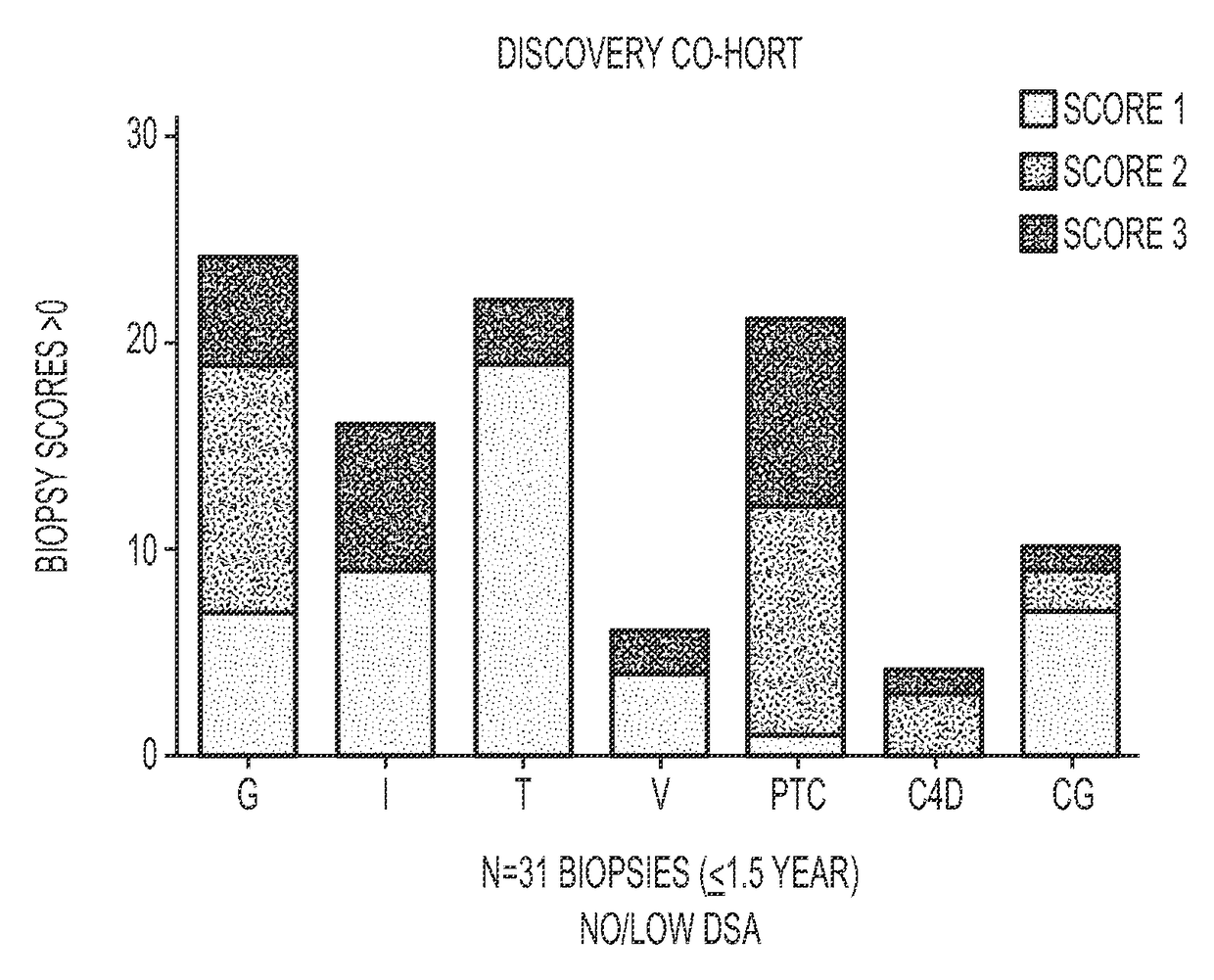
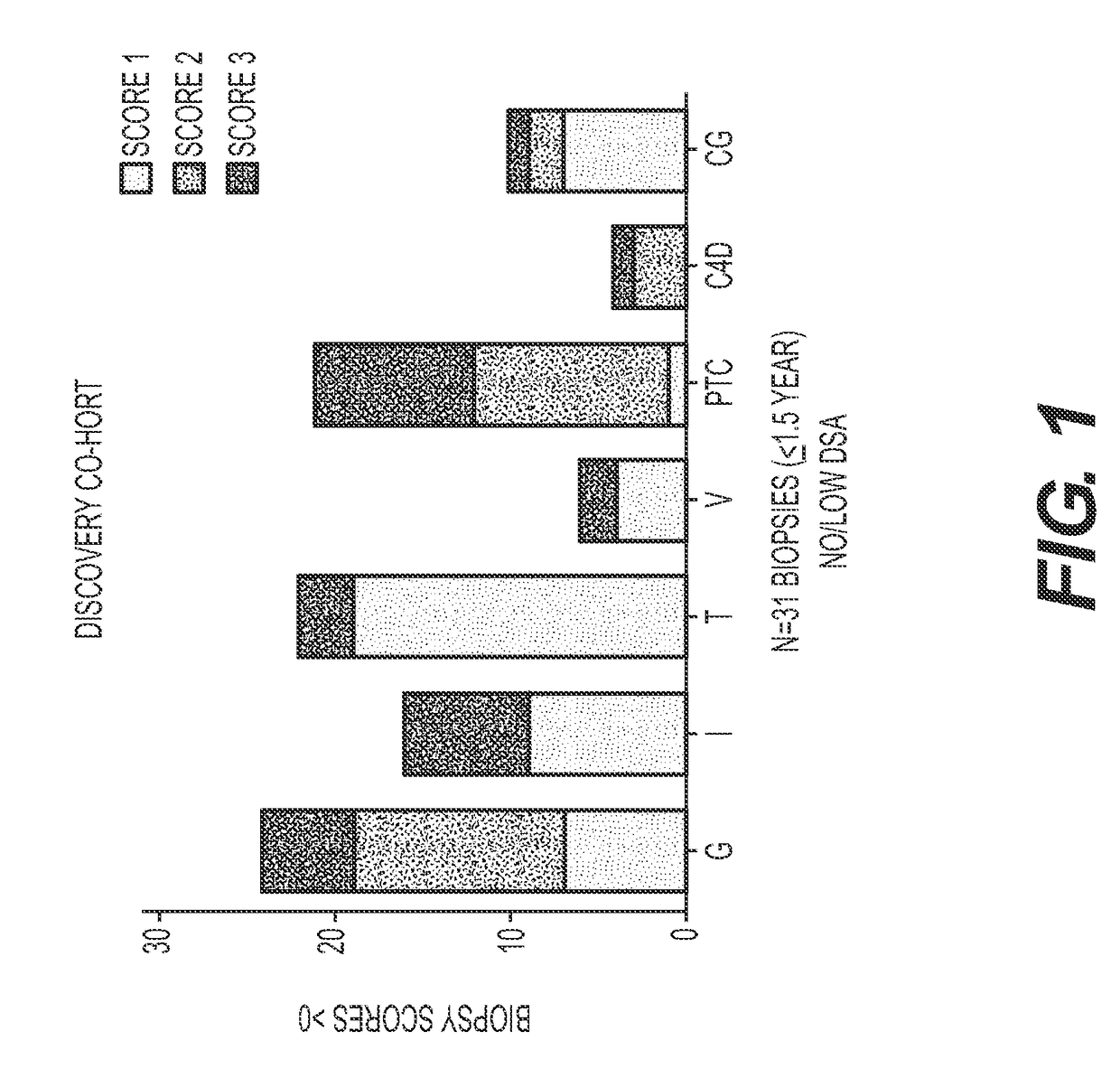
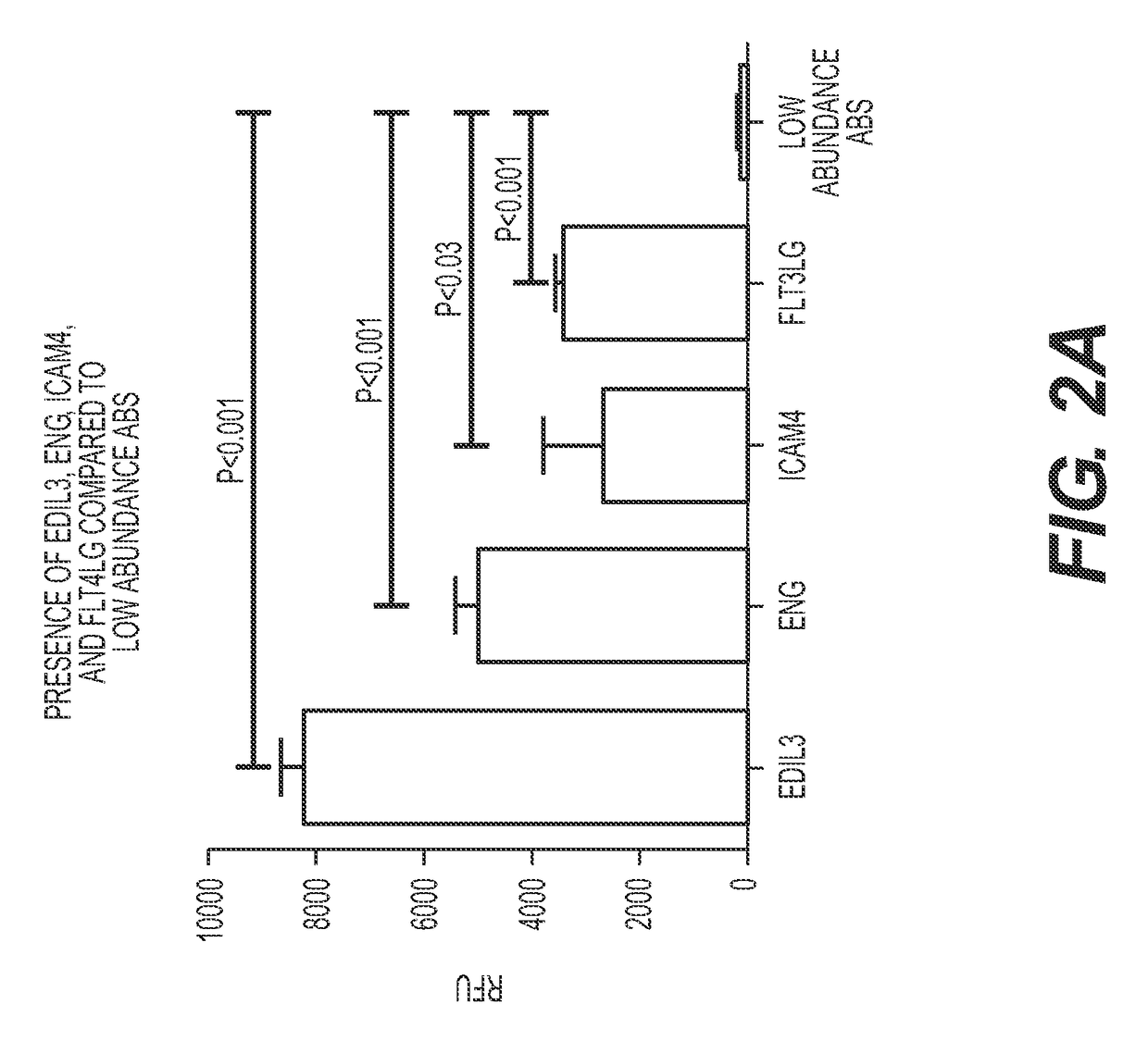
Method for identifying kidney allograft recipients at risk for chronic injury
A method for identifying a renal allograft recipient at risk for chronic allograft damage or interstutial fibrosis and tubular atrophy (IF / TA) by comparing the transcription level of a preselected gene signature set with the transcription level of a comparison standard, and diagnosing the recipient as being at risk for chronic allograft damage if the transcription level of the preselected gene signature set is significantly higher than the transcription level of the comparison standard.
Owner:WESTERN SYDNEY LOCAL HEALTH DISTRICT +1
Method for proliferating NK cells in vitro by virtue of magnetic beads coupled with multiple stimulating proteins and application of NK cells
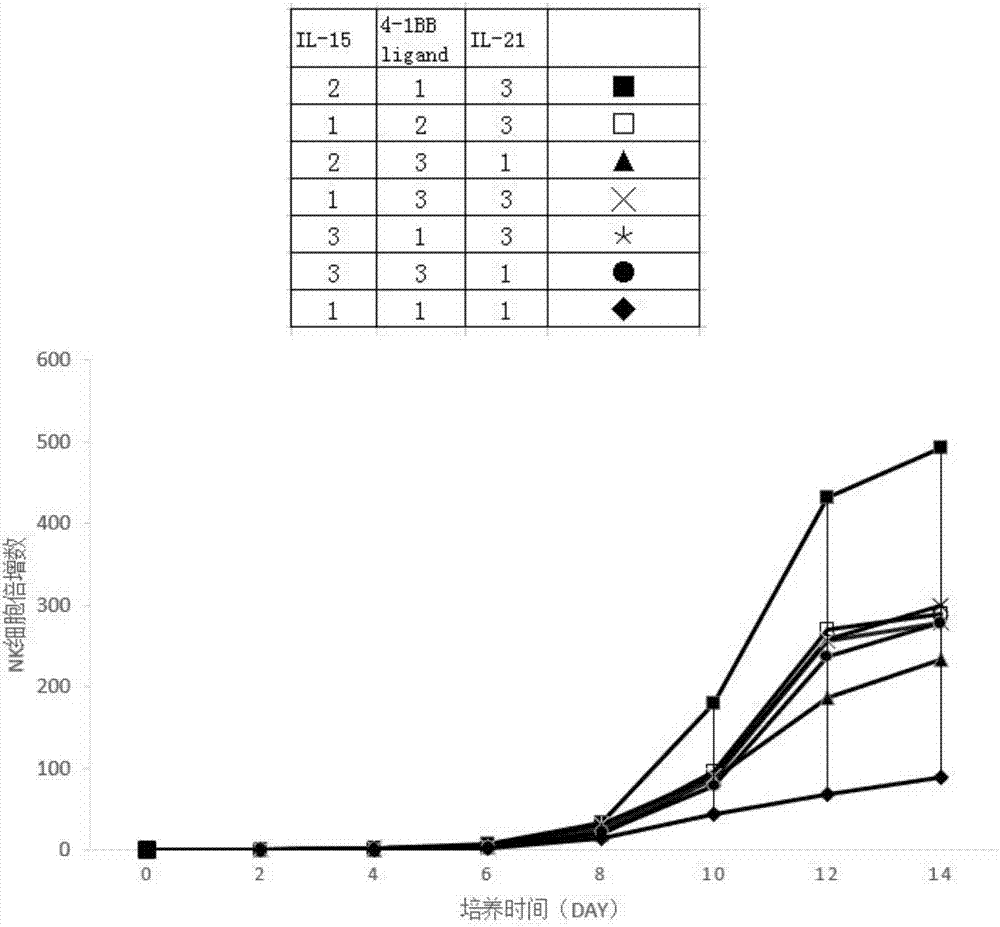
ActiveCN107354133AReduce usageOptimal culture conditionsBlood/immune system cellsCell culture active agentsNatural Killer Cell Inhibitory ReceptorsMagnetic bead
The invention discloses a method for proliferating NK cells in vitro by virtue of magnetic beads coupled with multiple stimulating proteins. The method comprises the following steps: culturing NK cells by adopting an NK cell culture medium, adding magnetic beads coupled with IL-15, 4-1BBligand and IL-21 (of which the molar ratio is (1-3):(1-3):(1-3)), culturing for 12-15 days, and harvesting and washing the NK cells, wherein the NK cell culture medium is prepared by adopting a method comprising the step of adding 10% of human AB serum or autologous serum, 500IU / mL of IL-2 and 5ng / ml of IL-15 into an X-VIVO 20 basic culture medium. The method disclosed by the invention has the advantages that the magnetic beads are coupled with three specificity stimulating factors, the aim of stimulating the NK cells by multiple factors is achieved, and usage of a trophoblastic cell line is also avoided, so that GMP production requirement and amplification efficiency requirement are considered at the same time, and the harvested NK cells can be used for NK allotransplantation.
Owner:YINFENG BIOLOGICAL GRP
Supperssor of the endogenous interferon-gamma
InactiveUS20110135602A1Suppressing hIFN-γ activityNervous disorderPeptide/protein ingredientsDiseaseAutoimmune responses
The invention relates to suppressor of the endogenous human interferon-gamma (hlFN-γ) applicable in treatment of diseases associated with impaired activity of endogenous hlFN-γ, especially autoimmune diseases and for prevention of graft arteriosclerosis and rejection of organs in allograft transplanted patients. It is based on inactive analogues of the hlFN-γ with pre-served affinity to the hlFN-γ receptor, genetically modified in the domain responsible for triggering the signal transduction pathway.
Owner:MILLICOM
Chimeric antigen receptor adipose-derived stem cell and preparation method thereof
InactiveCN105567640ALow immunogenicitySusceptible to infectionTumor necrosis factorNGF/TNF-superfamilyFatty liverAntigen receptors
The invention discloses a chimeric antigen receptor adipose-derived stem cell and a preparation method thereof. The chimeric antigen receptor adipose-derived stem cell is composed of a chimeric antigen receptor, an adipose-derived stem cell body and a tumor killing factor. The high-targeting characteristic that the antigen receptor is specifically combined with a specific tumor type, the characteristics that a fatty liver cell is low in immunogenicity, prone to be infected with viruses and capable of being amplified in a large scale within a short time and the tumor resisting function of the tumor killing factor are combined, and therefore the problems of immune storms, low chimeric antigen expression efficiency and allotransplantation immunological rejection during existing CART tumor treatment can be solved, and the tumor treating effect is improved. The improved adipose-derived stem cell has the efficient targeting performance of being specifically transferred to a specific tumor position, the risk that an immune system is excessively activated, and consequently immunological rejection is caused is avoided, the tumor killing factor carried by the adipose-derived stem cell can efficiently kill a tumor at the position of the tumor, and then the aim of treating the tumor is achieved.
Owner:BIOTOWNTEK CO LTD
Application of exosomes derived from menstrual blood stem cells on preparation of medicament for treating intrauterine adhesion
ActiveCN109893541APromote repairGood treatment effectSurgical drugsMammal material medical ingredientsDiseaseTissue repair
The invention belongs to the field of biological medicines, and particularly relates to application of exosomes derived from menstrual blood stem cells on preparation of a medicament for treating intrauterine adhesion. After primary isolation, culture and passage of the menstrual blood stem cells are carried out, 3rd-6th generations of menstrual blood stem cells are selected for exosomes preparation. When the density of the menstrual blood stem cells reaches 80%, serum components are removed by PBS washing, serum-free DMEM-F12 culture medium is added, and the menstrual blood stem cells are cultured in an incubator at the conditions of 37 DEG C, 5% CO2 and saturated humidity for 24 hours. The supernate is collected, the exosomes are prepared by ultracentrifugation under GMP conditions, andsterile PBS is collected and stored at the temperature of 80 DEG C. The cells have the advantages of being high in survival rate, stable in characters and easy to acquire and expand, and having an obvious treatment effect on intrauterine adhesion. The exosomes can even completely simulate the biological function of the cells to achieve the effects of promoting tissue repair and treating diseases.The exosomes are safer than the cells where the exosomes are derived from, do not lose functions after cryopreservation, can be used for allotransplantation and are suitable for mass production, and have important clinical application values and social and economic benefits.
Owner:SHENGJING HOSPITAL OF CHINA MEDICAL UNIV
Methods of diagnosing chronic cardiac allograft rejection
InactiveUS20110190151A1Chronic allograft rejectionMicrobiological testing/measurementLibrary screeningCardiac allograftGenome
The present invention relates to methods of diagnosing chronic rejection of a cardiac allograft using genomic expression profiling, proteomic expression profiling, or a combination of genomic and proteomic expression profiling.
Owner:THE UNIV OF BRITISH COLUMBIA
Method for identifying kidney allograft recipients at risk for chronic injury
ActiveUS10941446B2Microbiological testing/measurementFermentationCardiac allograftAllotransplantation
A method for identifying a renal allograft recipient at risk for chronic allograft damage or interstutial fibrosis and tubular atrophy (IF / TA) by comparing the transcription level of a preselected gene signature set with the transcription level of a comparison standard, and diagnosing the recipient as being at risk for chronic allograft damage if the transcription level of the preselected gene signature set is significantly higher than the transcription level of the comparison standard.
Owner:WESTERN SYDNEY LOCAL HEALTH DISTRICT +1
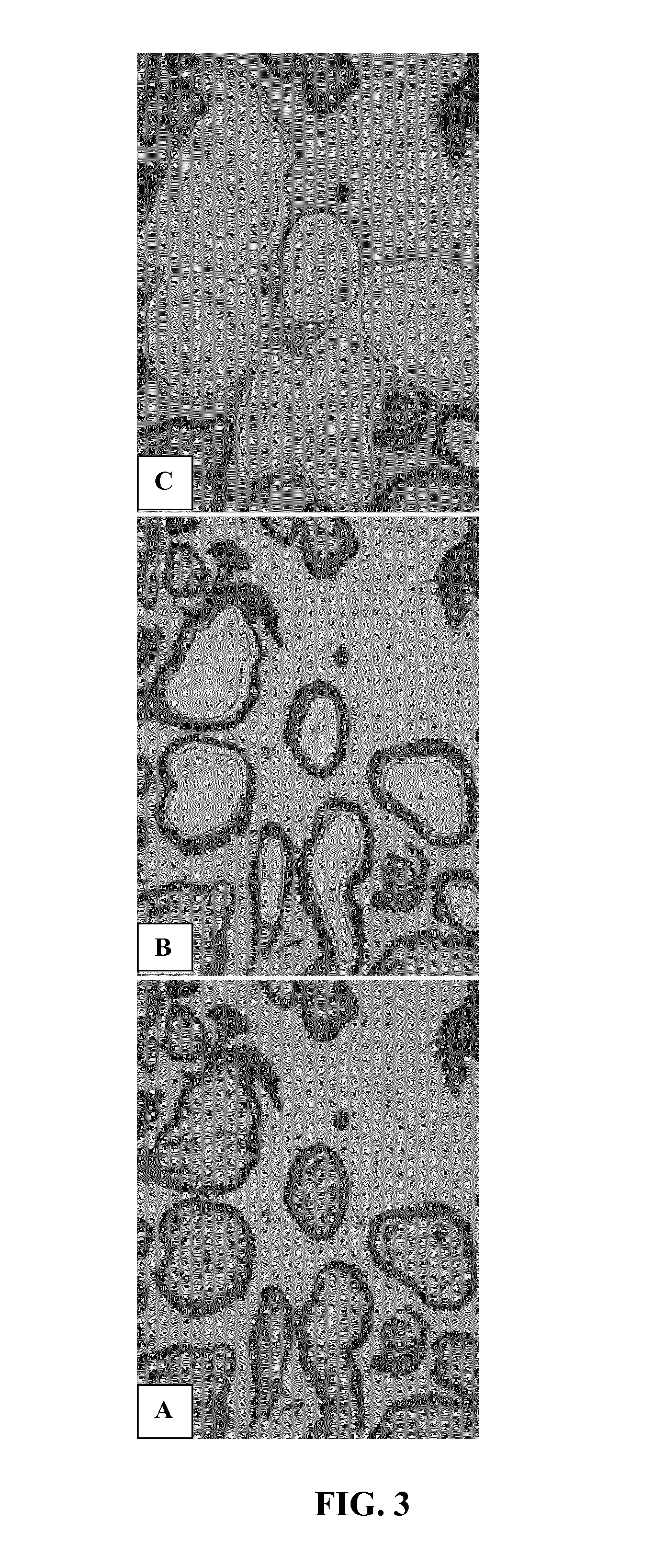
Process for obtaining a functional dermal substitute of decellurized amniotic membrane from the placenta combination with keratinocytes and its use as an agent for tissue regeneration of the skin
InactiveUS20200078492A1Stimulate early regeneration and reconstructionLow costTissue regenerationCoatingsMuscle contractionFibrosis
This invention provides a process of obtaining a functional dermal substitute from decellularized amniotic membrane from placenta in combination with mammalian keratinocytes and its use as a tissue regeneration agent of skin, which the process for the generation of an artificial skin graft in the laboratory using specialized techniques in biotechnology such as the mammalian cells culture, preferably human, in which keratinocytes are cultured in aseptic conditions that form part of the epidermal layer of the skin, which are combined with a mesh rich in collagen which is obtained from cells that compose the structure of the skin in combination with the amniotic membrane of the placenta to generate two layers that can be used in burn patients or with skin lesions. Additionally, this technology considerably reduces the cost of treatment compared to the cost of current methods such as skin graft treatments (autografts or allograft) and the result of this process is a prompt cellular reorganization, remodeling, and re-epithelization of the wound resulting in the formation of new skin tissue and avoiding the formation of fibrotic tissue that results from contraction muscle.
Owner:PEREZ CHAVEZ FERNANDO
Compositions and methods for detecting Anti-endothelial cell antibodies in allograft rejection
ActiveUS20170276687A1Increased riskReduce riskDisease diagnosisBiological testingEnd stage diseaseAllogeneic graft
Provided herein are methods, compositions, and kits for diagnosing allograft rejection of organ transplants by identifying the presence of anti-endothelial cell antibodies. Such methods and compositions are independent of external confounders such as recipient age, transplant center, assay, cause of end-stage disease, co-morbidities, immunosuppression usage, and the like.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Method to prevent transplant rejection by stable expression of heme oxygenase-1
InactiveUS20050142111A1BiocideGenetic material ingredientsTransplant arteriosclerosisTransplant rejection
The present invention relates to a method for the prevention of allograft rejection in clinical transplantation. Specifically, the method of the present invention relates to the prevention of transplant arteriosclerosis and interstitial fibrosis by stable and long-term expression of HO-1 in grafts. The present invention represents a novel therapeutic approach to prevent allograft rejection in clinical transplantation.
Owner:AGTC GENE TECH CO LTD
Compound Chinese traditional medicine preparation for inducing immune tolerance and preparing method and application thereof
ActiveCN105031555ASuppress immune rejectionEffective induction ofImmunological disordersPlant ingredientsMotilityCD86
The invention discloses a compound Chinese traditional medicine preparation for inducing immune tolerance and preparing method and application thereof. The compound Chinese traditional medicine preparation comprises 2-30 parts of roots of Chinese thorowax, 3-31 parts of moutan bark, 4-32 parts of radix paeoniae alba, 5-33 parts of radix curcumae and 6-35 parts of liquorice. The above components are prepared according to the proportion, to be smashed, soaked with water, decocted, filtered, centrifuged and dried to be made into capsules, oral liquid, tablets and the like. The compound Chinese traditional medicine preparation can induce generation of DC regs of high-expression CD11b and low-expression HLA-DR / CD86 / CD80, the DCregs generated through inducing can effectively inhibit DC-mediated lymphopoiesis and promote secretion of cytokines IL-10; a receptor mice is induced to tolerate the iso-allotransplantation skin; no influence on the motility rate of DC cells and the hepatorenal function of the receptor mice is generated, and the compound Chinese traditional medicine preparation is effective and low-toxic and induces immune tolerance.
Owner:TIANJIN MEDICAL UNIV
Methods and materials for immunomodulation of tissue grafts
PendingUS20200139012A1Organic active ingredientsPharmaceutical non-active ingredientsLocal immunitySpinal cord
Embodiments described herein relate to restorative solutions for segmental peripheral nerve (PN) defects using allografted PNs for stimulating PN repair. More specifically, embodiments described herein provide for localized immunosuppression (LIS) surrounding PN allografts as an alternative to systemically suppressing a patient's entire immune system. Methods described herein provide for injection of ISV agents into a nerve graft prior to implantation of the nerve graft into a recipient. Methods described herein also provide for injection of ISV agents with a polymerizable carrier into a nerve graft, polymerization of the carrier within the graft, and implantation of the nerve graft into a recipient. Certain embodiments provide for reinnervation of central nervous system (CNS) axons in a spinal cord utilizing a peripheral nerve graft.
Owner:UNIVERSITY OF WYOMING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com