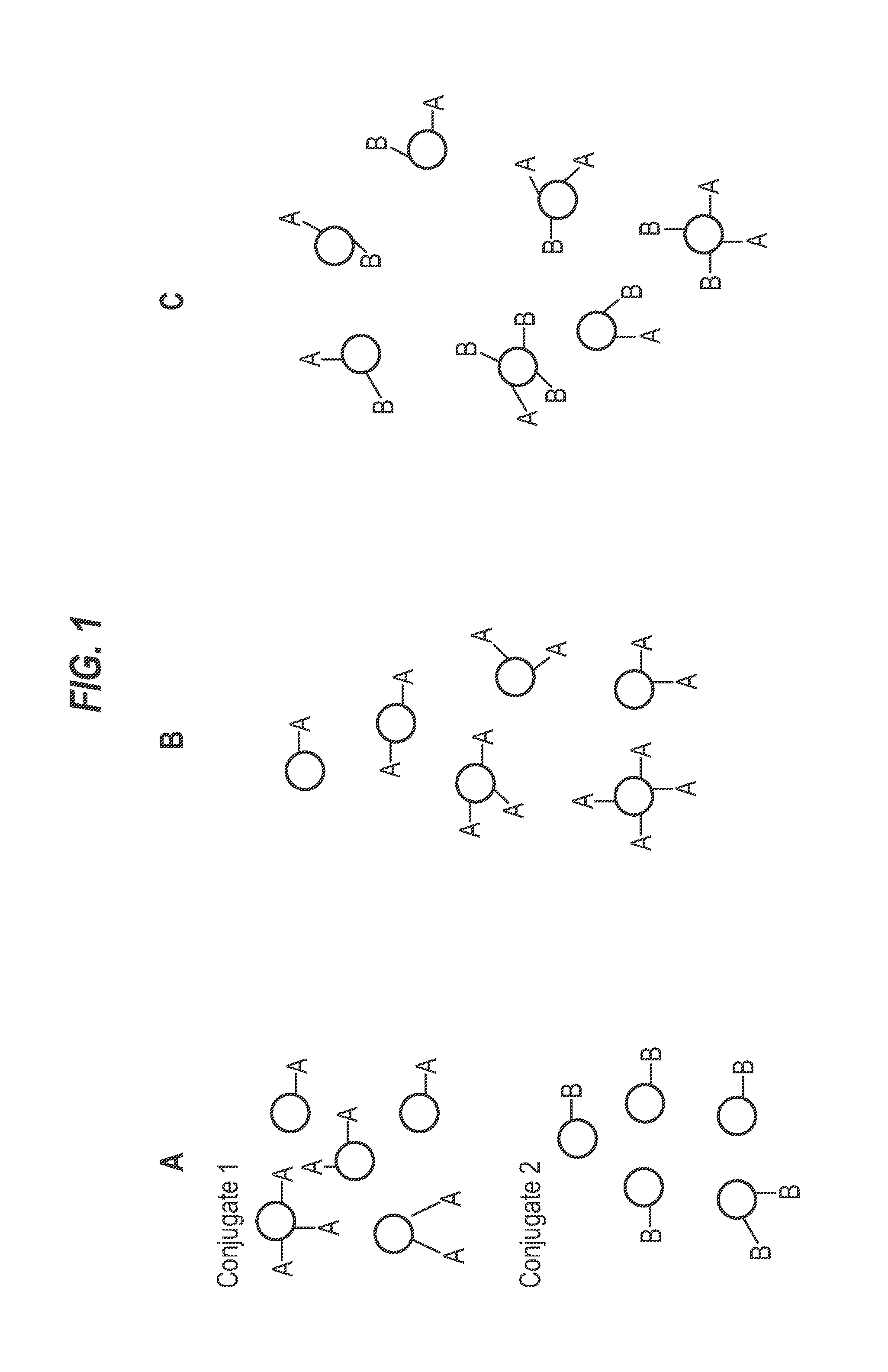
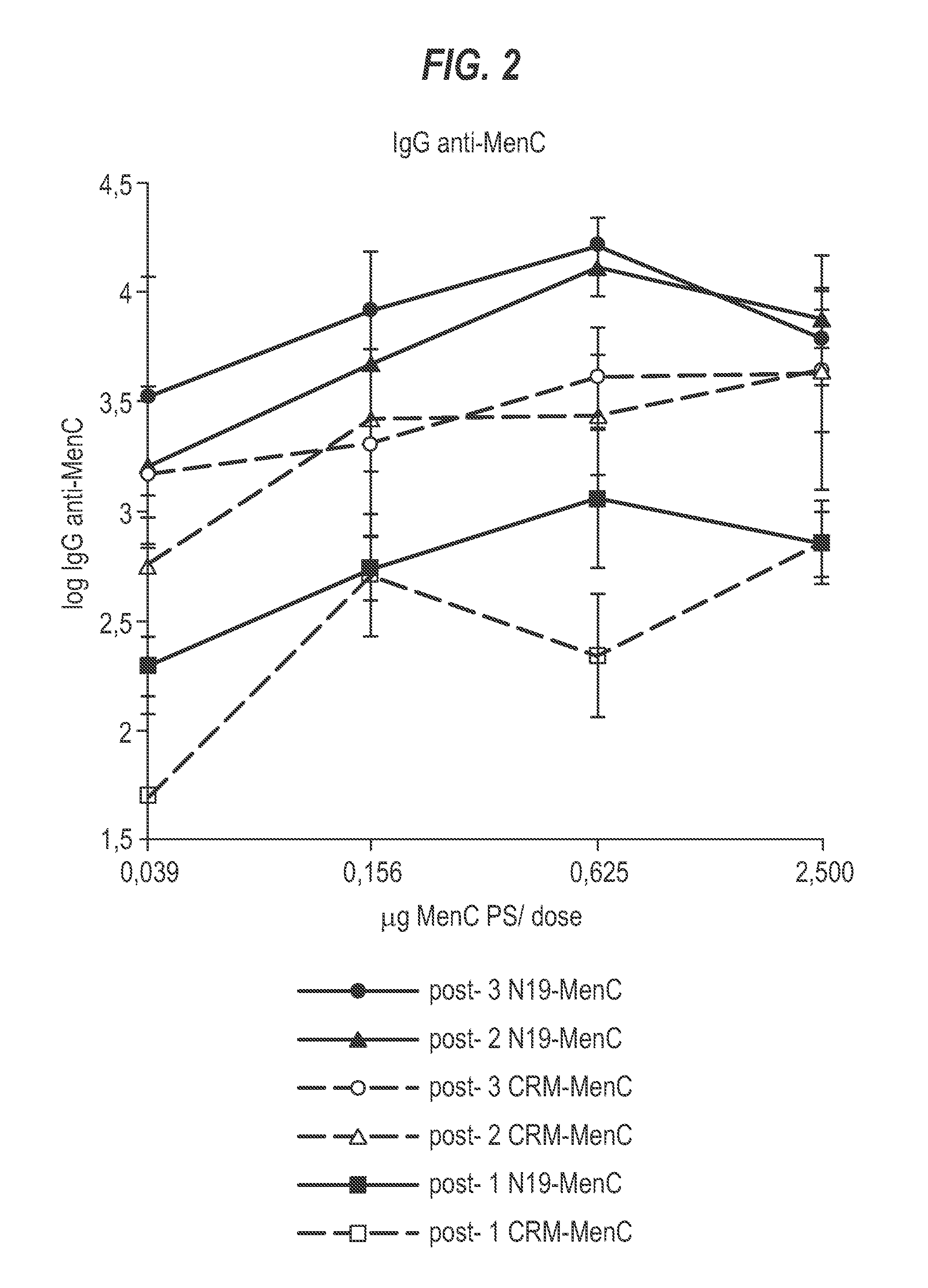
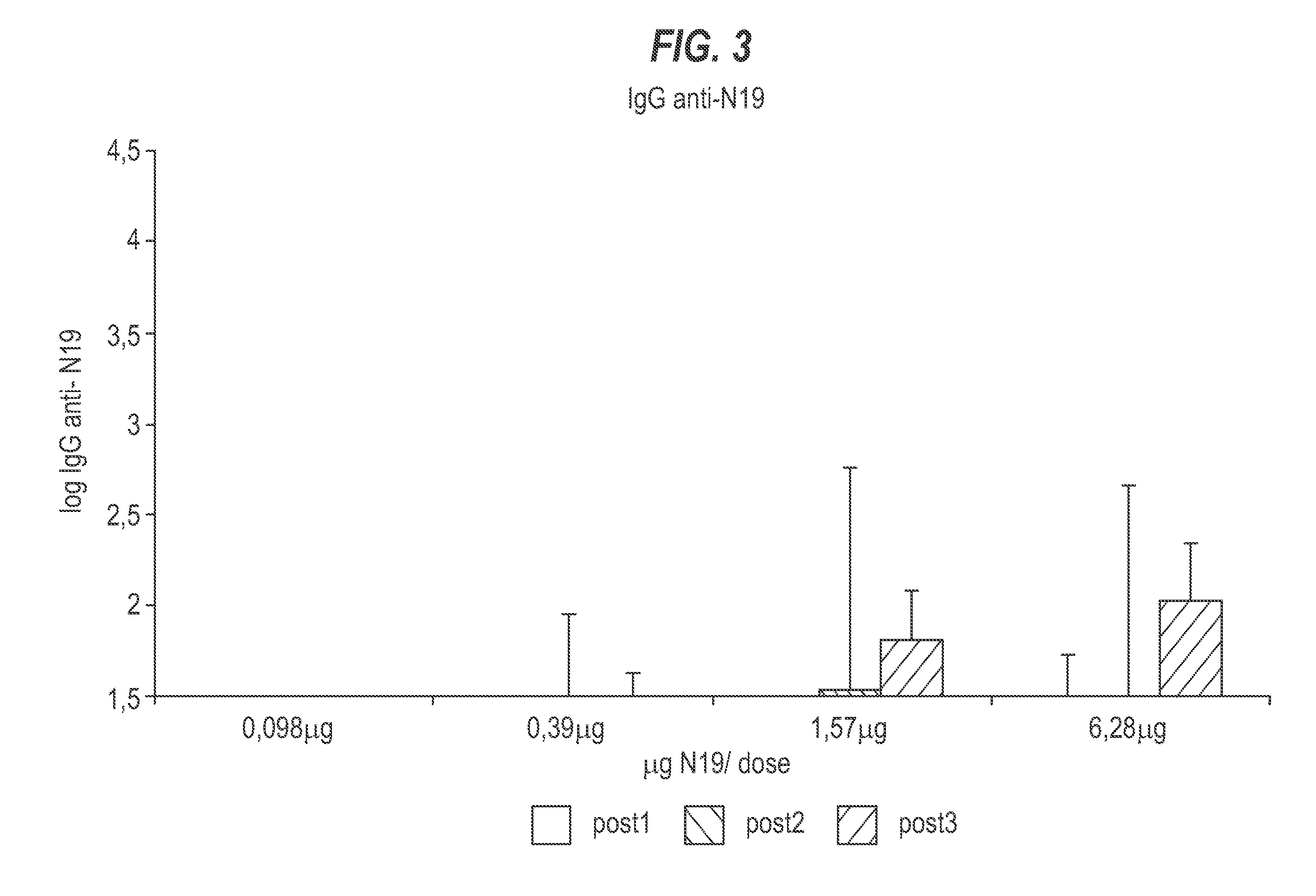
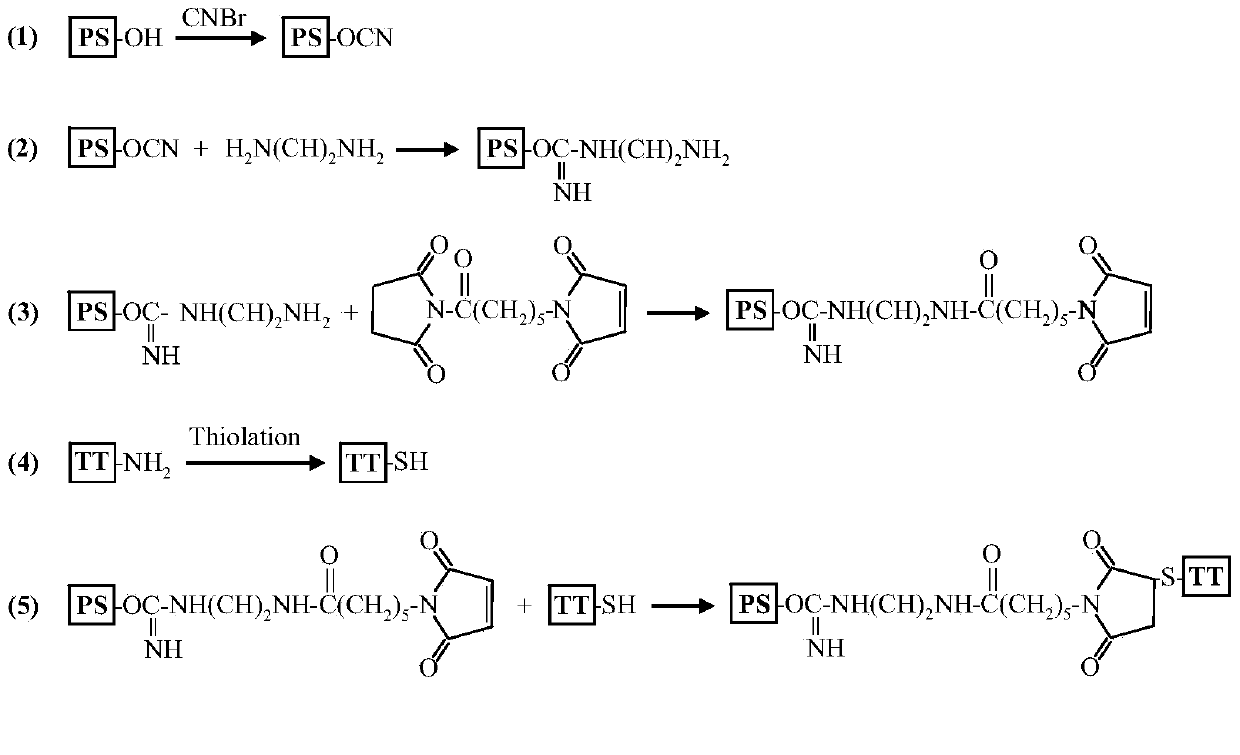
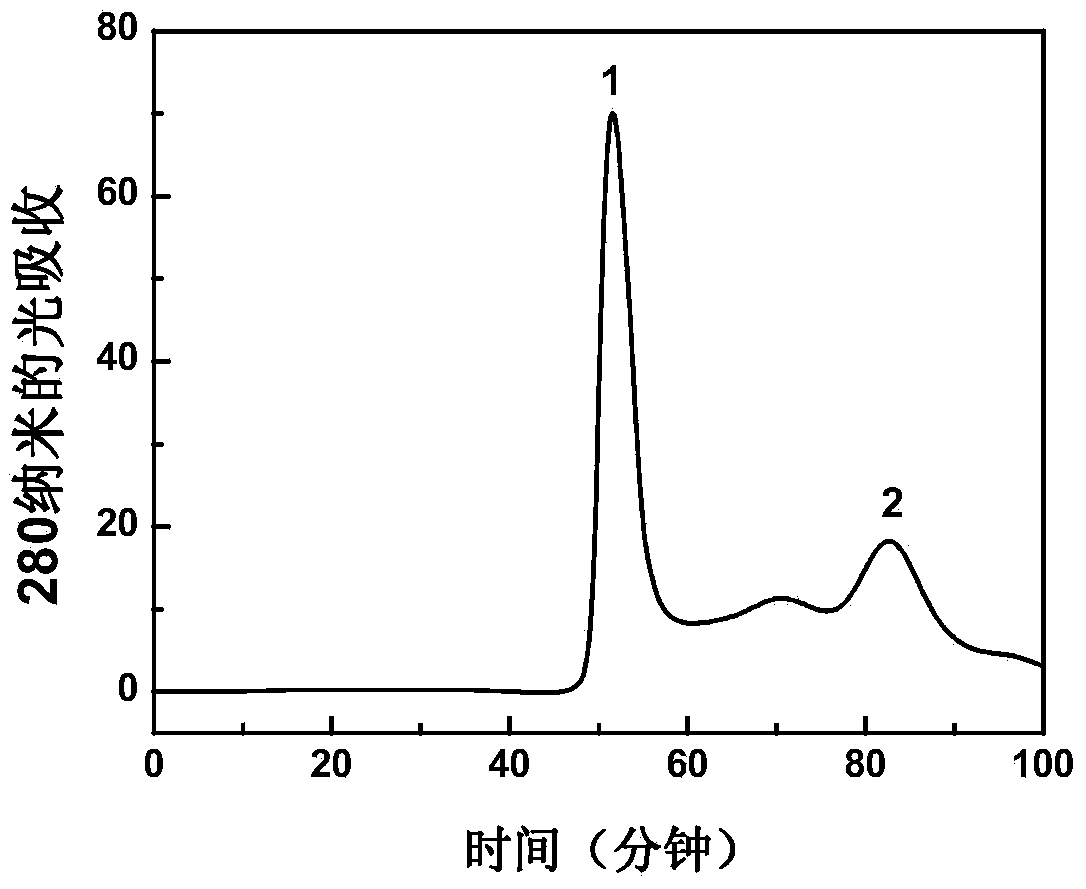
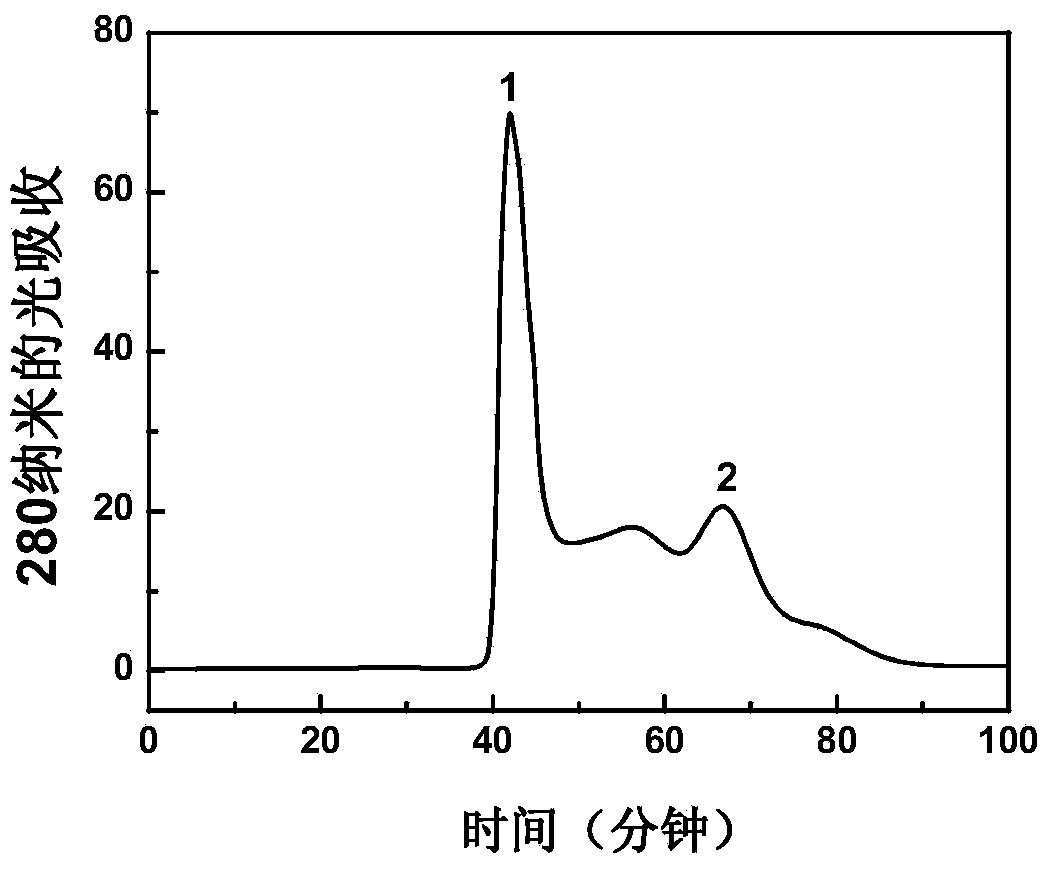
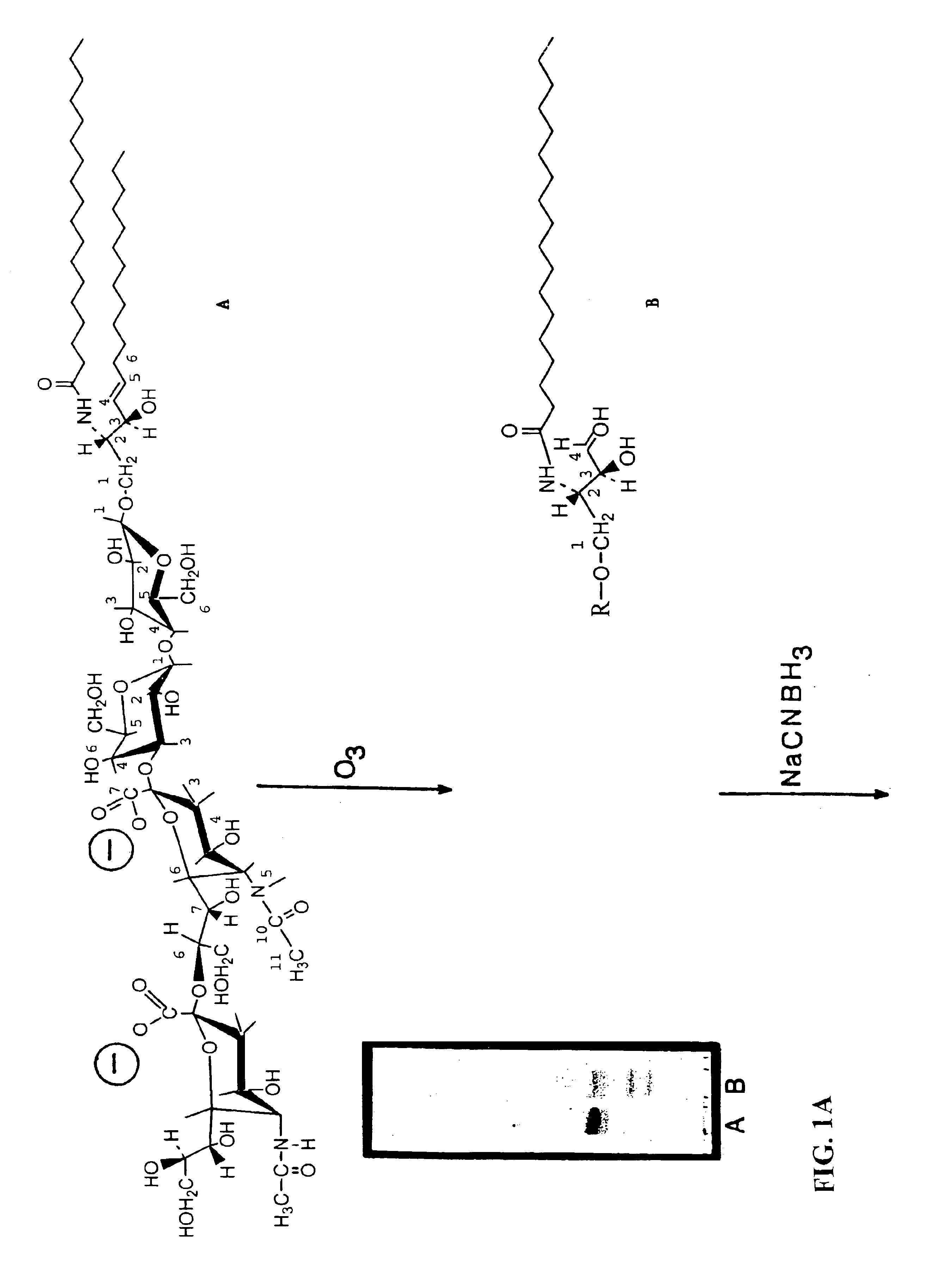
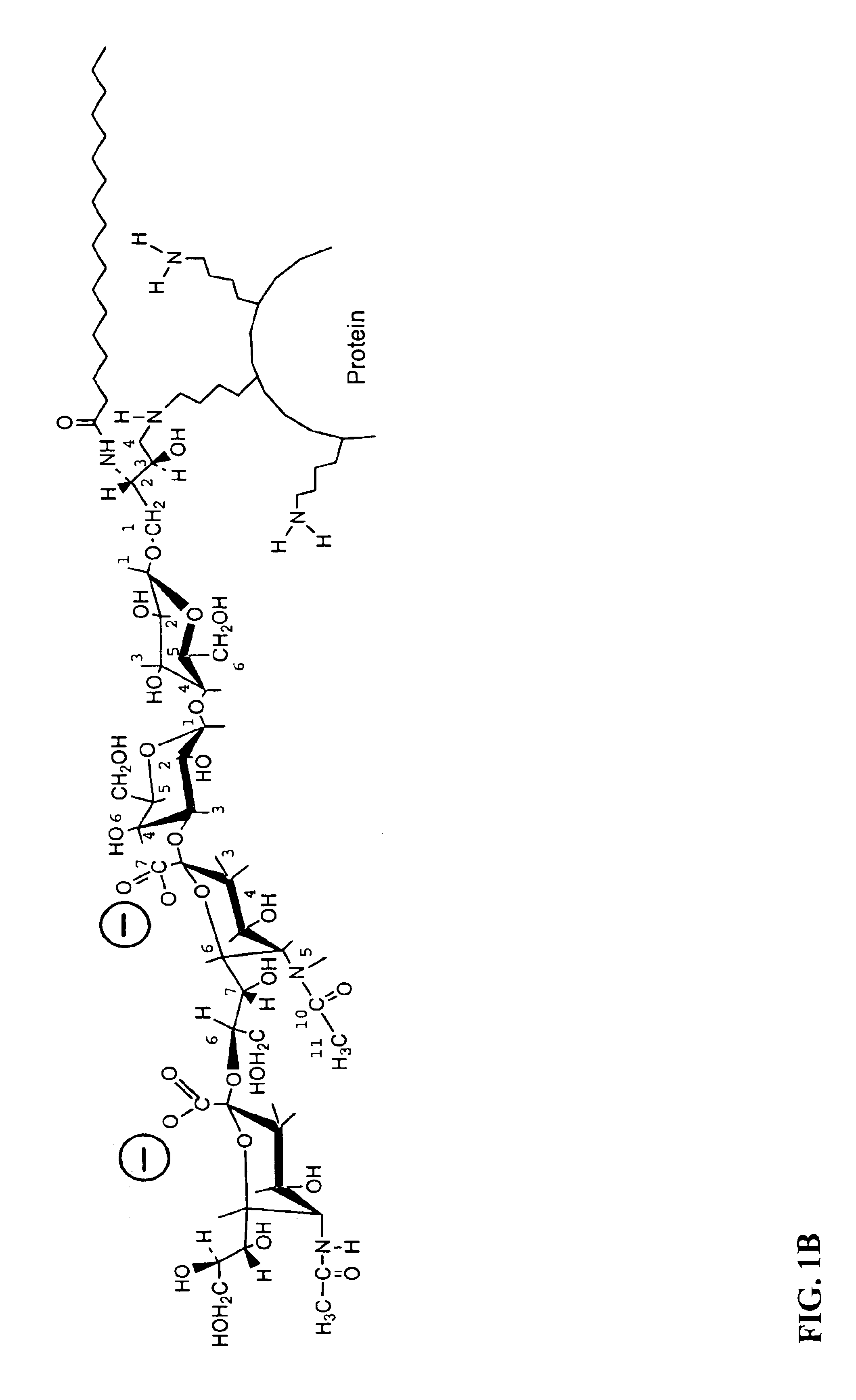
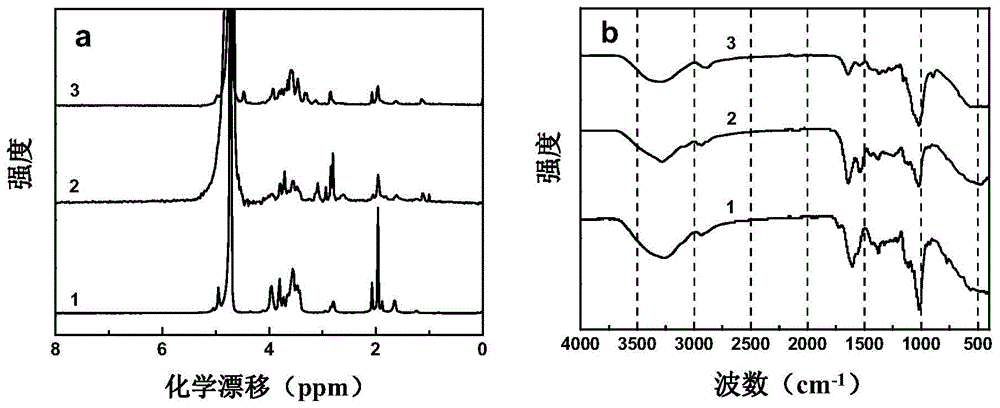
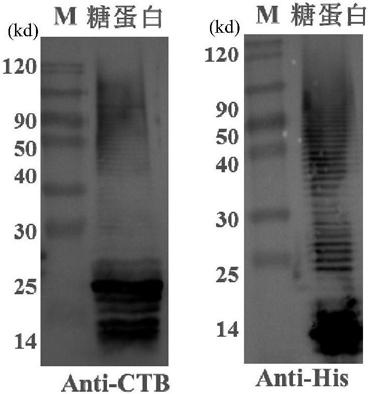
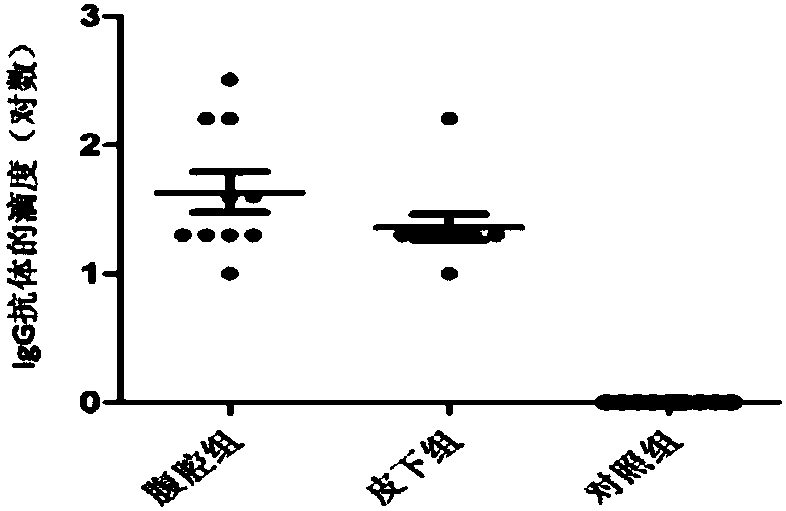
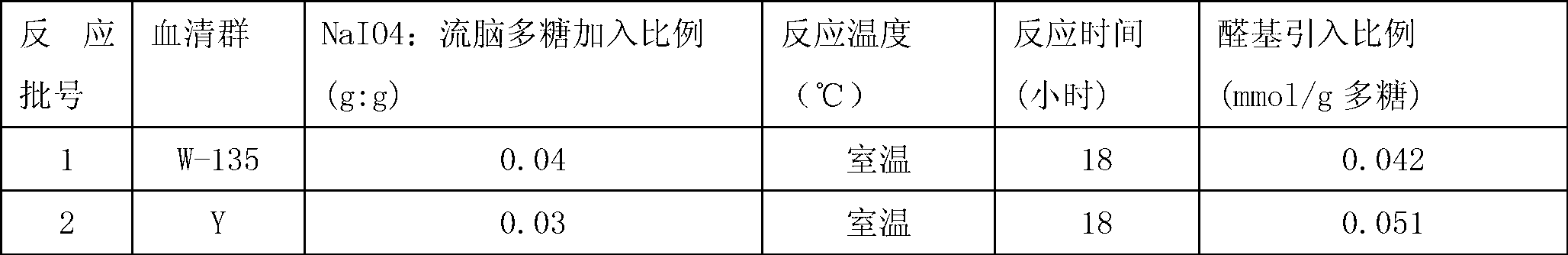
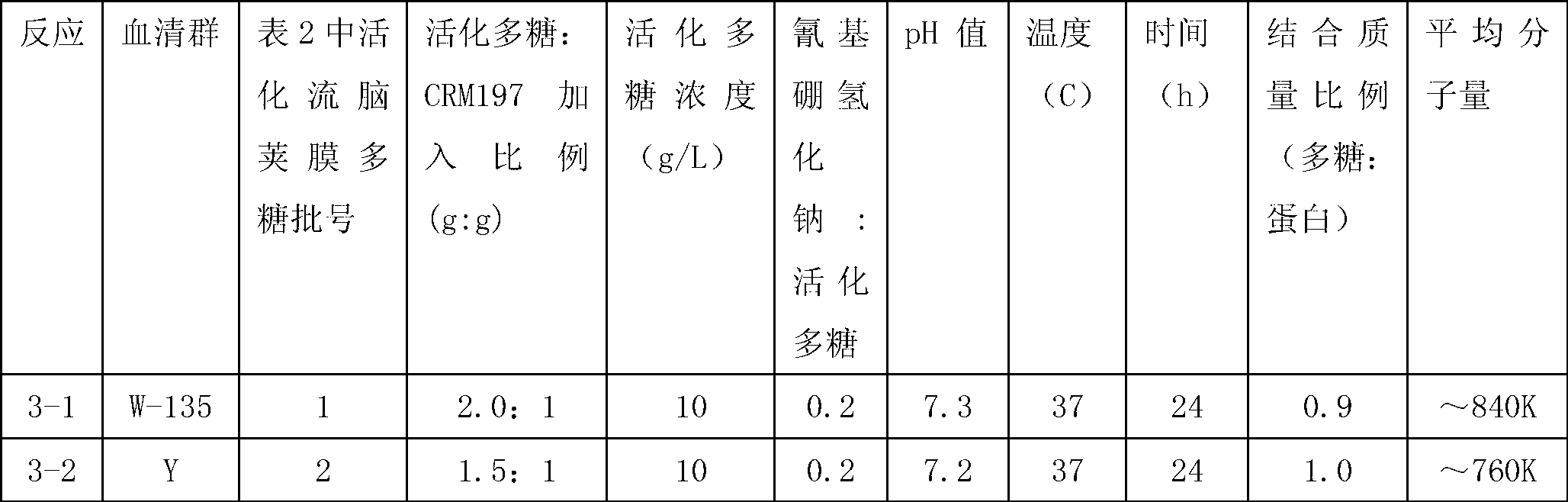
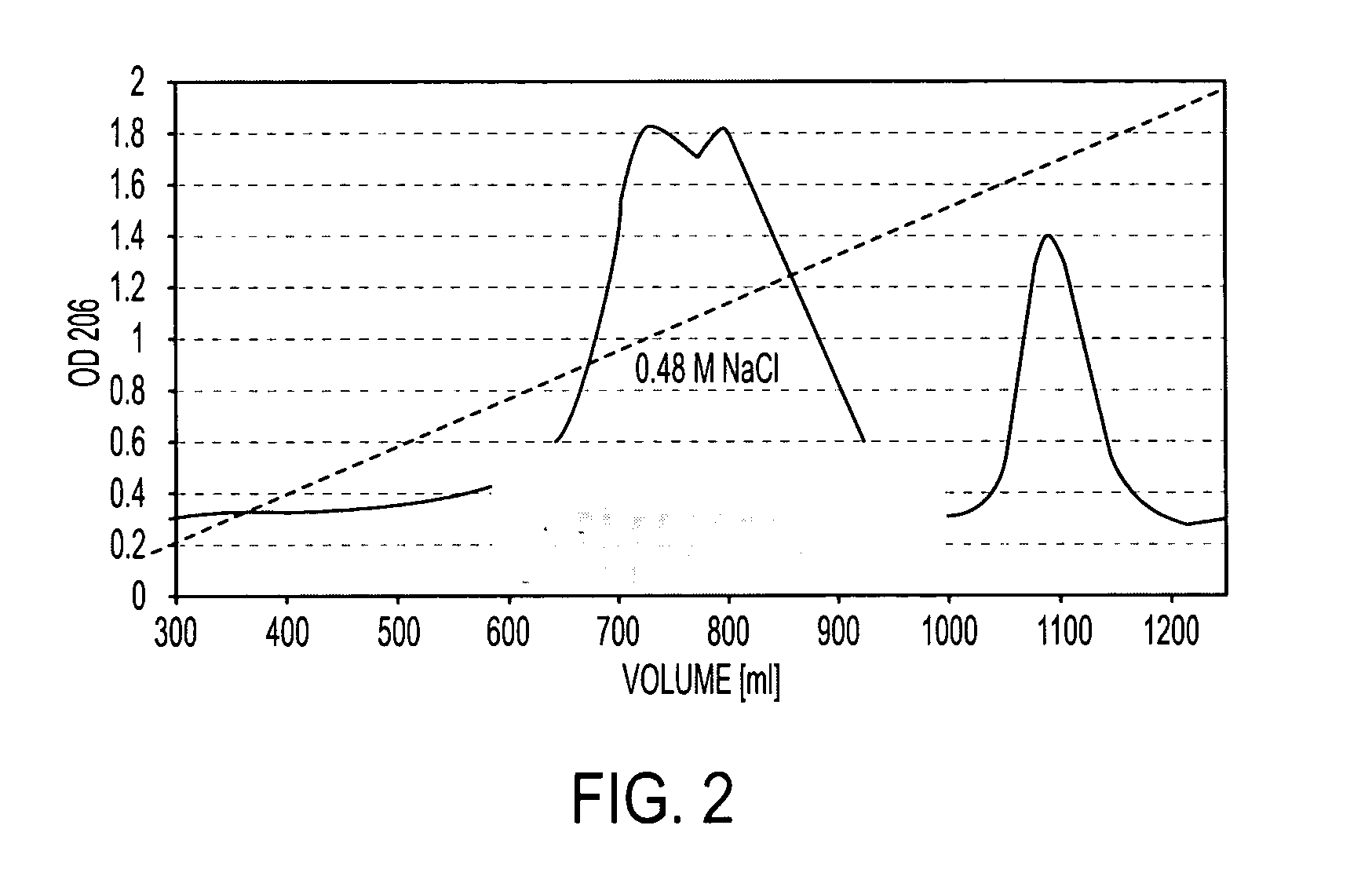
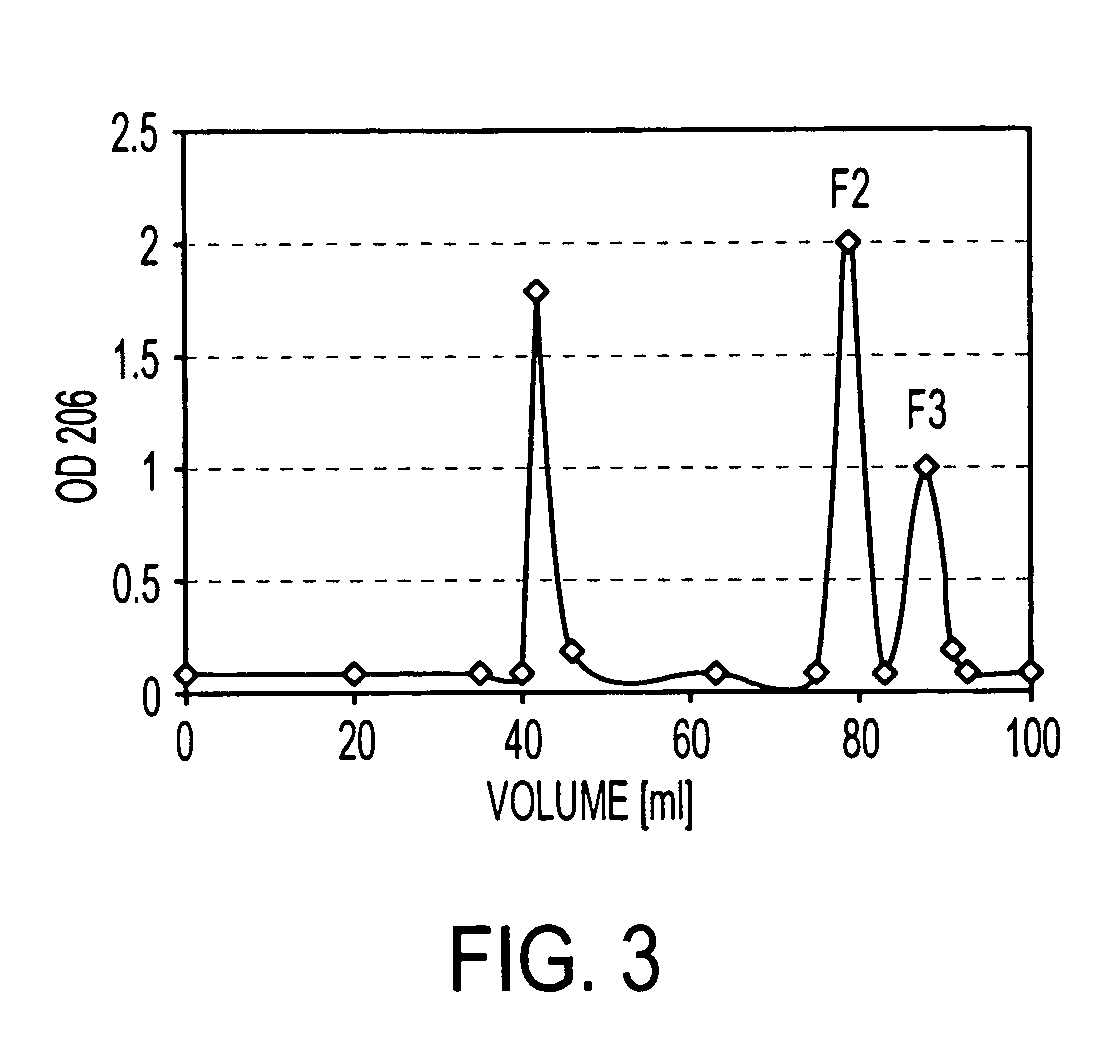
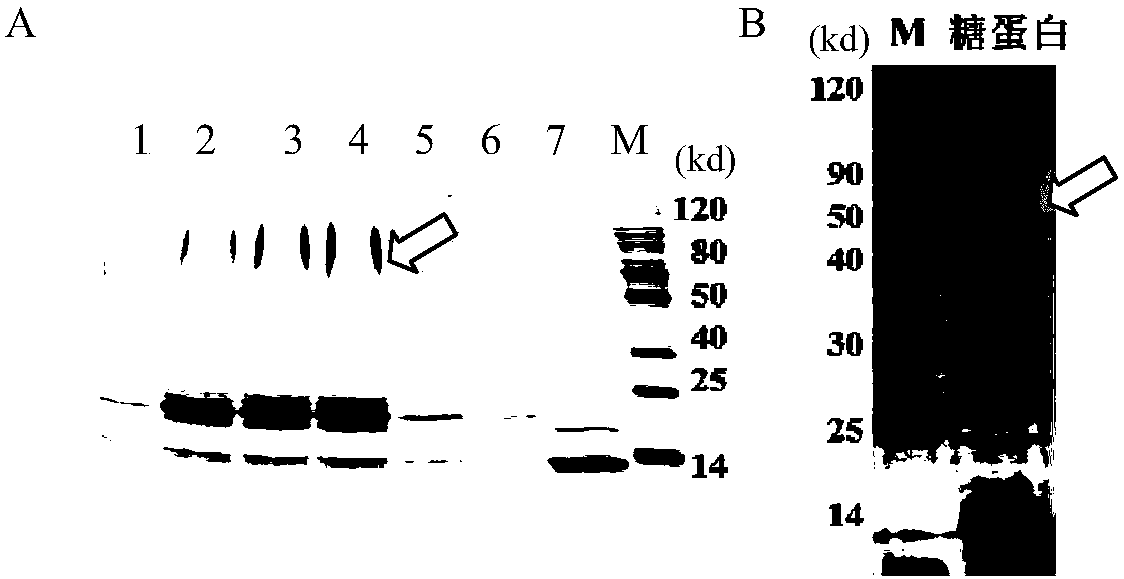Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
127 results about "Conjugated vaccines" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medicine, a conjugate vaccine, or conjugated vaccine, is a type of vaccine that is created by joining an antigen to a protein molecule. Conjugated vaccines are usually used to immunize babies and children against certain bacterial infections.
Conjugate vaccines for non-proteinaceous antigens
InactiveUS20070231344A1Strong and rapid effectStrong and rapid immune responseBacterial antigen ingredientsLipid/lipoprotein ingredientsAntigenConjugate vaccine
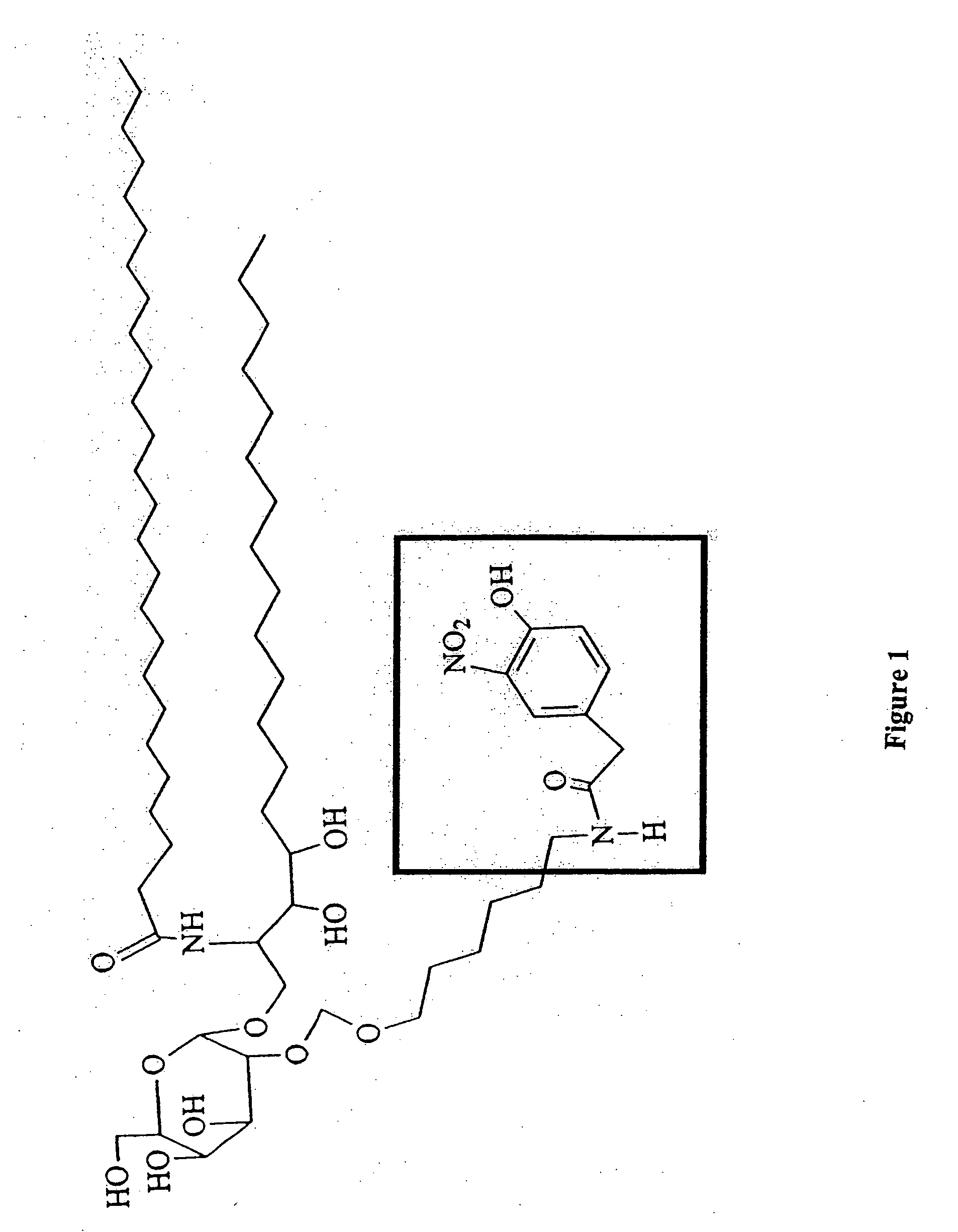
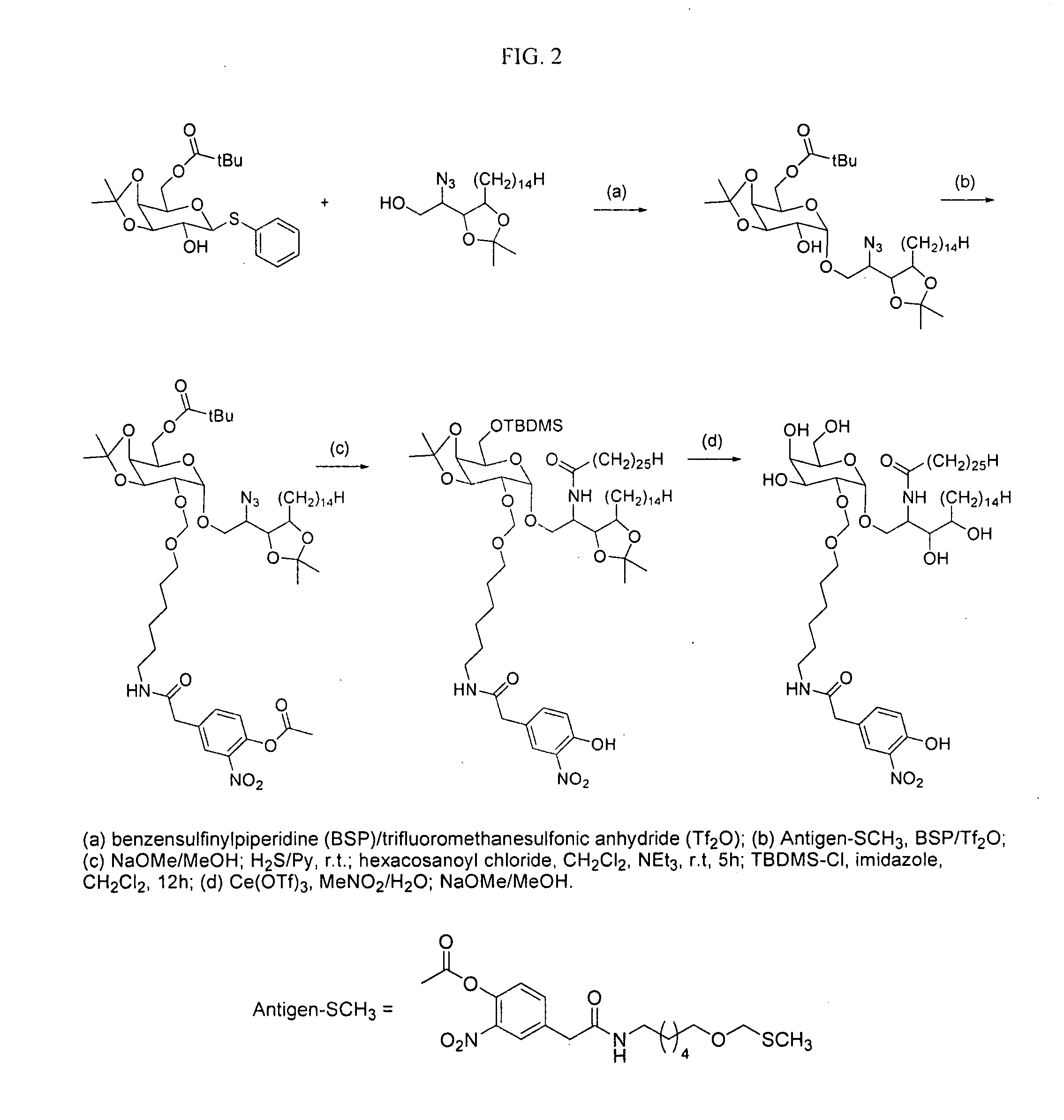
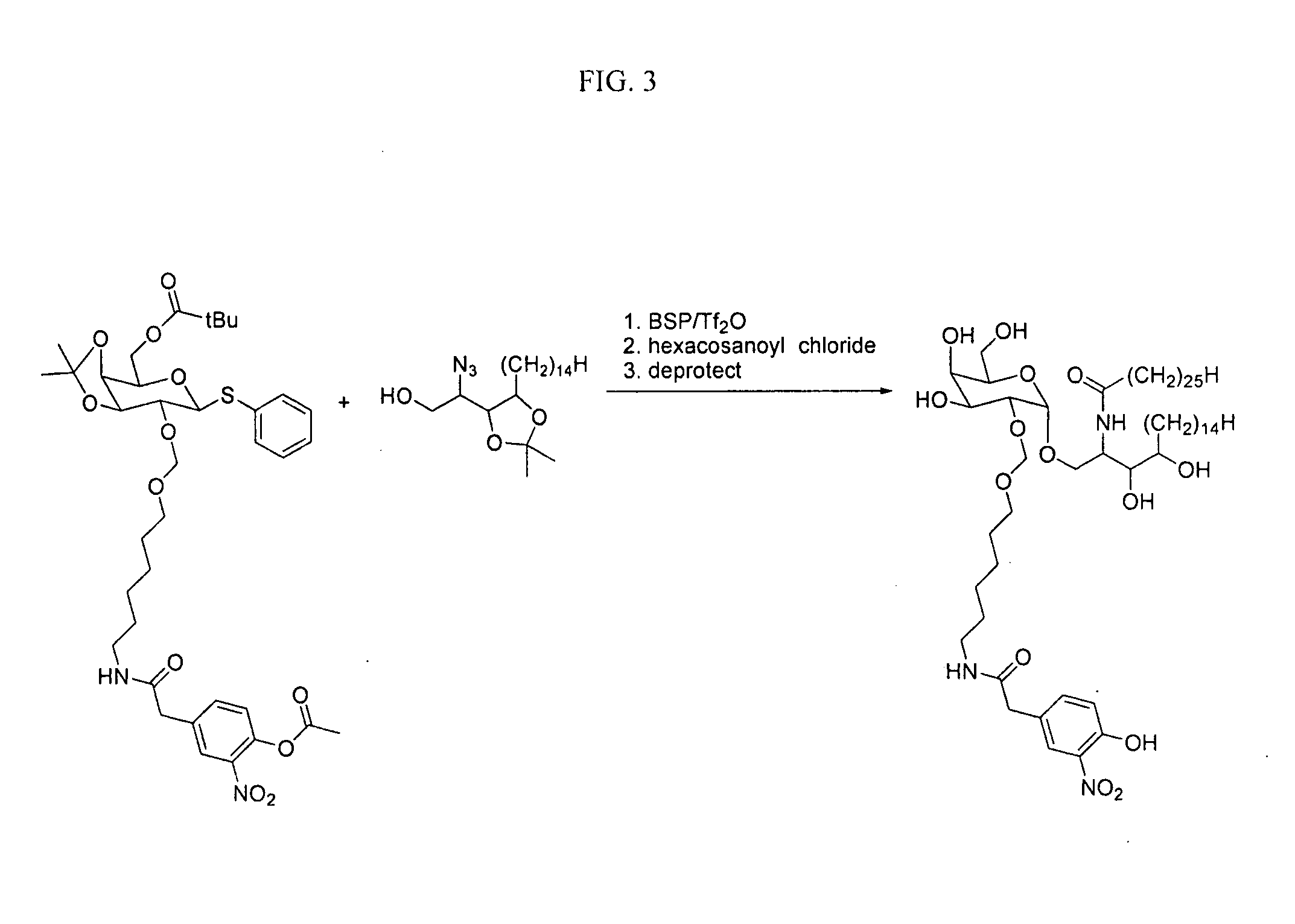
The present invention is directed to pharmaceutical compositions that can be used to immunize subjects using, for example, lipid, glycan, or nucleic acid antigens. These antigens are conjugated to a glycosphingolipid.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Saccharide Conjugate Vaccines
InactiveUS20080260773A1Improve bioavailabilityImprove efficacyAntibacterial agentsPeptide/protein ingredientsConjugate vaccineCarrier protein
The invention provides compositions comprising a combination of two or more monovalent conjugates, each of said two or more monovalent conjugates comprising a carrier protein comprising T cell epitopes from two or more pathogens conjugated to saccharide antigen. The invention also provides a multivalent conjugate comprising two or more antigenically distinct saccharide antigens conjugated to the same carrier protein molecule, wherein the carrier protein comprises T cell epitopes from two or more pathogens. Further compositions comprise one or more of said monovalent conjugates and one or more of said multivalent conjugates. The invention further provides methods for making said compositions and uses for said compositions.
Owner:NOVARTIS AG
Vaccine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Bacterial polysaccharide-protein conjugate vaccine and preparation method thereof
The invention relates to a bacterial polysaccharide-protein conjugate vaccine with immunogenicity, in particular to a conjugate vaccine which is formed by connecting a recombinant rotavirus protein with a bacterial polysaccharide by using a covalent bond, a nucleotide sequence for coding the recombinant rotavirus protein, a recombinant expression system, a protein expressed by the recombinant expression system, a preparation method of the conjugate vaccine and a pneumococcus polysaccharide-recombinant rotavirus protein conjugate vaccine. The bacterial polysaccharide is connected with a recombinant rotavirus surface protein through a covalent bond. The recombinant rotavirus protein is selected from a partial or complete amino acid sequence of a P-gene rotavirus protein and a partial or complete amino acid sequence of a G-gene rotavirus protein.
Owner:普大生物科技(泰州)有限公司
Conjugate Vaccines
InactiveUS20080305127A1Improving immunogenicityReduce chain lengthAntibacterial agentsBacterial antigen ingredientsImmunologyHuman patient
The invention provides vaccines against Neisseria meningitidis, pneumococcus and DTPa / w. In particular, it provides vaccines based on conjugated capsular saccharides from multiple meningococcal and / or pneumococcal serogroups. It further provides vaccine administration schemes for the immunisation of human patients with two or more of these vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent pneumococcal capsular polysaccharide composition as well as preparation method and application thereof
ActiveCN103656632AStable physical and chemical propertiesPrevent diseaseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
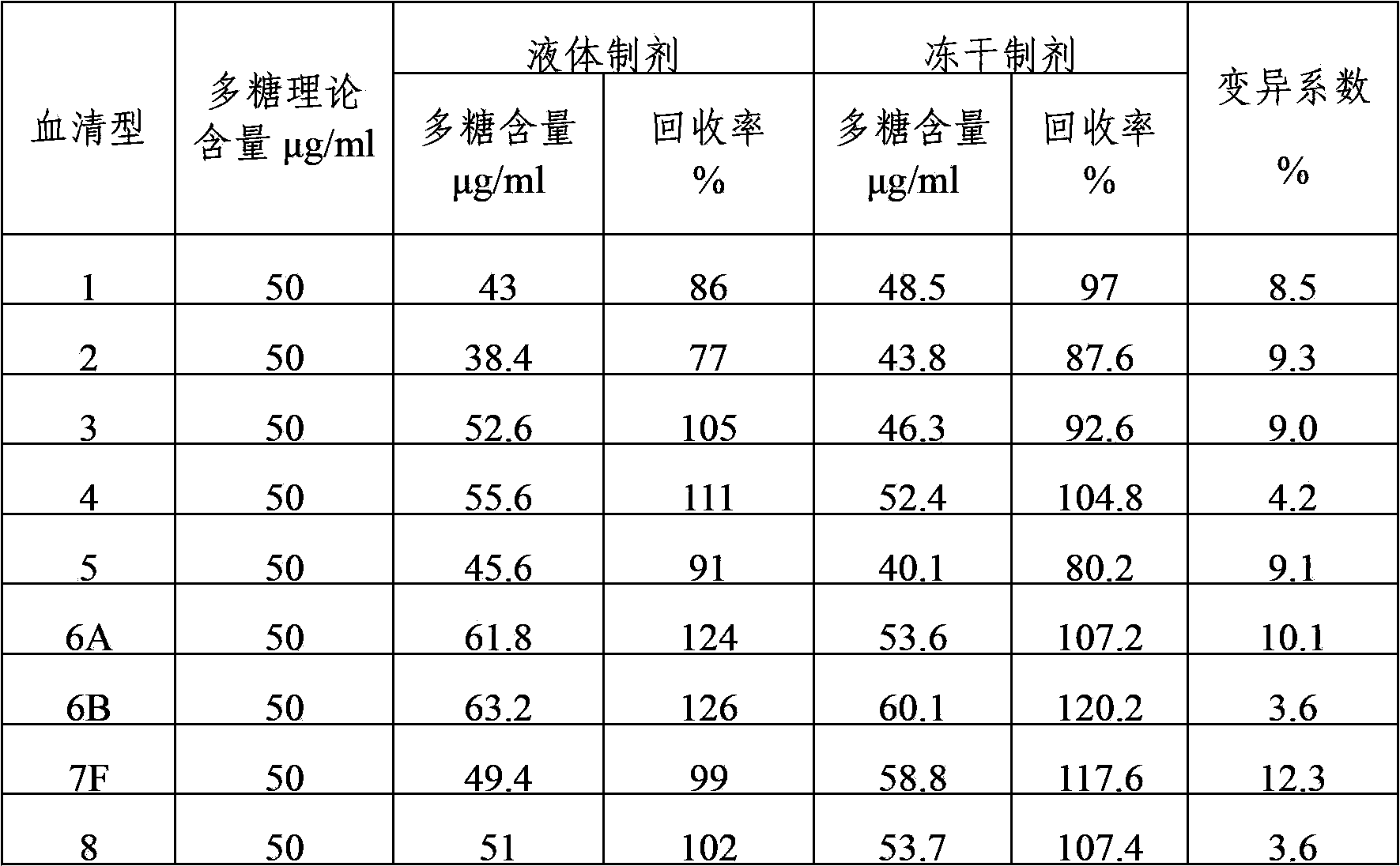
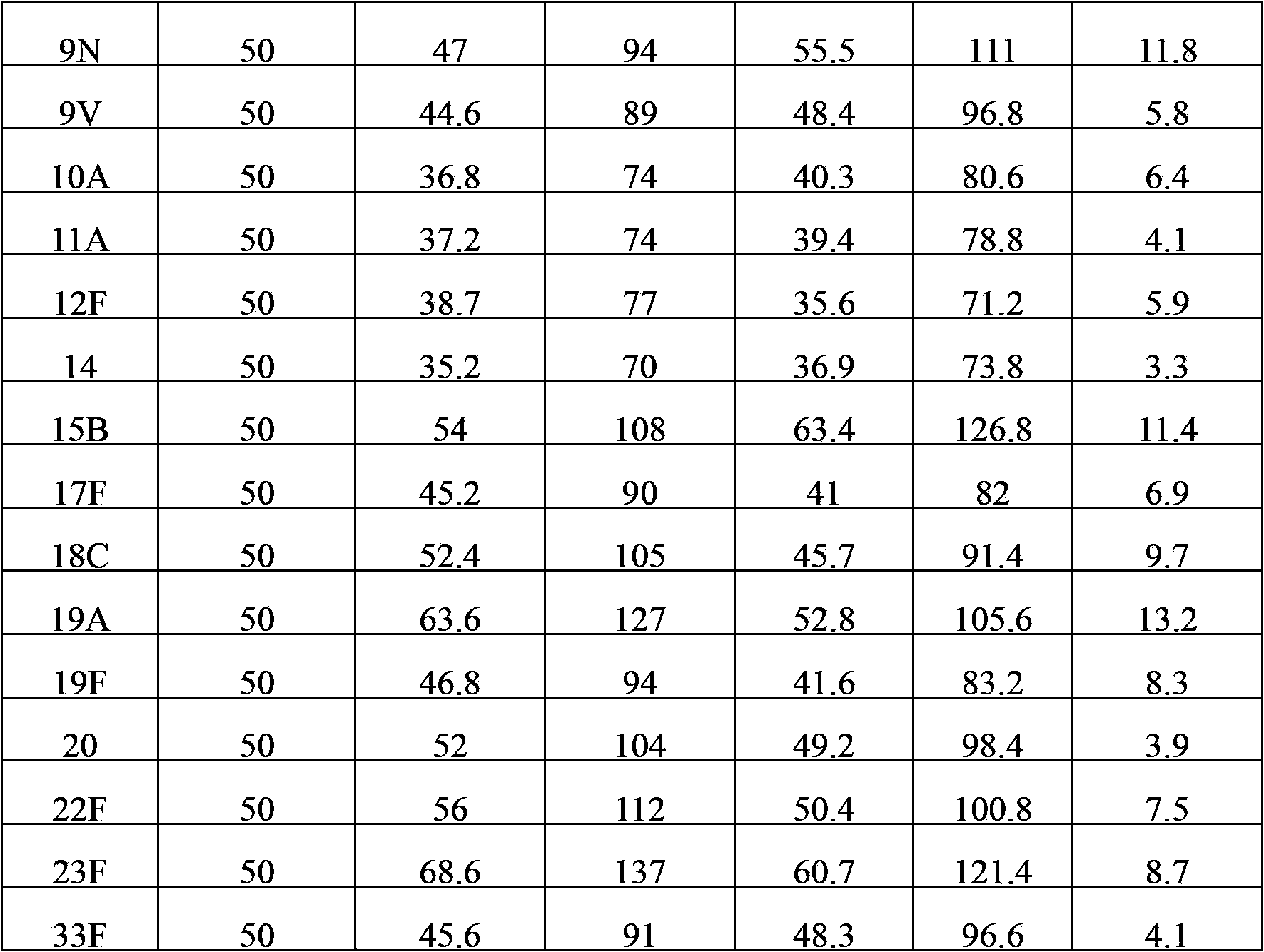
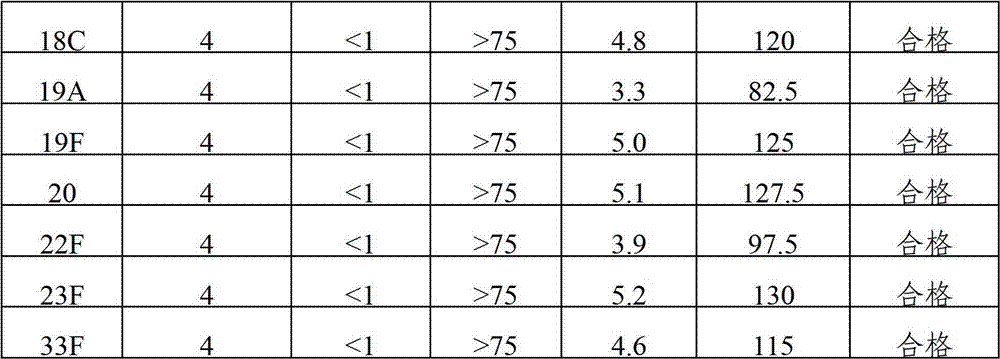
The invention provides a multivalent pneumococcal capsular polysaccharide composition as well as a preparation method and application thereof. The multivalent pneumococcal capsular polysaccharide composition contains a serotype 6A and at least one extra serotype selected from the group consisting of 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The multivalent pneumococcal capsular polysaccharide composition provided by the invention can be used for inducing an organism to generate humoral immunity, can generate a relatively good protecting effect for infectious diseases caused by the 24 common serotype pneumococcuses and is wide in immunity coverage rate and better in effect as comparison with various existing pneumococcal polysaccharide vaccines and conjugate vaccines sold on the market.
Owner:SINOVAC RES & DEV
Vaccine
InactiveUS20050214329A1Elicit immune responsePrevention and ameliorationAntibacterial agentsBacterial antigen ingredientsTGE VACCINEConjugated vaccines
The present invention provides an optimal formulation of multiple serotype Streptococcus pneumoniae conjugate vaccines.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Multivalent pneumococcus capsular polysaccharide-protein conjugated composition and preparation method thereof
InactiveCN104069488AImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseConjugate vaccine
The invention provides a multivalent pneumococcus capsular polysaccharide-protein conjugated composition and a preparation method thereof. The conjugated composition is formed by covalent linkage of multivalent pneumococcus capsular polysaccharides of 14 different serotypes and carrier protein, wherein the 14 serotypes include 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F and 33F. The conjugated composition has good adsorption effect and good stability, has multiple immunogenicity and protective performance against invasion of the pneumococcus of 14 serotypes, is superior to on-sale low-valent pneumonia compositions, and the immune response of the conjugated composition disclosed by the invention is higher than that of an uncombined composition. The inoculating injection frequency can be reduced by using the multivalent pneumococcus capsular polysaccharide conjugate vaccine containing the conjugated composition, the immune process can be simplified, and diseases of human and animals caused by the 14 serotypes of pneumococcal bacteria can be effectively prevented. The conjugated composition has wider coverage and better immune effect.
Owner:SINOVAC RES & DEV
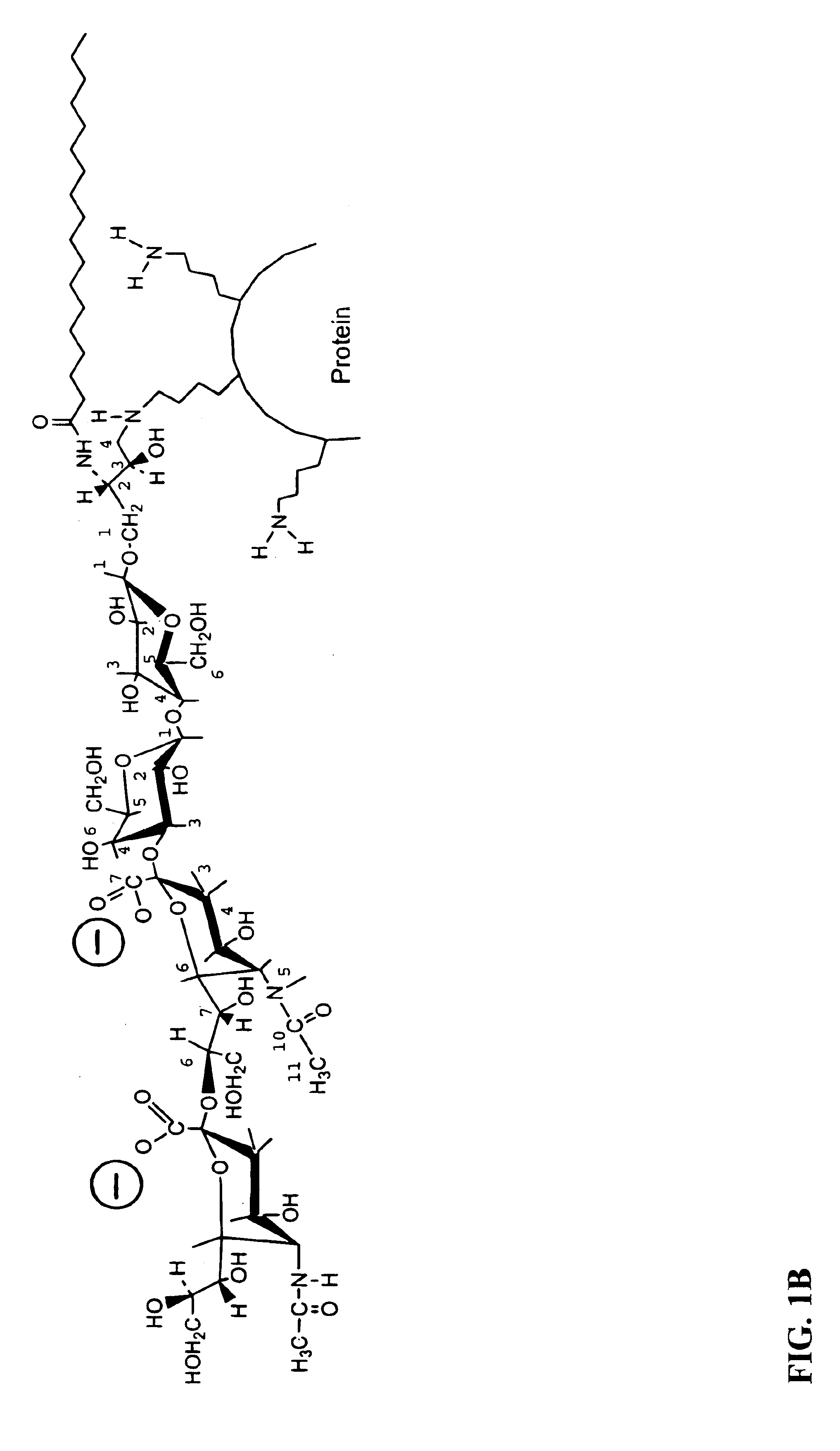
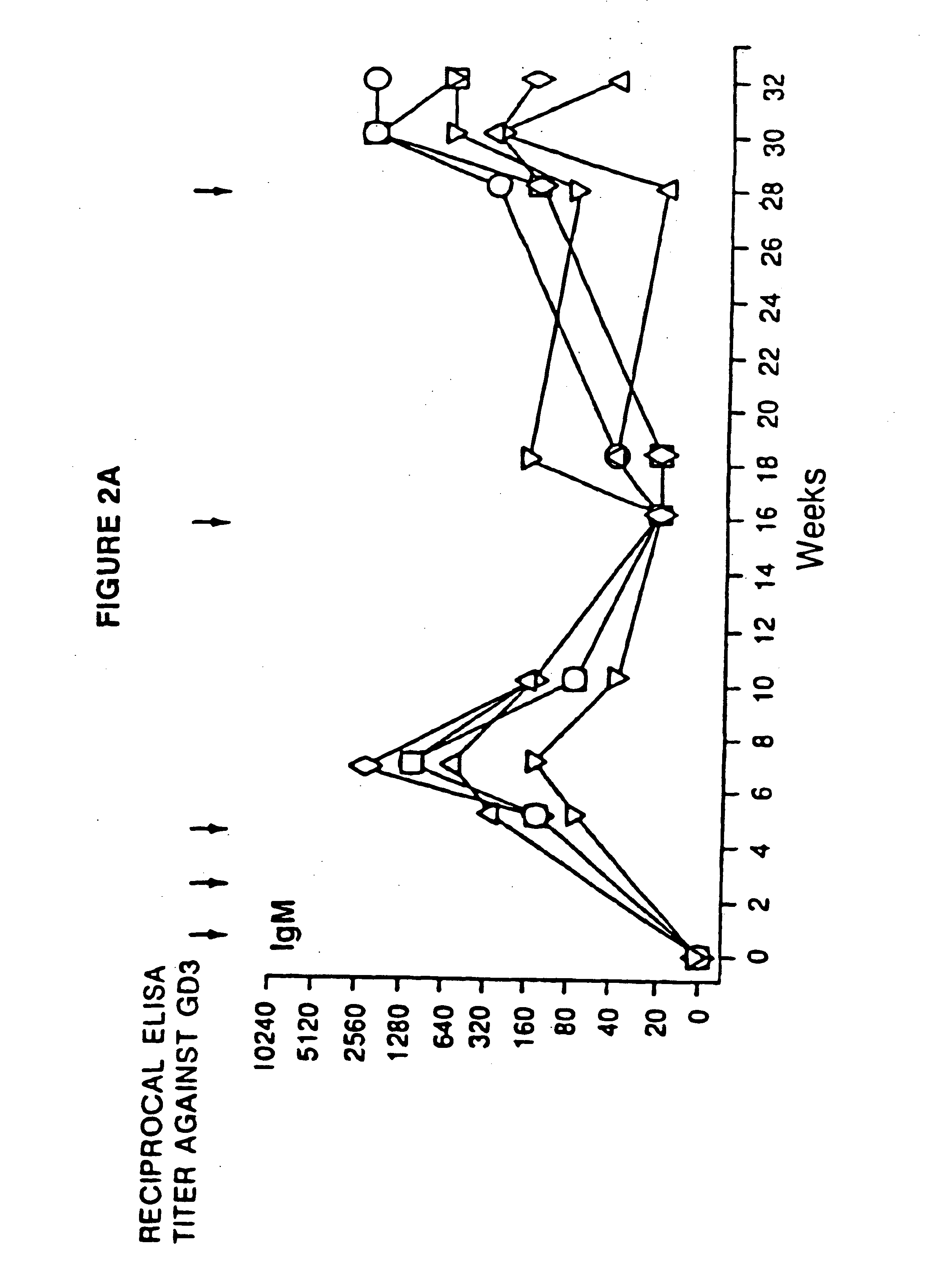
Ganglioside-KLH conjugate vaccines plus QS-21
This invention provides a vaccine for stimulating or enhancing in a subject to which the vaccine is administered, production of an antibody which recognizes a ganglioside, comprising an amount of ganglioside or oligosaccharide portion thereof conjugated to an immunogenic protein effective to stimulate or enhance antibody production in the subject, an effective amount of adjuvant and a pharmaceutically acceptable vehicle.
Owner:SLOAN KETTERING INST FOR CANCER RES
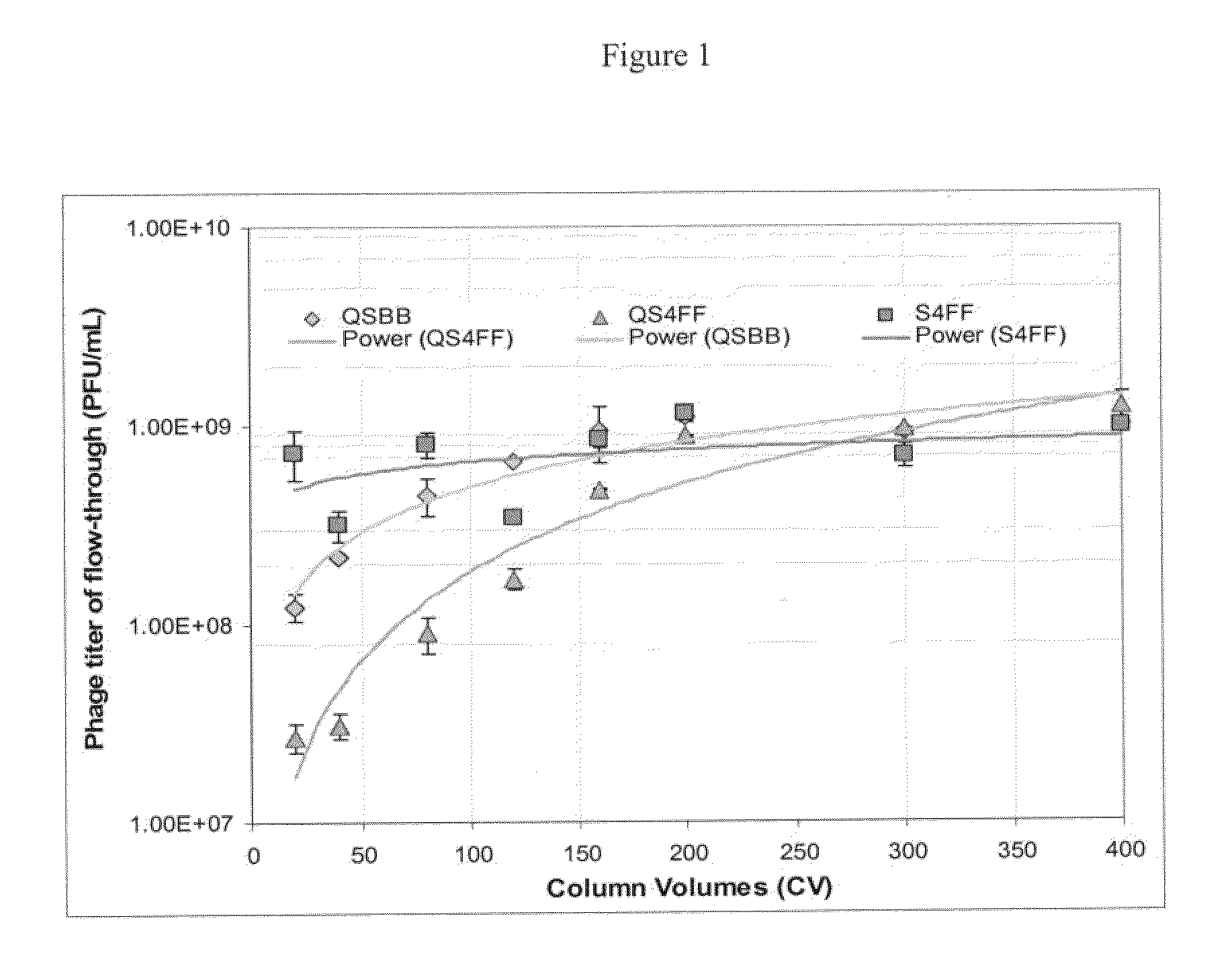
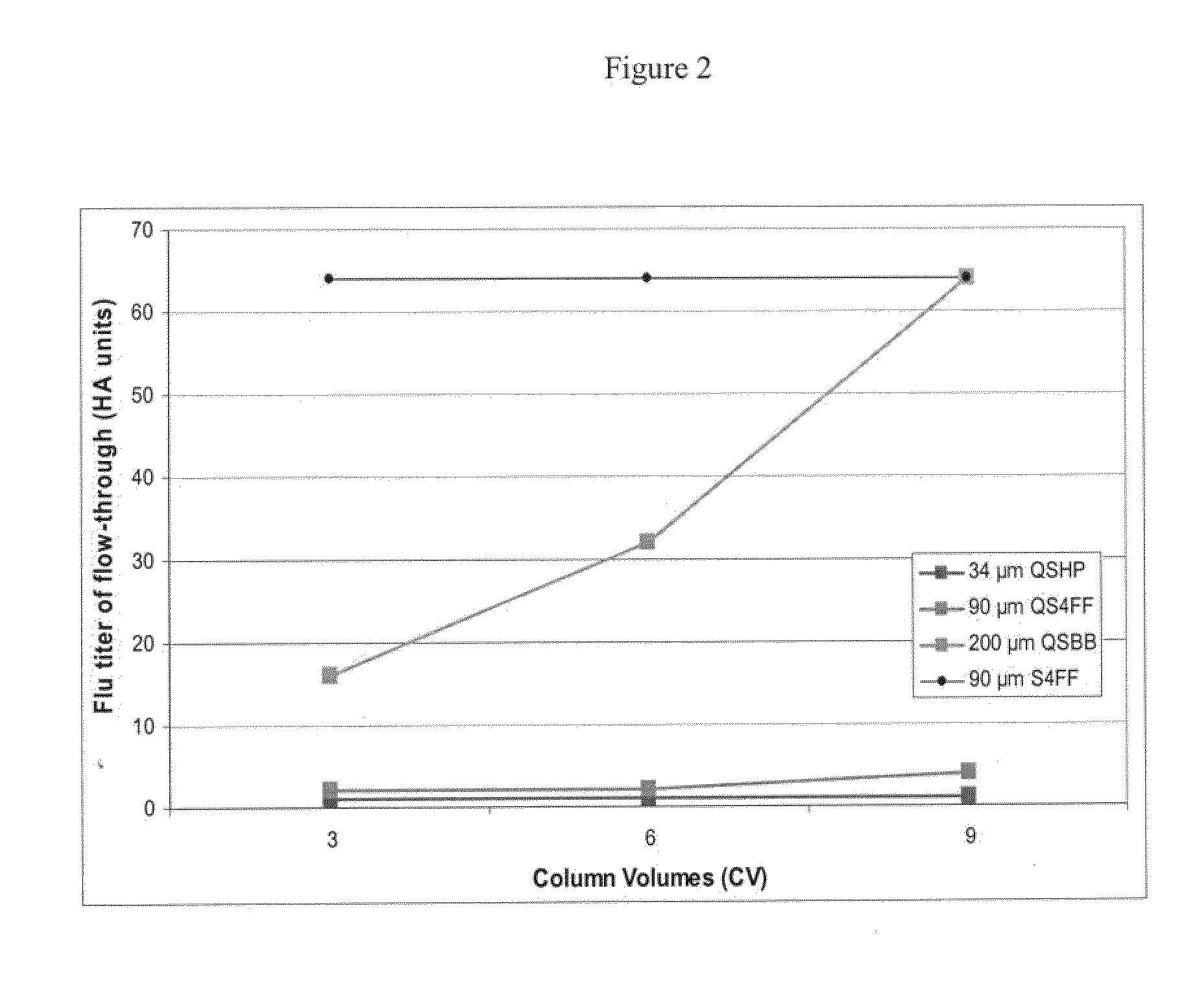
Flow through purification processes for large biomolecules
InactiveUS20110142863A1Maximum recoveryPromote recoverySsRNA viruses negative-senseSnake antigen ingredientsConjugate vaccinePurification methods
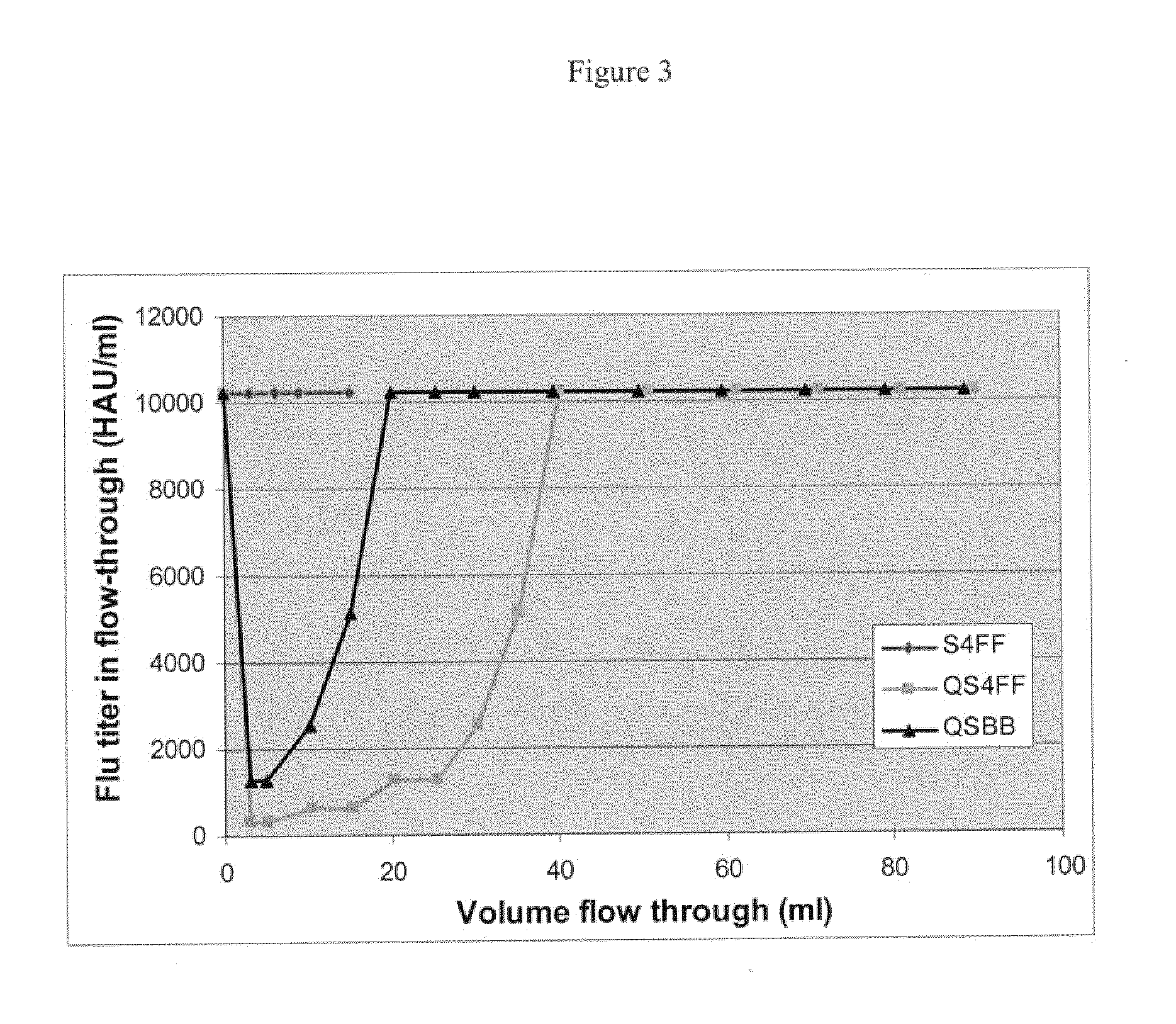
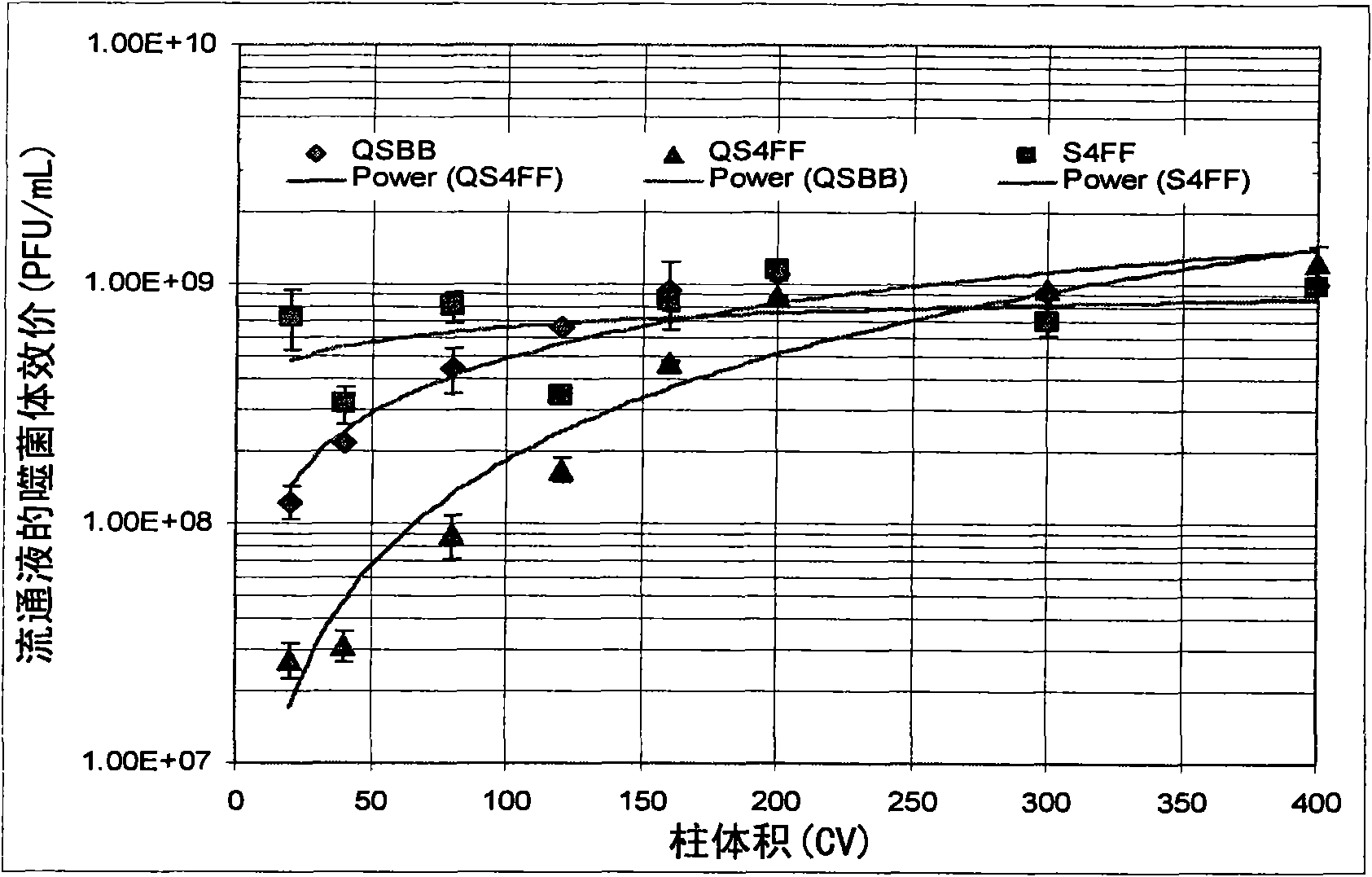
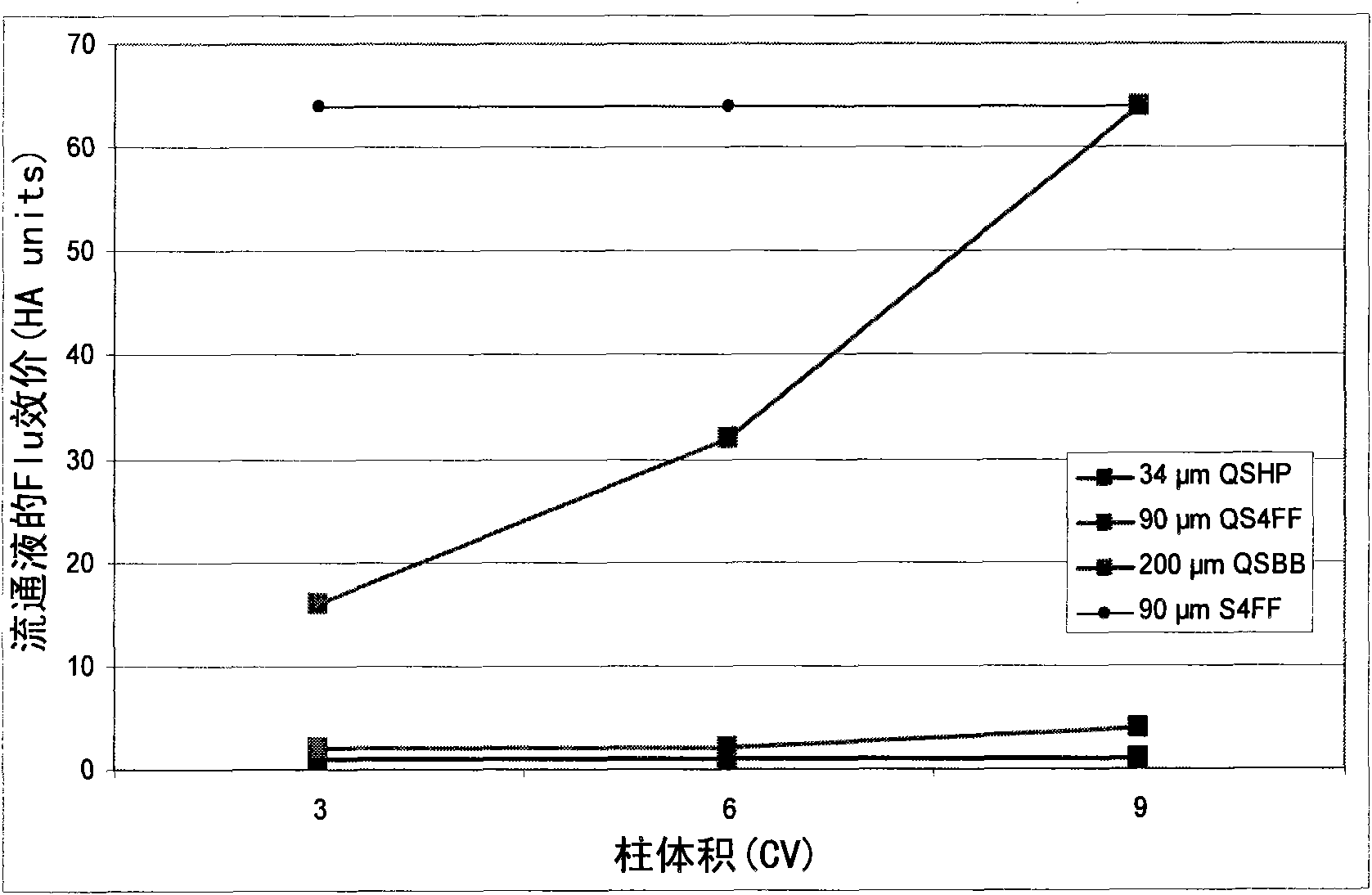
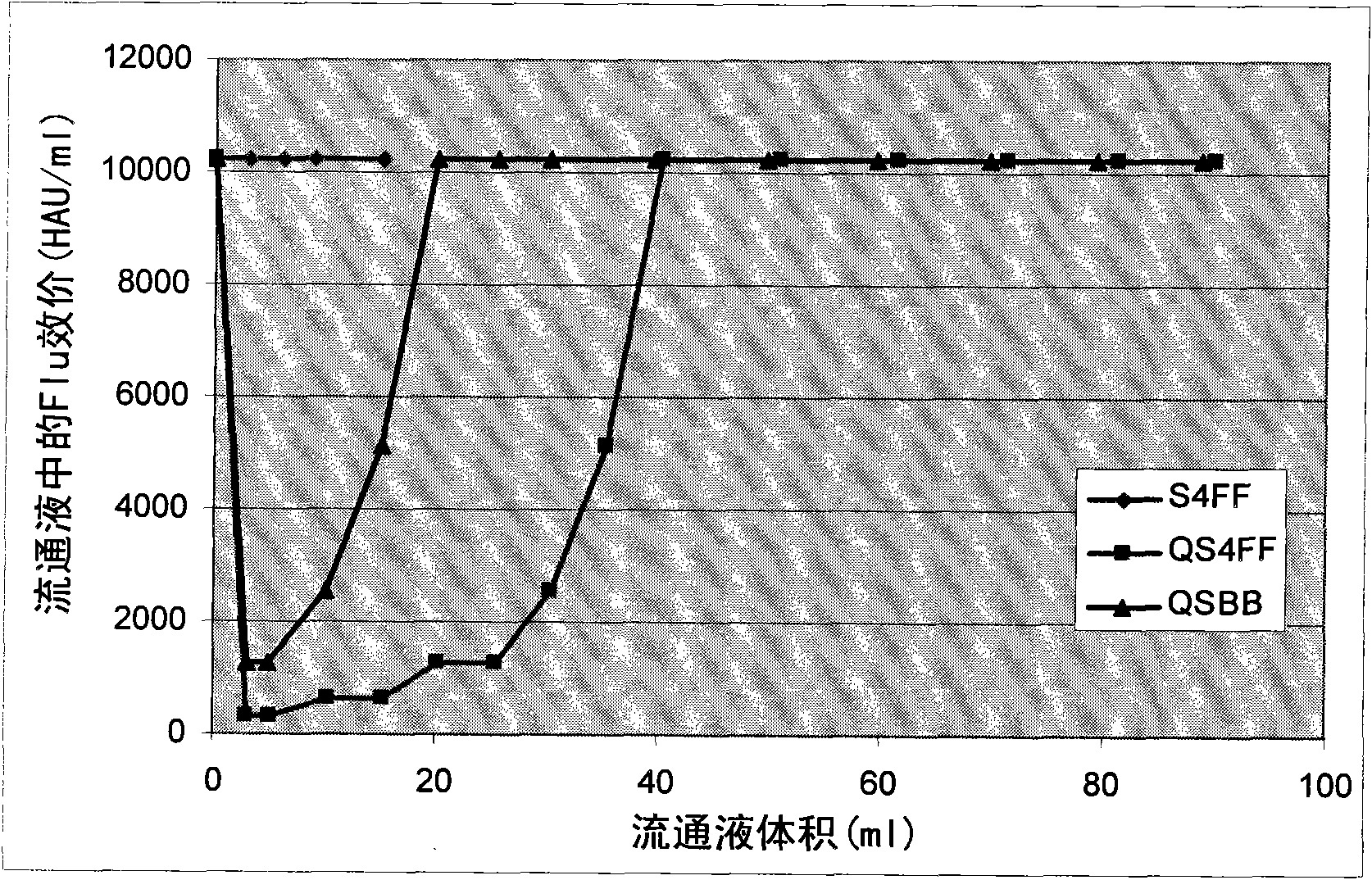
The present invention relates, at least in part, to novel and improved flow-through purification processes for separating large biomolecules, such as, for example, encapsulated viruses, virus-like particles and conjugate vaccines from one or more contaminants in a sample, where the process employs the use of at least one population of a solid porous particle which comprises a minimized external surface area per unit volume of the particles and an internal surface area per unit volume which is not decreased by more than 25% relative to a population of a similar particle which does not have a minimized external surface area.
Owner:MILLIPORE CORP
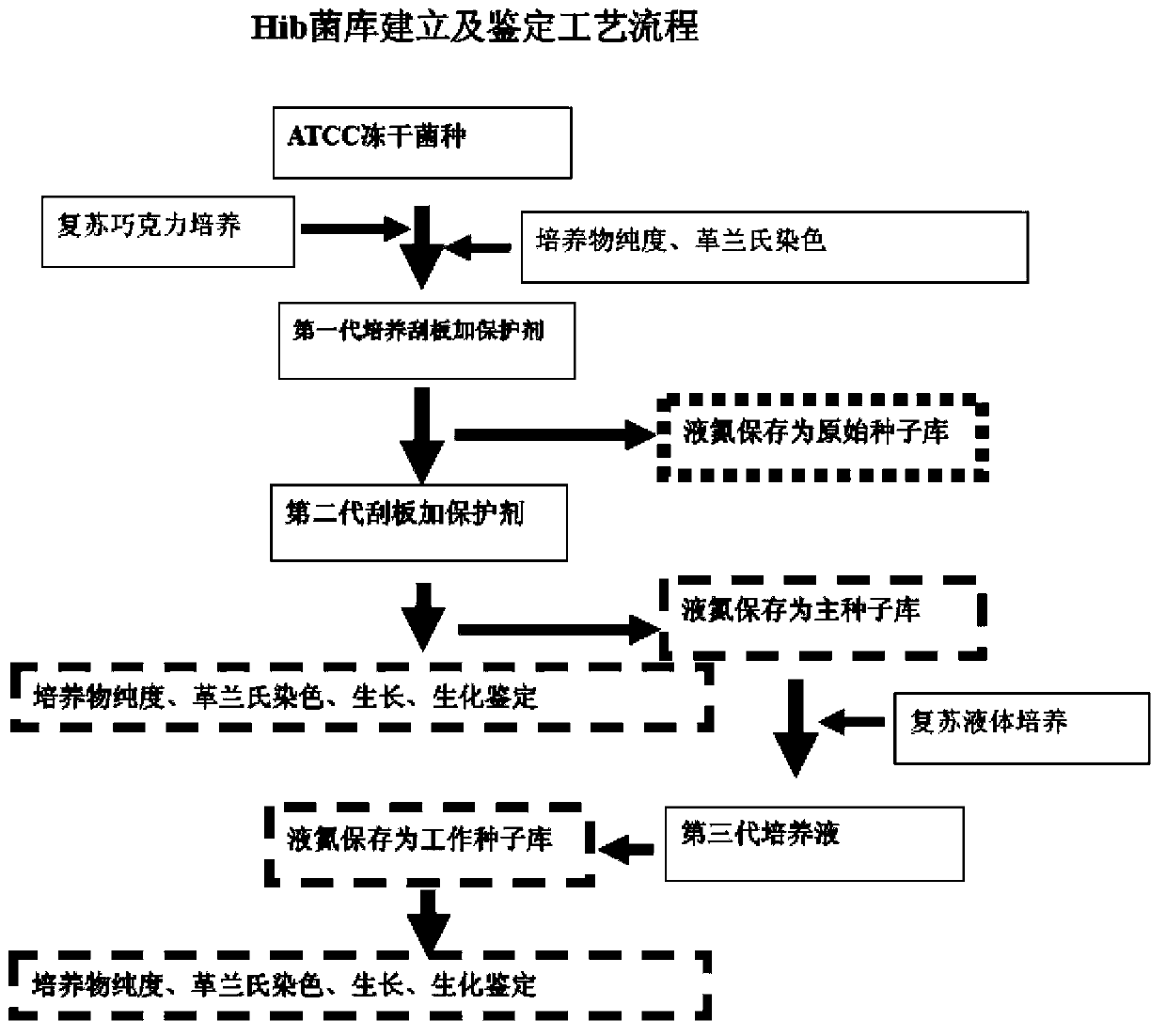
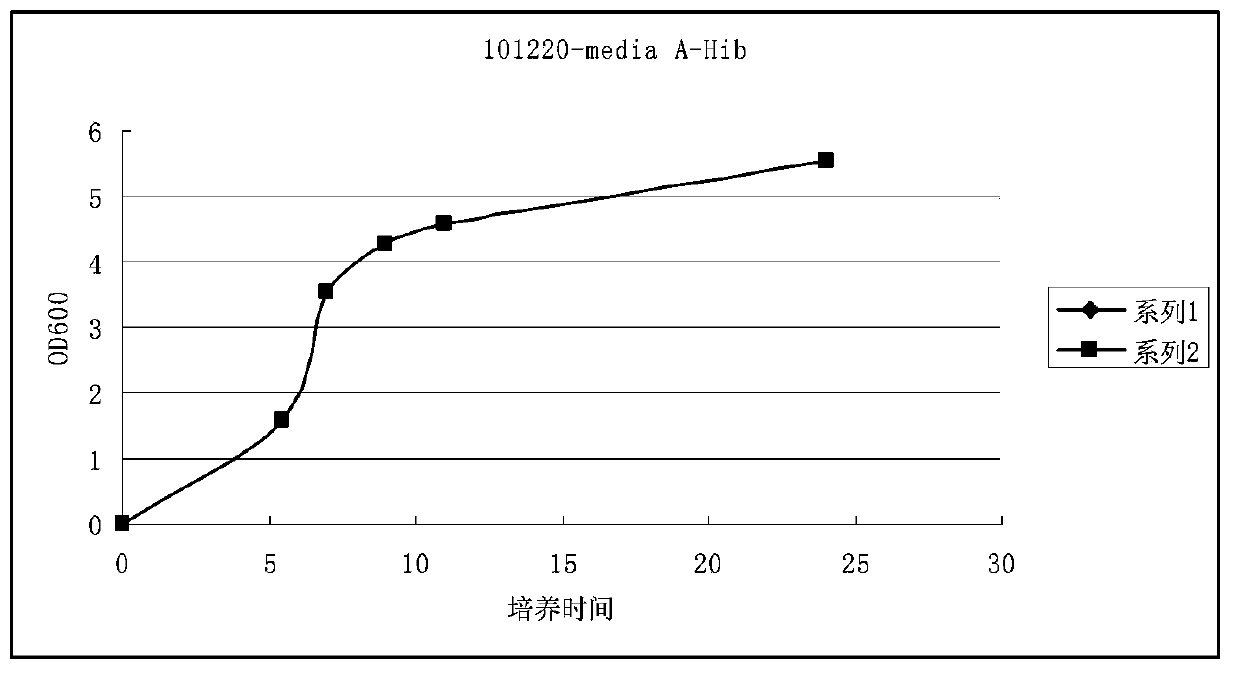
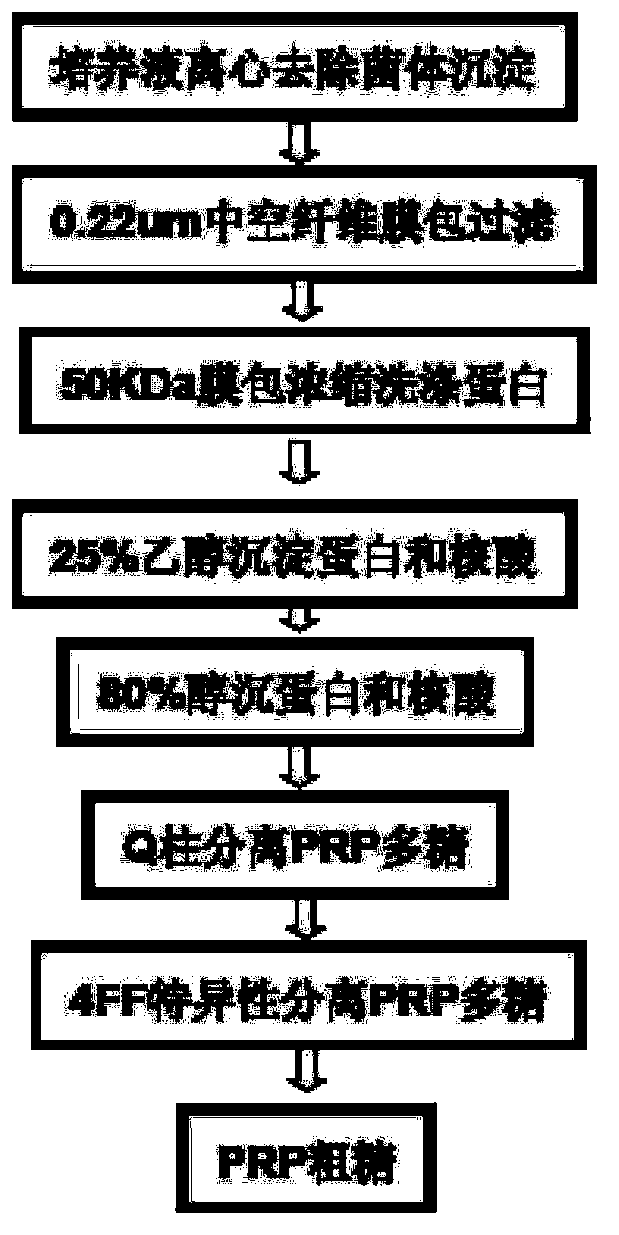
Haemophilus influenzac type B polysaccharide conjugate vaccine preparation method
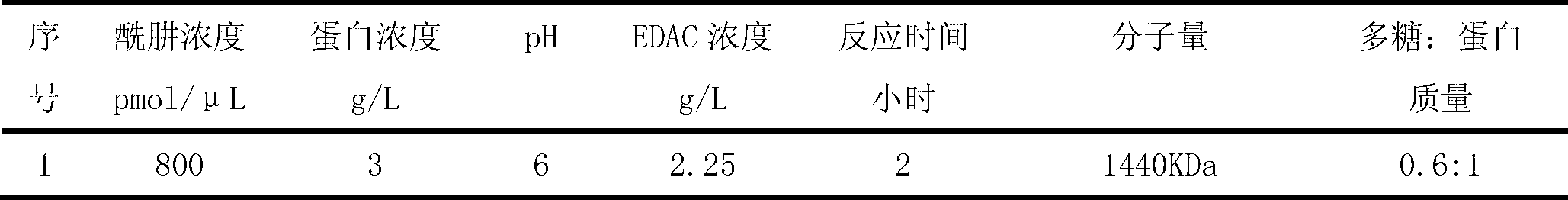
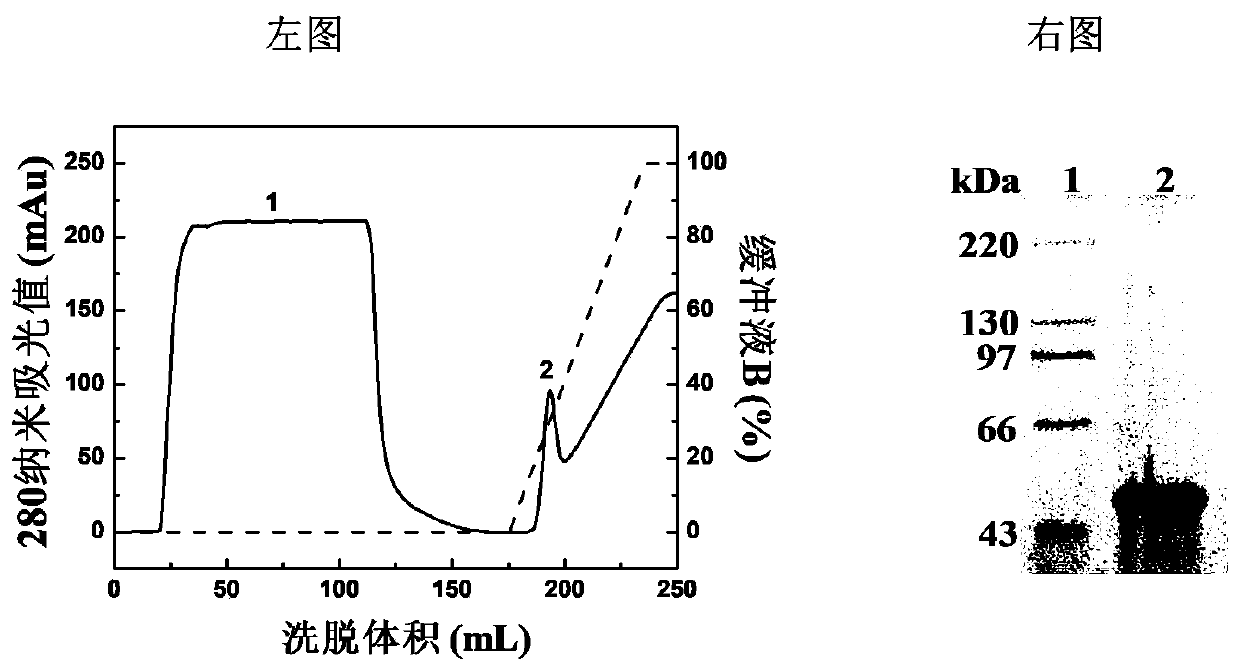
The present invention discloses a Haemophilus influenzac type B polysaccharide conjugate vaccine preparation method, which comprises: A, preparing a Haemophilus influenzac type B polysaccharide extract (PRP), adopting ultrapure water or water for injection to dissolve the PRP polysaccharide at a room temperature until achieving a concentration of 20 mg / ml, adding CDAP according to a mass ratio of PRP to CDAP of 1, maintaining for 1.5 min, adjusting the pH value to 9.5 with 0.3 M NaOH, maintaining for 3 min, adding a solid ADH according to a mass ratio of ADH to the PRP polysaccharide of 4:1, adjusting the pH value to 9.5 with 0.3 M NaOH, maintaining for 1 h, and carrying out dialysis at a temperature of 2-8 DEG C with 0.2 M NaCl; and B, mixing the obtained PRP-AH and a 20 mg / ml TT solution according to a mass ratio of 1:2, adjusting the pH value to 5.0 with 0.1 M HCL, adding a solid EDAC according to a mass ratio of EDAC to PRP of 10:1, maintaining the pH value of 5.0 for 60 min at a room temperature, adjusting the pH value to 7.5 with 0.3 M NaOH, and carrying out a reaction for 30 min.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
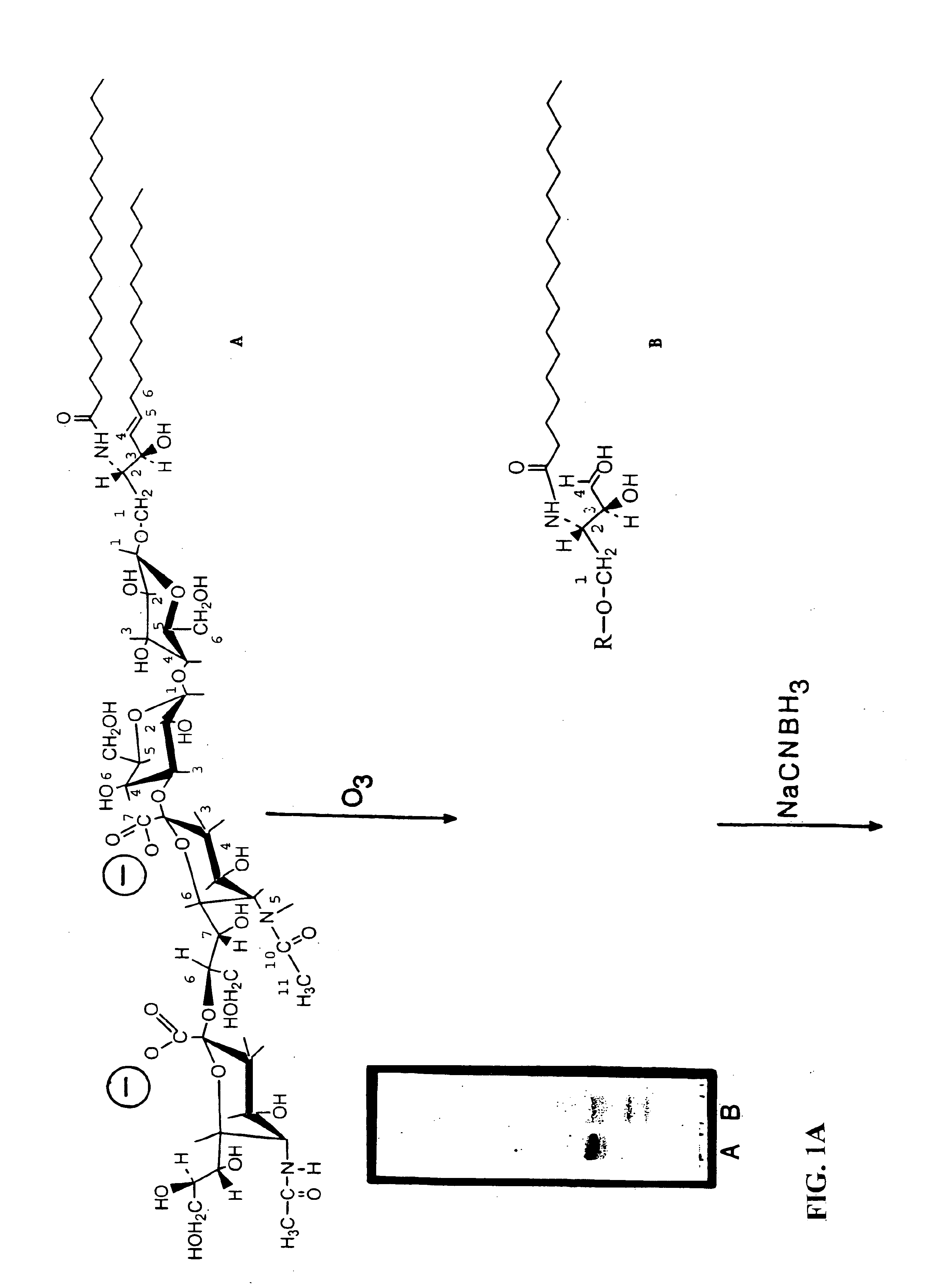
Modified Polysaccharides for Conjugate Vaccines
InactiveUS20130197203A1Good antigenicityContribute to the valency of the vaccineAntibacterial agentsDepsipeptidesCarrier proteinImmunogenicity
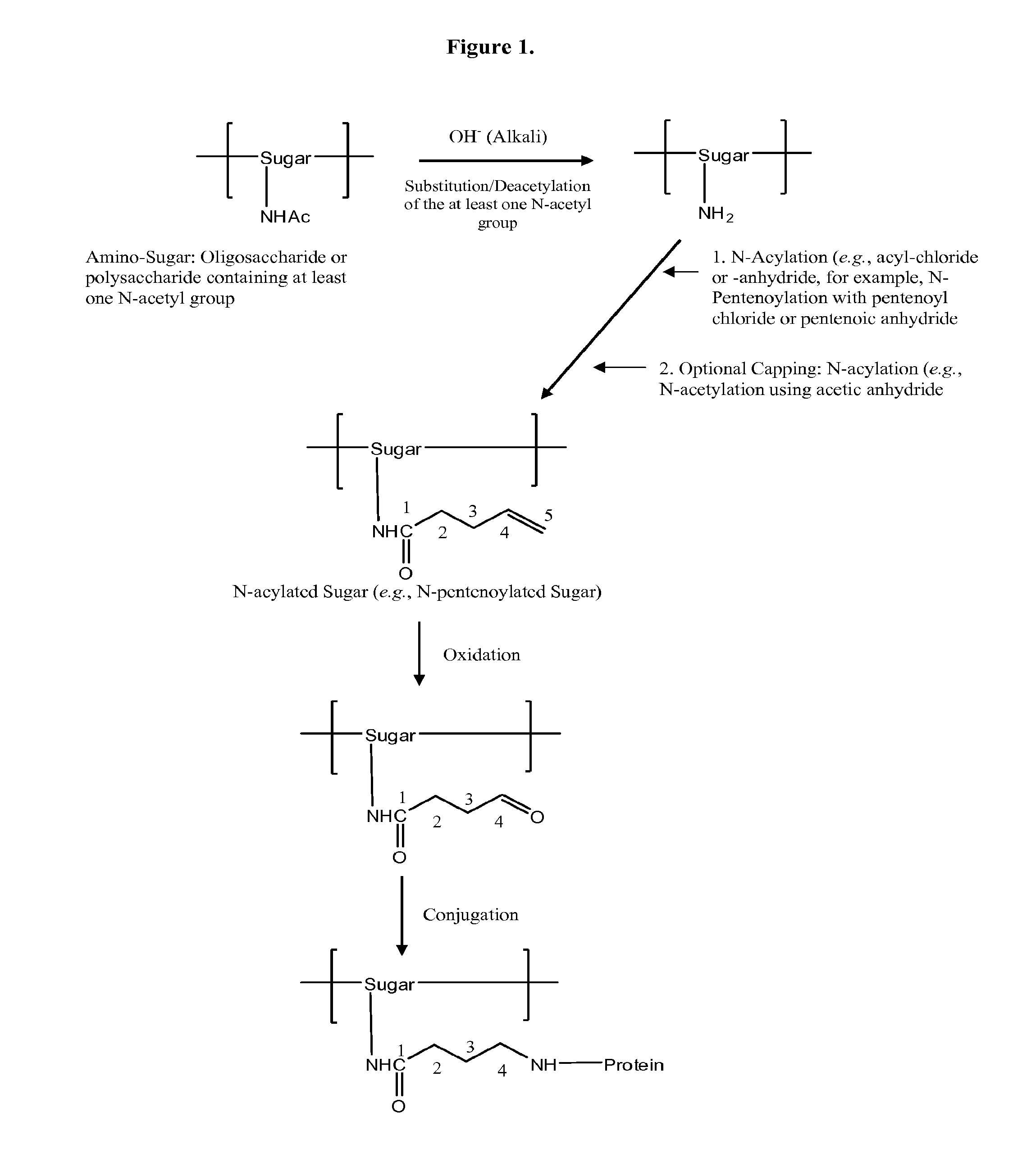
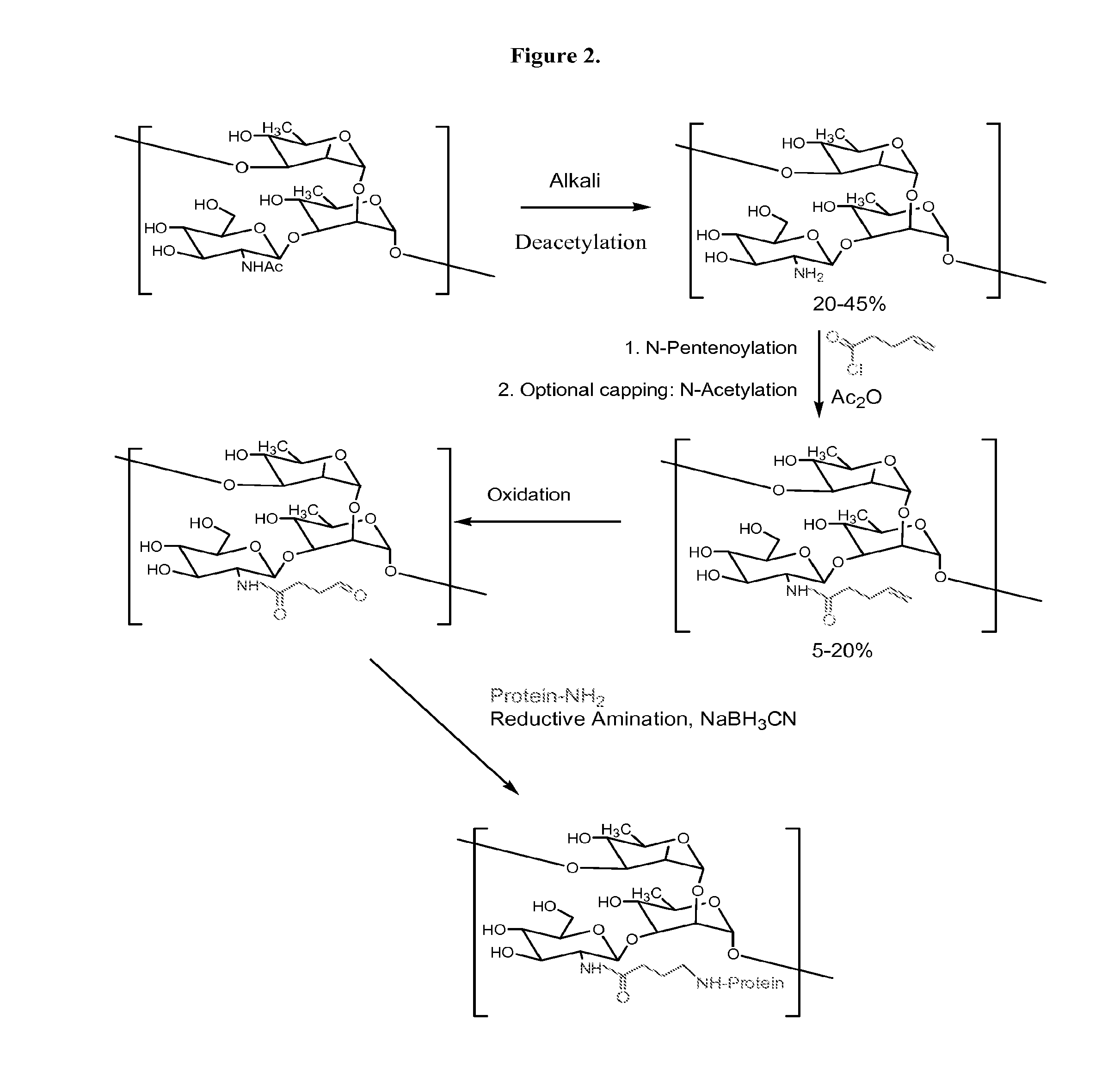
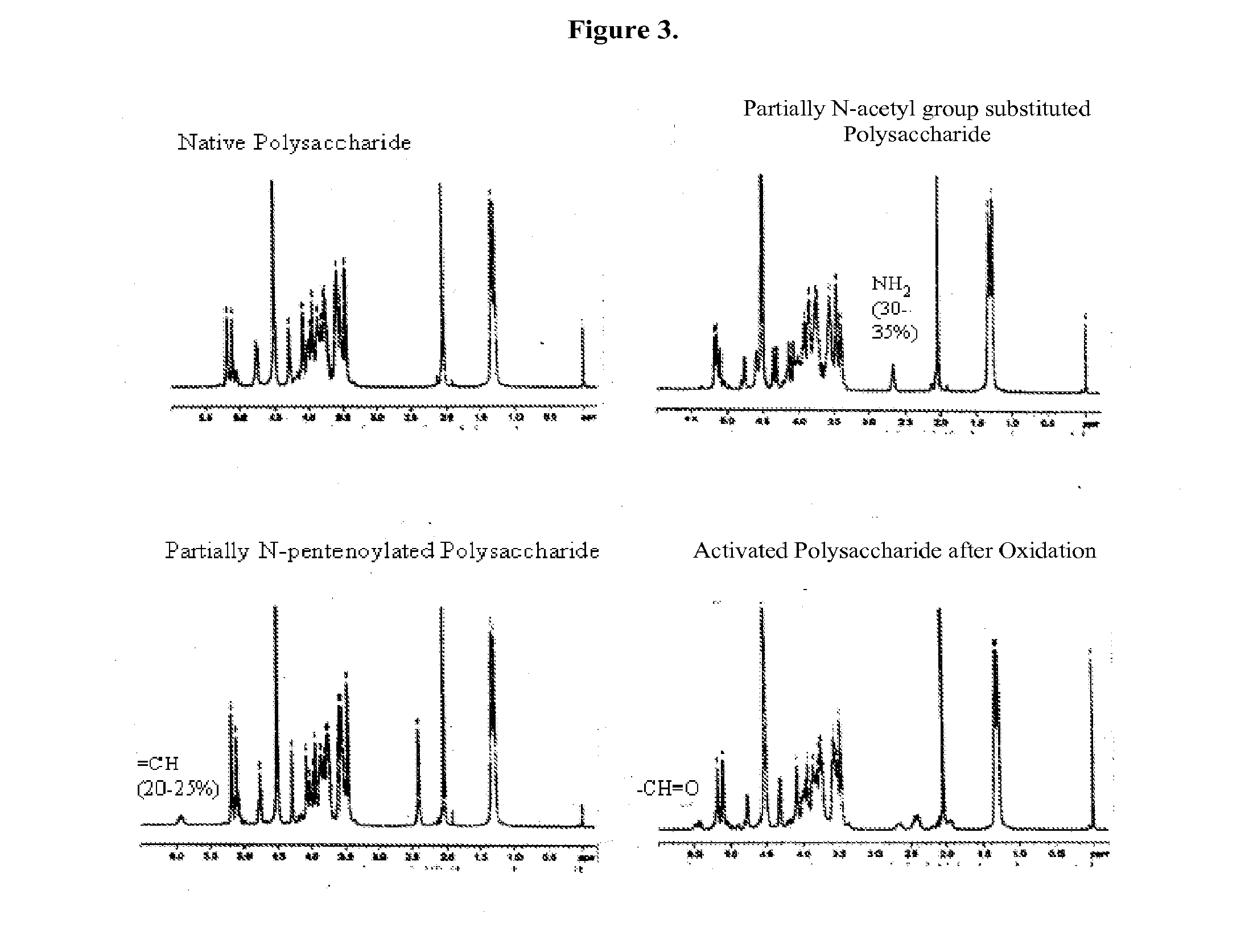
The present invention relates to methods of manufacture of immunogenic glycoconjugates, in particular for use in pharmaceutical compositions for inducing a therapeutic immune response in a subject. The immunogenic glycoconjugates of the invention comprise one or more oligosaccharides or polysaccharides that are conjugated to one or more carrier proteins via an active aldehyde group. Accordingly, the invention provides methods of making (i) unsaturated microbial N-acyl derivative oligosaccharides or polysaccharides; (ii) novel conjugates of unsaturated N-acyl derivatives; and (iii) glycoconjugate compositions comprising conjugate molecules of fragments of microbial unsaturated N-acyl derivatives that serve as a covalent linker to one or more proteins. The invention further encompasses the use of the immunogenic glycoconjugates pharmaceutical compositions for the prevention or treatment of an infectious disease.
Owner:PFIZER IRELAND PHARM CORP
Carrier protein of bacterial polysaccharide conjugate vaccine and application thereof
ActiveCN106511994AUniform polysaccharide binding siteQuality controllableAntibacterial agentsImmunoglobulins against bacteriaConjugate vaccineCarrier protein
The invention discloses a bacterial polysaccharide O-glycosylation modified recombinant cholera toxin B subunit fusion protein and an application thereof. The present invention provides a conjugate of polysaccharide and protein which is coupled by the bacterial polysaccharide and recombinant cholera toxin B subunit fusion protein. The bacterial polysaccharide is connected with the glycosylation sites of the recombinant cholera toxin B subunit fusion protein in the form of O-glucosidic bond. The experiment indicates that the preparation of the bacterial polysaccharide protein conjugate vaccine from O-glycosylation modified recombinant cholera toxin B subunit fusion protein can enhance the ability of inducing animals to generate anti-polysaccharide antibodies, avoid miscellaneous problems of pathogen culture, enhance the vaccine homogeneity and production efficiency, and reduce the vaccine preparation cost, therefore possessing wide application prospect.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Polyvalent pneumococcus polysaccharide conjugate vaccine and preparation method thereof
InactiveCN108144056AImmunogenicity hasAntibacterial agentsBacterial antigen ingredientsProtein compositionStreptococcus mitis
The invention provides a polyvalent pneumococcus polysaccharide conjugate vaccine and a preparation method thereof. For a polysaccharide-protein conjugate with immunogenicity, polysaccharide coming from streptococcus pneumoniae and protein form the conjugate, wherein the polysaccharide belongs to capsular polysaccharide with the serotype being 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 16F, 17F, 18C, 19A, 19F, 20, 20F, 23A, 23F and 33F. For the conjugate provided by the invention, the serum coverage rate achieves 90% or above, and a protection effect can be generated on people of 2 months or older.
Owner:BRAVOVAX
Analysis of Saccharide Vaccines Without Interference
InactiveUS20080286300A1Improve quality controlQuicker and easy to performAntibacterial agentsRadiation pyrometryConjugate vaccineSialic acid
The invention is based on methods that allow analysis of mixed meningococcal saccharides from multiple serogroups even though they share monosaccharide units. With a combination of saccharides from serogroups C, W135 and Y, the invention analyses sialic acid, glucose and galactose content. The glucose and galactose results are used to directly quantify saccharides from serogroups Y and W135, respectively, and the combined glucose and galactose content is subtracted from the sialic acid content to quantify saccharides from serogroup C. The three serogroups can thus be resolved even though their monosaccharide contents overlap. The three different monosaccharide analyses can be performed on the same material, without interference between the monosaccharides and without interference from any other saccharide materials in the composition (e.g. lyophilisation stabilisers). The method can be used to analyse total and free saccharide in conjugate vaccines and simplifies quality control of vaccines containing capsular saccharides from multiple serogroups.
Owner:NOVARTIS AG
Attenuated Salmonella enterica serovar paratyphi A and uses thereof
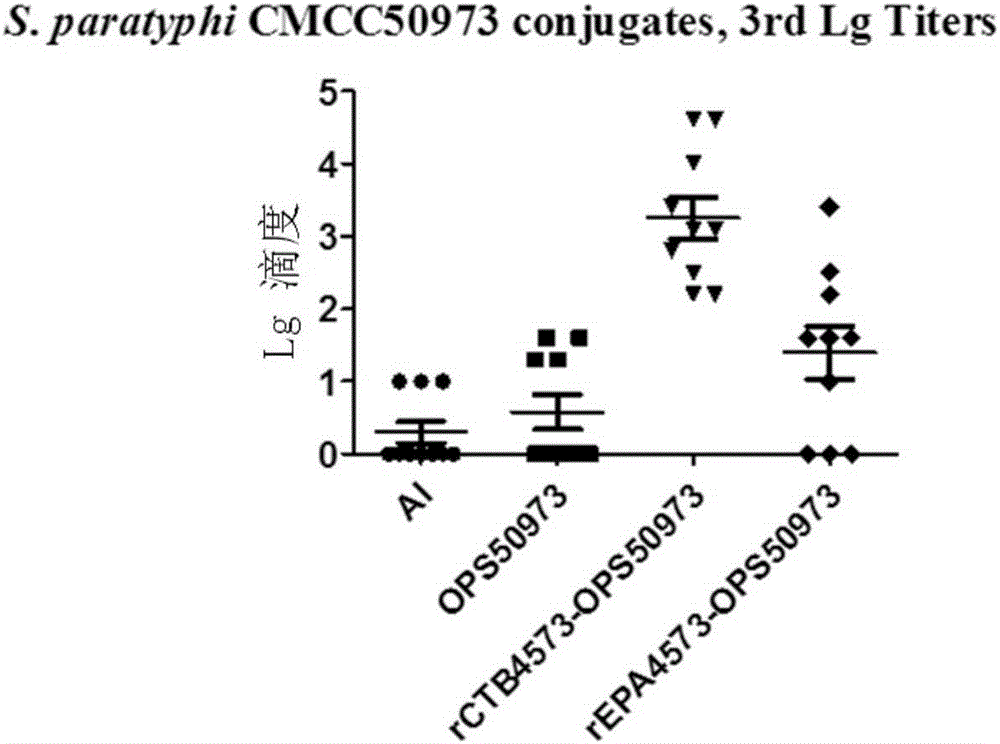
ActiveUS8137930B2Peptide preparation methodsDepsipeptidesSalmonella enterica serovar Paratyphi AConjugate vaccine
The present invention is drawn to a live, attenuated S. Paratyphi A strain, a live, attenuated S. Paratyphi A strain comprising a stabilized plasmid expression system, an S. Paratyphi conjugate vaccine, and methods of using these strains and conjugate vaccine.
Owner:MARYLAND UNIV OF
Saccharide Conjugate Vaccines
InactiveUS20150165019A1Improve bioavailabilityImprove efficacyAntibacterial agentsPeptide/protein ingredientsCarrier proteinTGE VACCINE
The invention provides compositions comprising a combination of two or more monovalent conjugates, each of said two or more monovalent conjugates comprising a carrier protein comprising T cell epitopes from two or more pathogens conjugated to saccharide antigen. The invention also provides a multivalent conjugate comprising two or more antigenically distinct saccharide antigens conjugated to the same carrier protein molecule, wherein the carrier protein comprises T cell epitopes from two or more pathogens. Further compositions comprise one or more of said monovalent conjugates and one or more of said multivalent conjugates. The invention further provides methods for making said compositions and uses for said compositions.
Owner:NOVARTIS AG
Meningitis polysaccharide conjugate vaccine and preparing method thereof
InactiveCN103690944AImproving immunogenicityRelieve painAntibacterial agentsCarrier-bound antigen/hapten ingredientsConjugate vaccineEpitope
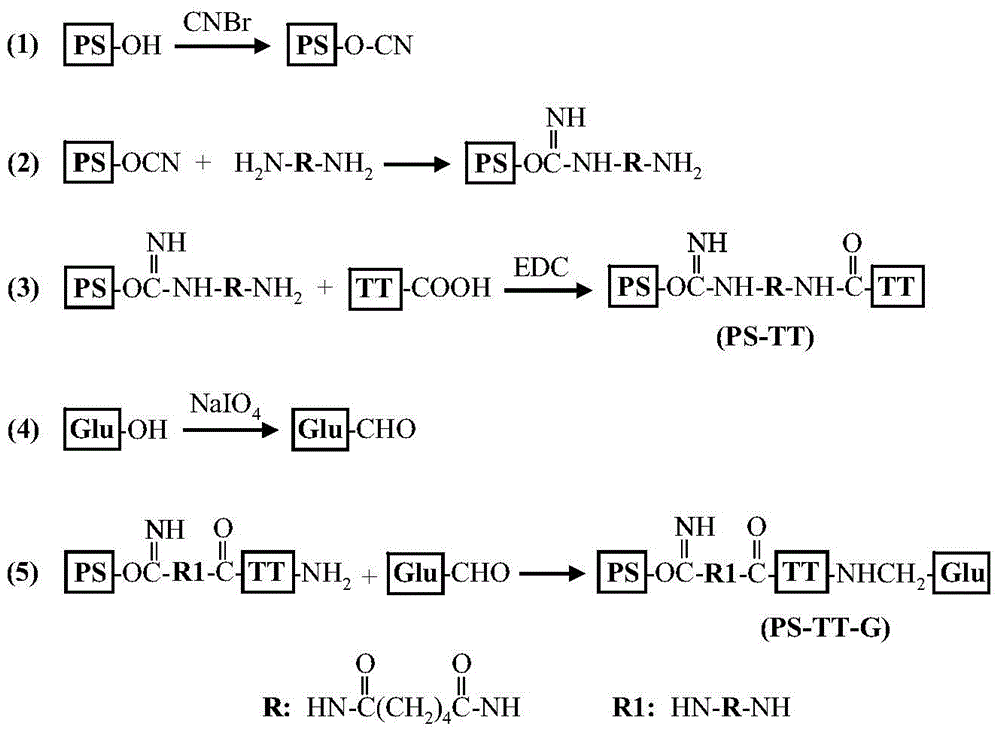
The invention describes a method for preparing a meningitis polysaccharide conjugate vaccine. The meningitis polysaccharide conjugate vaccine is developed based on the method. The method comprises the following steps: (1) activating meningococcus polysaccharides by cyanogen bromide, then deriving the meningococcus polysaccharides which are activated by the cyanogen bromide by ethanediamine, finally deriving the polysaccharides by a reagent which is of a structure of succinimidyl ester-R-maleimide; (2), sulfhydrylating carrier proteins; (3) combining the derived meningococcus polysaccharides with the sulfhydrylated carrier proteins. As conjugation bridges between the meningococcus polysaccharides and the carrier proteins are very long, the spatial shielding effect of the carrier proteins on the antigenic epitopes of the polysaccharides is reduced, and the original immunization property of the polysaccharide conjugate vaccine is improved.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Ganglioslide-KLH conjugate vaccine plus QS-21
This invention provides a vaccine for stimulating or enhancing in a subject to which the vaccine is administered, production of an antibody which recognizes a ganglioside, comprising an amount of ganglioside or oligosaccharide portion thereof conjugated to an immunogenic protein effective to stimulate or enhance antibody production in the subject, an effective amount of adjuvant and a pharmaceutically acceptable vehicle.
Owner:SLOAN KETTERING INST FOR CANCER RES
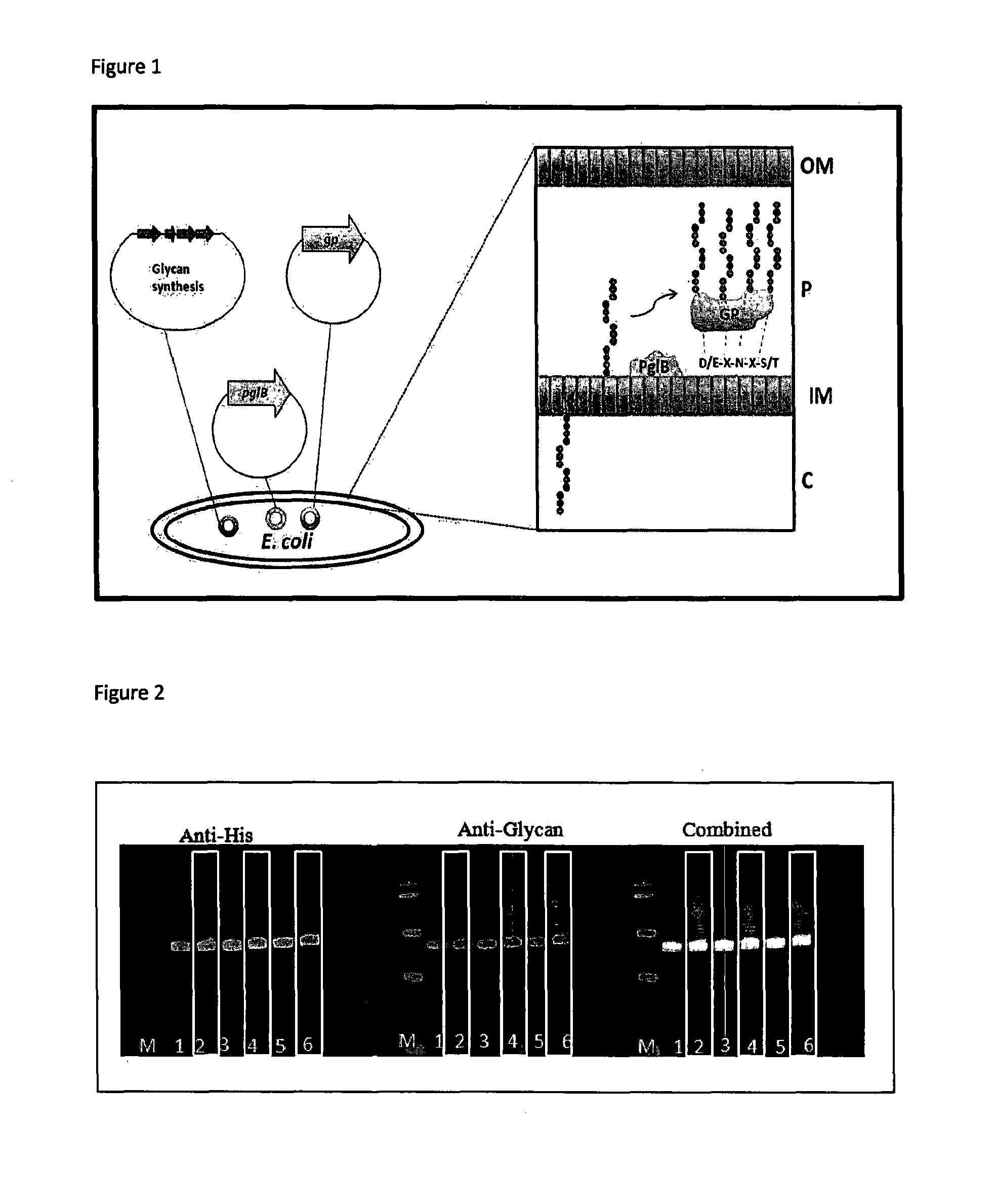
Glycosylation method
ActiveUS20150344928A1Maintain stabilityIncrease typeBacteriaMicrobiological testing/measurementBiotechnologyHost genome
The invention relates to microbial host cells engineered to produce glycoconjugate vaccines by stable integration of an acceptor protein and an oligosaccharyltransferase into the host's genome, wherein expression of the oligosaccharyltransferase is regulated.
Owner:LONDON SCHOOL OF HYGIENE & TROPICAL MEDICINE
Beta-glucan modified meningitis polysaccharide conjugate vaccine and preparation method thereof
ActiveCN104548090AImproving immunogenicityReduce the number of vaccinationsAntibacterial agentsCarrier-bound antigen/hapten ingredientsCyanogen bromideMeningococcal meningitis
The invention relates to a beta-glucan modified meningitis polysaccharide conjugate vaccine and a preparation method thereof. The preparation method of the beta-glucan modified meningitis polysaccharide conjugate vaccine comprises the following steps: (1) activating meningococcus polysaccharide by cyanogen bromide, and then deriving by adopting adipic dihydrazide; (2) combining a derived meningococcus polysaccharide derivative with carrier protein; (3) activating beta-glucan; and (4) modifying polysaccharide-protein conjugate by the activated beta-glucan. By virtue of the steps, a novel and efficient meningitis polysaccharide conjugate vaccine can be prepared and can be used for preventing infection caused by epidemic cerebrospinal meningitis Neisseria gonorrhoeae.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Vaccine for preventing and treating diseases caused by Acinetobacter baumannii and preparation method thereof
ActiveCN109420165AImprove uniformityIncrease productivityAntibacterial agentsPolypeptide with localisation/targeting motifDiseaseConjugate vaccine
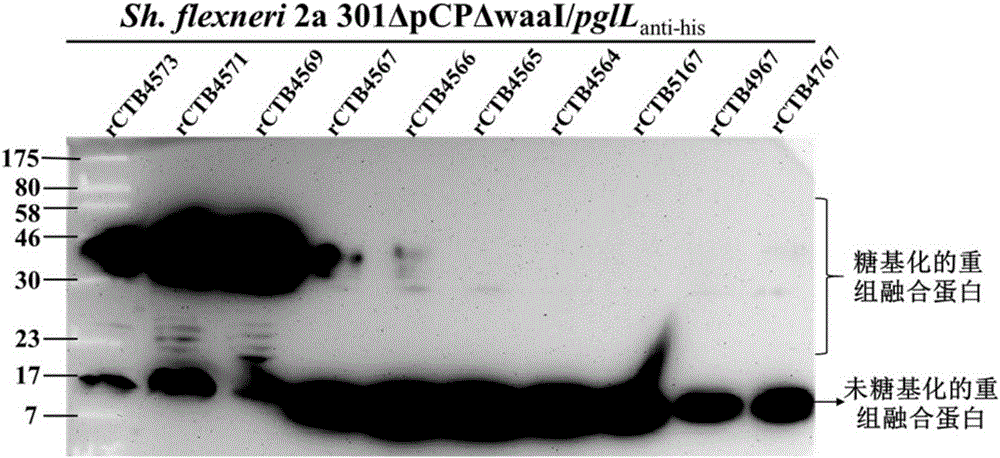
The invention discloses a vaccine for preventing and treating diseases caused by Acinetobacter baumannii and a preparation method thereof. The vaccine for preventing and treating the diseases caused by the Acinetobacter baumannii provided by the invention has an active component which is an Acinetobacter baumannii glycoprotein obtained through glycosylation of a protein composed of bases from site20 to site 156 of SEQ ID No. 1 or bases from site 1 to site 156 of SEQ ID No. 1. According to the invention, Acinetobacter baumannii glycoprotein modified rCTB4573 prepared by a genetic engineering method is used for preparation of an Acinetobacter baumannii glycoprotein protein conjugate vaccine; uniformity of the vaccine can be improved; production efficiency of the vaccine is improved; the cost is reduced; extensive application prospect is achieved; and the vaccine can be used for preventing the diseases caused by pathogenic Acinetobacter baumannii.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Flow through purification processes for large biomolecules
InactiveCN102154228AEasy to recycleSsRNA viruses negative-senseRecovery/purificationPurification methodsMulti pollutant
Owner:MILLIPORE CORP
Low-dose multivalent conjugated vaccine composition and application
InactiveCN103007268AReduce manufacturing costImprove securityAntibacterial agentsBacterial antigen ingredientsConjugate vaccineVaccination
The invention discloses a low-dose multivalent conjugated vaccine composition and application. The low-dose multivalent conjugated vaccine composition comprises group-A meningococcus capsular polysaccharide-carrier protein conjugate with group-A meningococcus capsular polysaccharide between 2.0 and 4.8mu g, group-C meningococcus capsular polysaccharide-carrier protein conjugate with group-C meningococcus capsular polysaccharide between 2.0 and 4.8mu g, group-Y meningococcus capsular polysaccharide-carrier protein conjugate with group-Y meningococcus capsular polysaccharide between 2.0 and 4.8mu g, group-W135 meningococcus capsular polysaccharide-carrier protein conjugate with group-W135 meningococcus capsular polysaccharide between 2.0 and 4.8mu g, and type-B haemophilus influenzae capsular polysaccharide-carrier protein conjugate with type-B haemophilus influenzae capsular polysaccharide between 2.0 and 4.8mu g. According to the low-dose multivalent conjugated vaccine composition, the production cost can be reduced, and the content of impurities with toxic and side effects in the product can be reduced, so that the safety of the vaccine can be improved, the number of vaccination times for infants can be reduced, and the vaccination omission rate can be reduced.
Owner:CANSINO BIOLOGICS INC
Antimultiorganism Glycoconjugate vaccine
InactiveUS20050158346A1Enhanced interactionPromotes an increase in inductionBacterial antigen ingredientsSnake antigen ingredientsAntiendomysial antibodiesGlycoconjugate
The present invention relates, e.g., to a glycoconjugate composition comprising one or more polysaccharide types from a cell wall polysaccharide preparation from B. pumilus Sh 18, or variants thereof. Also disclosed are antibodies generated against the glycoconjugates, and methods of using the glycoconjugates and antibodies. An antimultiorganism vaccine which reacts against at least Haemophilus influenzae type a, Haemophilus influenzae type b, Staphylococcus aureus, and Staphylococcus epidermidis, is disclosed.
Owner:UNITED STATES OF AMERICA
Process for activating Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine
ActiveCN102626515AAvoid harmImprove securityAntibacterial agentsCarrier-bound antigen/hapten ingredientsTetrafluoroborateFreeze-drying
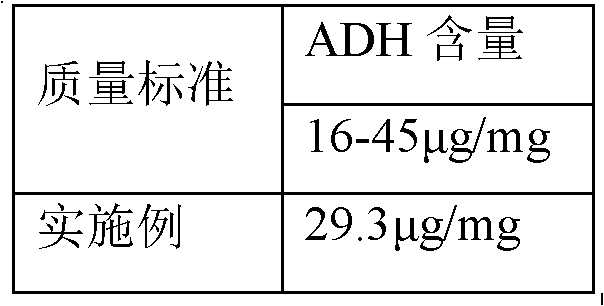
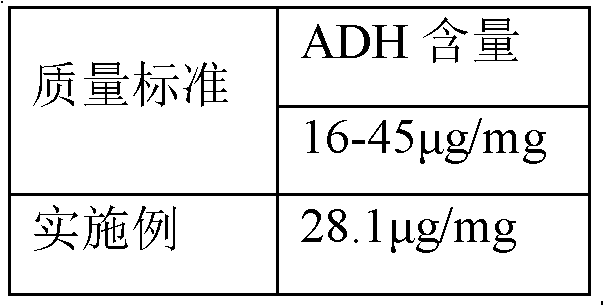
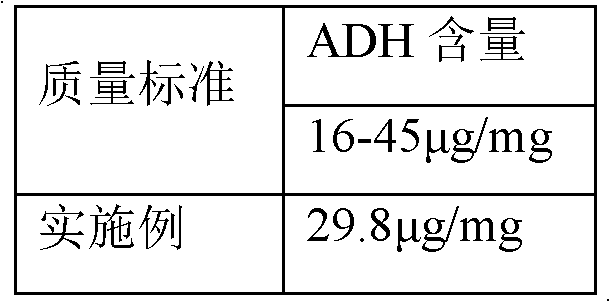
The invention discloses a process for activating a Haemophilus influenzae type b (Hib) polysaccharide conjugate vaccine, which comprises the following steps of: A, preparing Hib polysaccharide; B, dissolving 1-Cyano-4-dimethylaminopyridinium tetrafluoroborate (CDAP) by using acetonitrile into a solution; C, preparing the Hib polysaccharide into a solution; D, adding the CDAP solution to the Hib polysaccharide solution, and stirring for 2-5 minutes at the room temperature; E, dissolving adipic dihydrazide (ADH) by using NaHCO3 into a solution, adding the ADH solution to a mixed solution, and stirring for 0.5-2 hours at the room temperature; and F, collecting eluent of the void volume from a loading solution after the reaction is ended at a SephadexG-25 gel chromatography column balanced in advance by water for injection, and performing freeze drying to obtain a Hib polysaccharide-ADH derivative. The process for activating the Hib polysaccharide conjugate vaccine has the beneficial effects that the quality index of the prepared Hib polysaccharide-ADH can reach the industrial standard, and moreover, the safe and nontoxic CDAP is adopted to serve as an activating agent instead of cyanogen bromide which is greatly harmful to human and environment, and therefore, the safety is enhanced, and the harm to the human and the environment are avoided.
Owner:CHENGDU OLYMVAX BIOPHARM
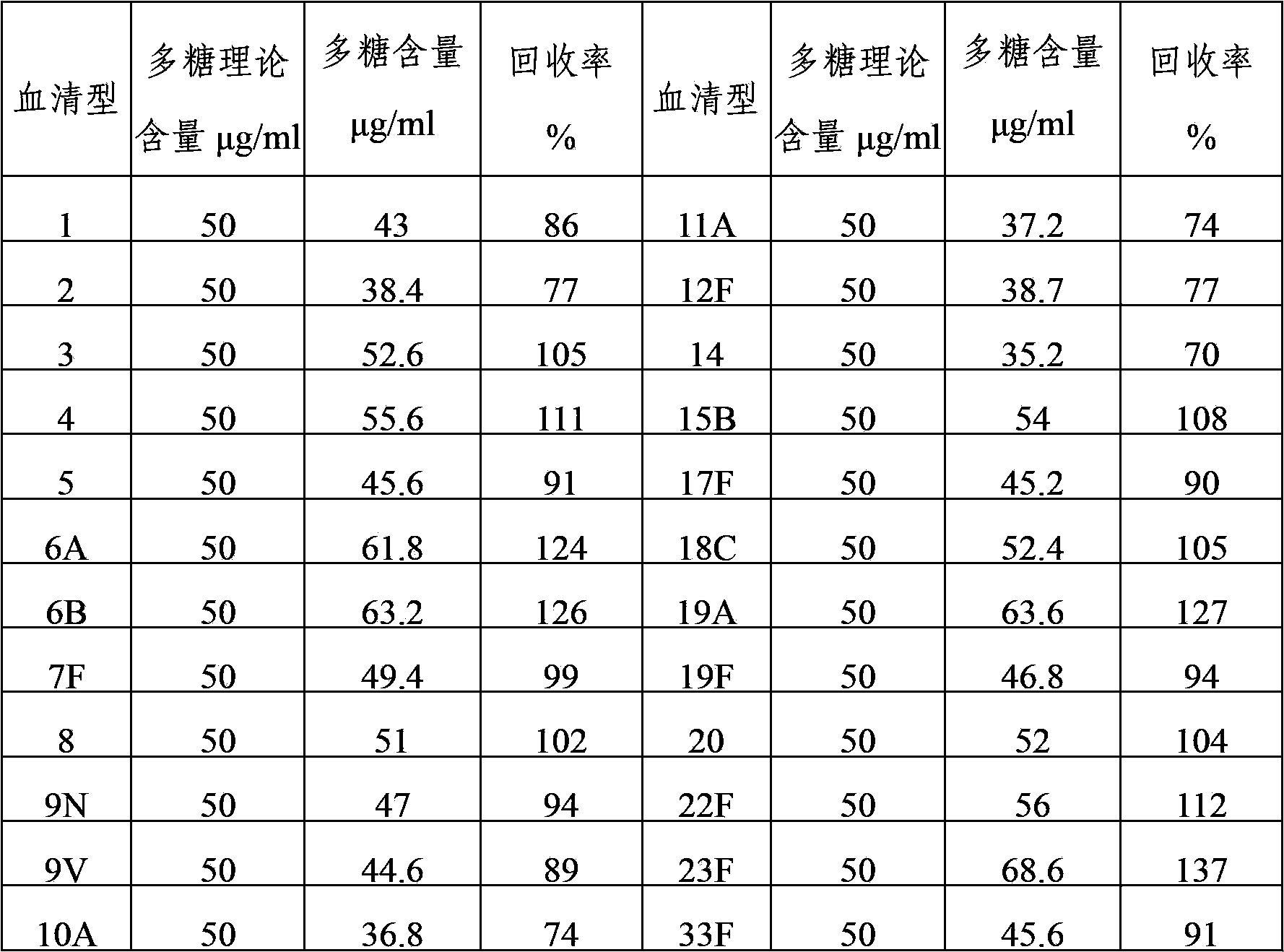
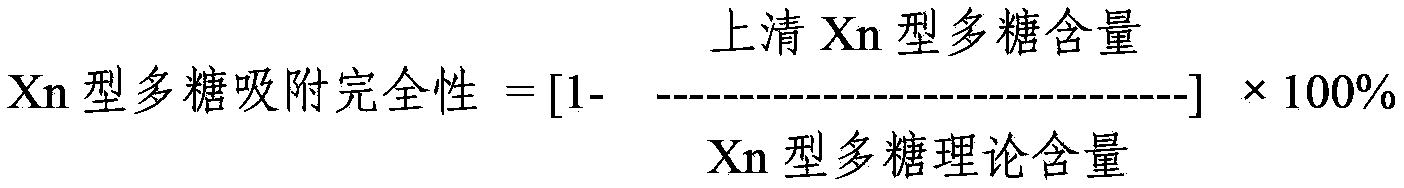
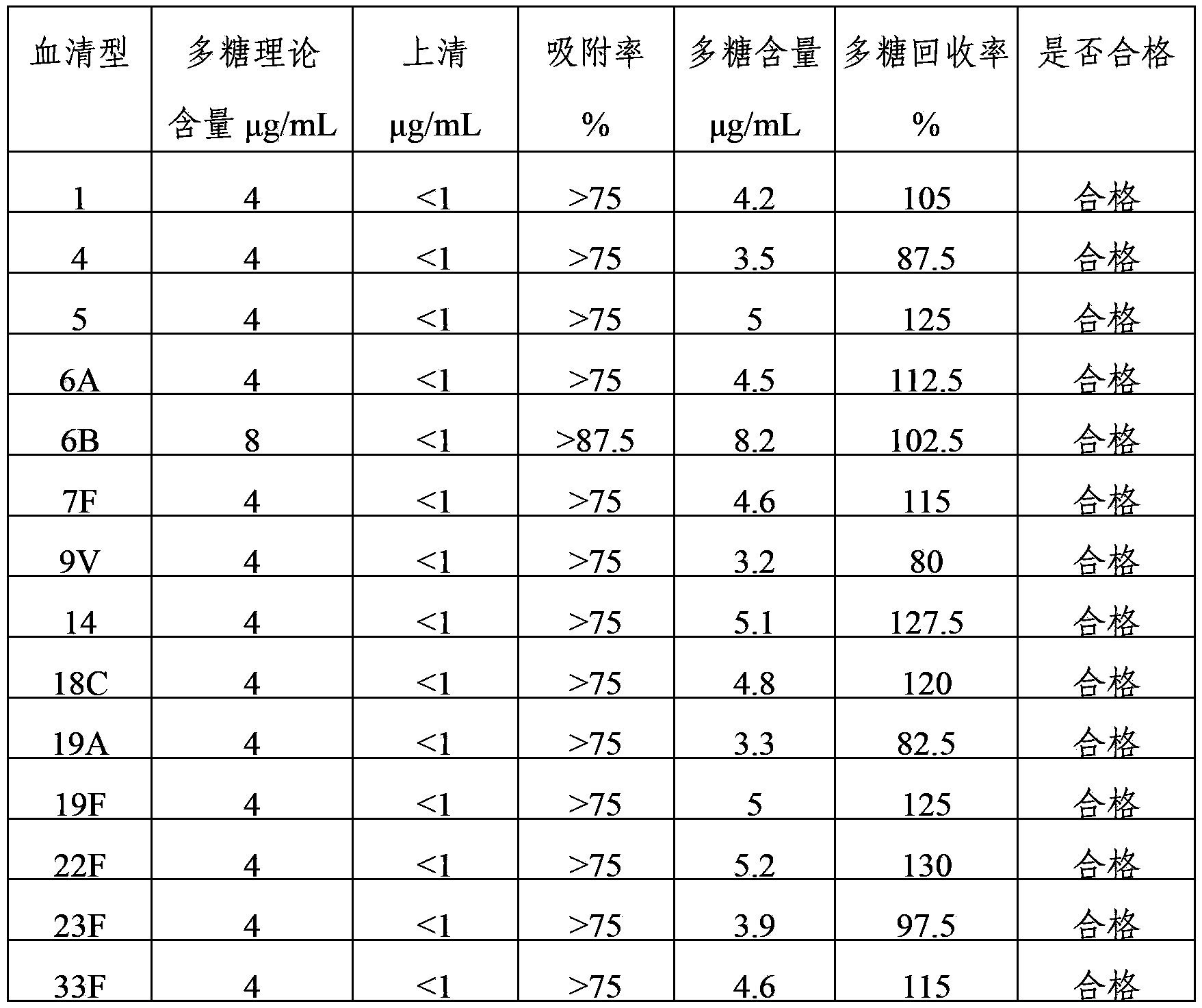
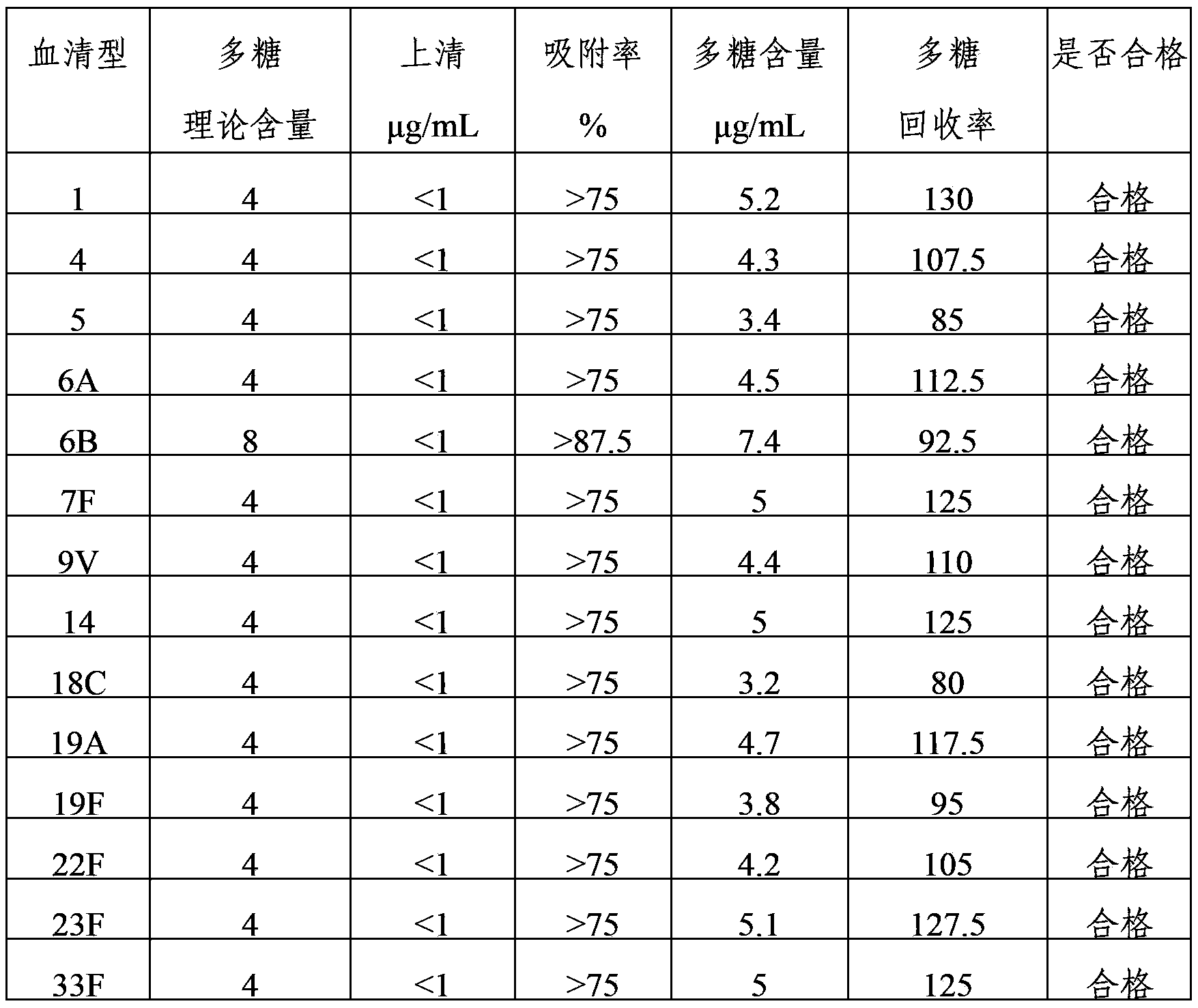
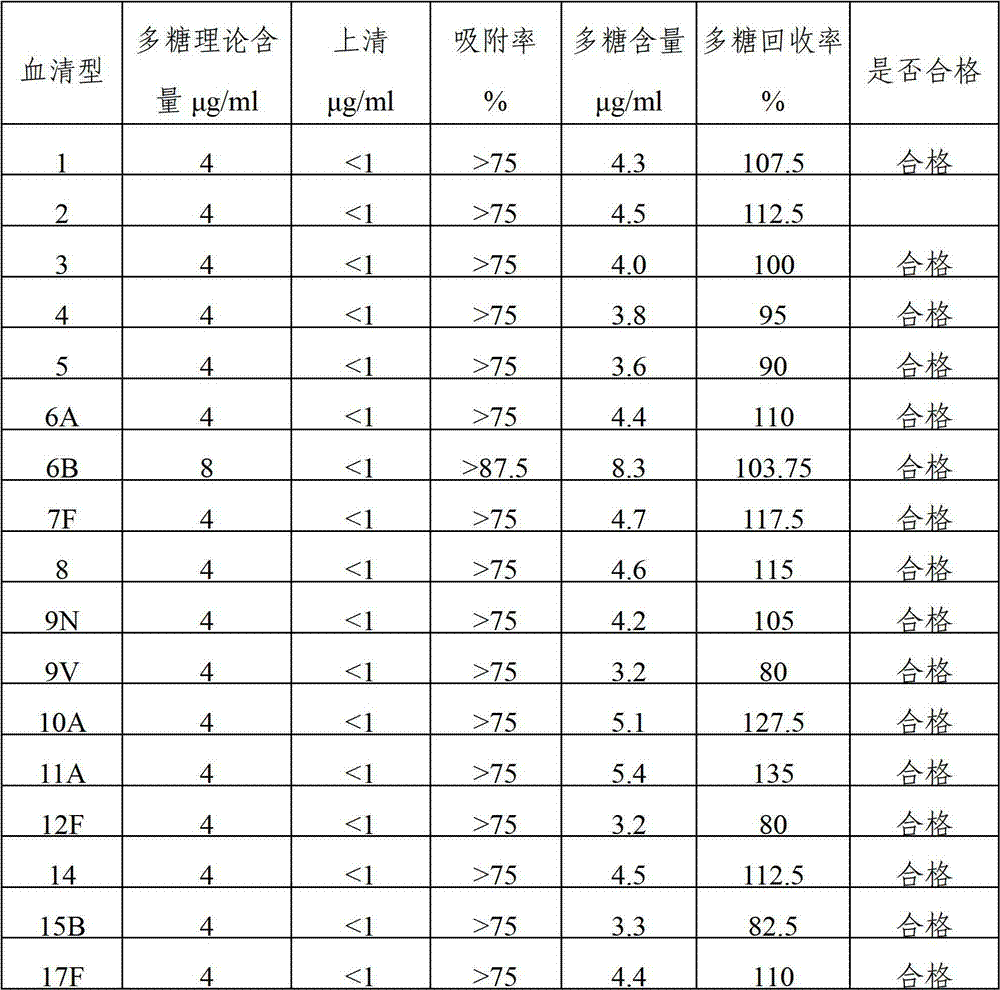
Method for determining content of polysaccharide of each group of meningococcus polysaccharide conjugate vaccine finished products
ActiveCN102809655AEliminate the effects ofAccurate measurementBiological testingCarrier proteinMENINGOCOCCAL POLYSACCHARIDE
The invention relates to a method for determining the content of the polysaccharide of each group of meningococcus polysaccharide conjugate vaccine finished products and belongs to the technical field of biology. The method comprises the following steps of: treating by using protease K during preparation of a detection product sample, wherein the total size of an enzymolysis reaction system is 420 mu l; and by using the total size as reference, adding the protease K, a protease K buffer solution which occupies one tenth of the total size and an ultra-filtration concentrated solution which occupies one sixth of the total size, uniformly mixing the protease K, the protease K buffer solution and the ultra-filtration concentrated solution, and then incubating, wherein the addition amount of the protease K is two to eight times of the content of proteins in the enzymolysis reaction system. By adoption of the method, influence of each group of conjugate carrier proteins on immunoelectrophoresis is eliminated, an appropriate detection system for detecting the polysaccharide of each of more than four groups of meningococcus polysaccharide conjugate vaccine finished products is established, and the content of the polysaccharide of each of more than four groups of meningococcus polysaccharide conjugate vaccine finished products can be accurately and quickly determined. The method has the characteristics of high durability, high accuracy and high precision. A method for evaluating the quality of meningococcus polysaccharide conjugate vaccine is established.
Owner:YUXI WALVAX BIOTECH CO LTD
Polyvalent pneumococcal capsular polysaccharide-protein conjugate composition and preparation method thereof
ActiveCN103656631BImprove adsorption capacityImprove stabilityAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
The invention provides a multivalence pneumococcus capsular polysaccharide-protein conjugate composition and a preparation method thereof. The conjugate composition is prepared from capsular polysaccharides of pneumococcus of 24 different serotypes and a carrier protein in a covalence connection manner, wherein the 24 different serotypes are 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F and 33F. The conjugate composition is good in adsorption effect and stability, has multiple immunogenicity and protection properties aiming at invasion of pneumococcus of the 24 serotypes, and is superior to the low-valence pneumonia composition in the market, and the immune response is higher than that of uncombined compositions. By inoculating a multivalence pneumococcus capsular polysaccharide conjugate vaccine prepared from the conjugate composition, the inoculation injection times can be reduced, the immunization procedure can be simplified, and meanwhile human beings and animals can be effectively prevented from diseases resulted from the pneumococcus of the 24 serotypes, and the conjugate composition is wide in coverage range and good in immune effect.
Owner:SINOVAC RES & DEV
Polysaccharide protein conjugate vaccine and identification method thereof
InactiveCN108904798AReduce concentrationIncrease demandAntibacterial agentsCarrier-bound antigen/hapten ingredientsVaccine ProductionReaction rate
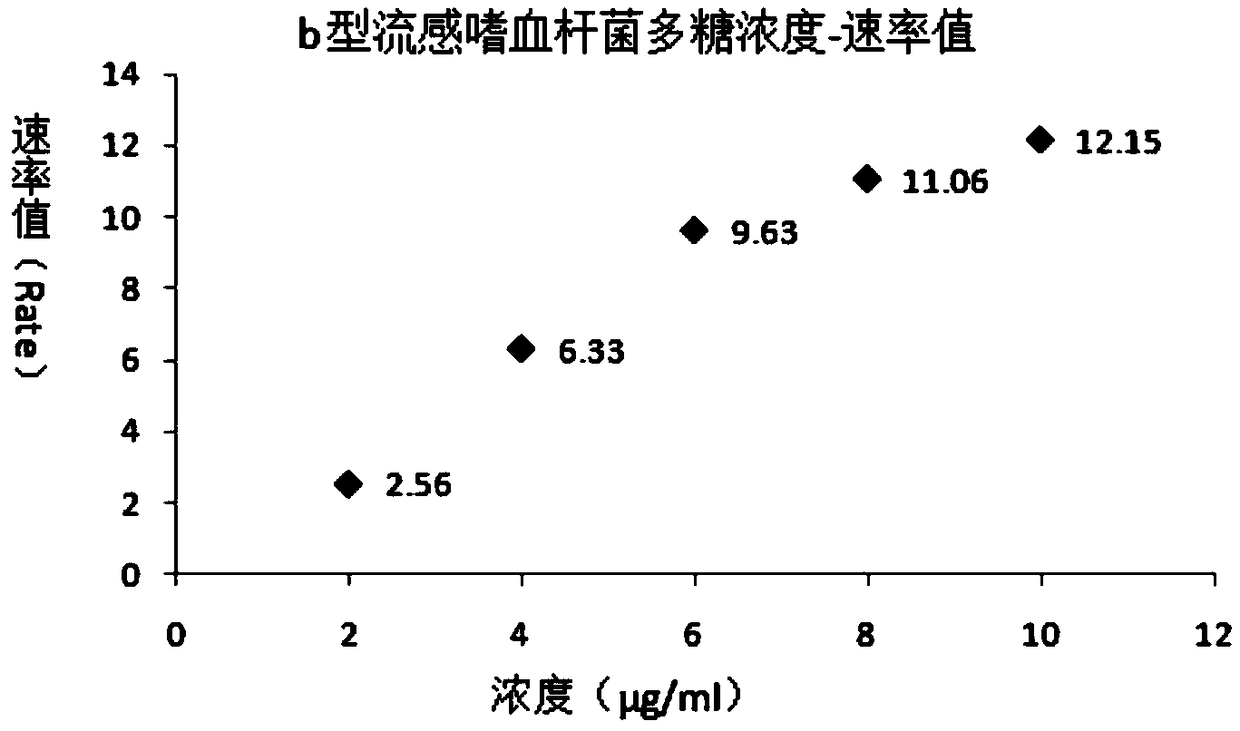
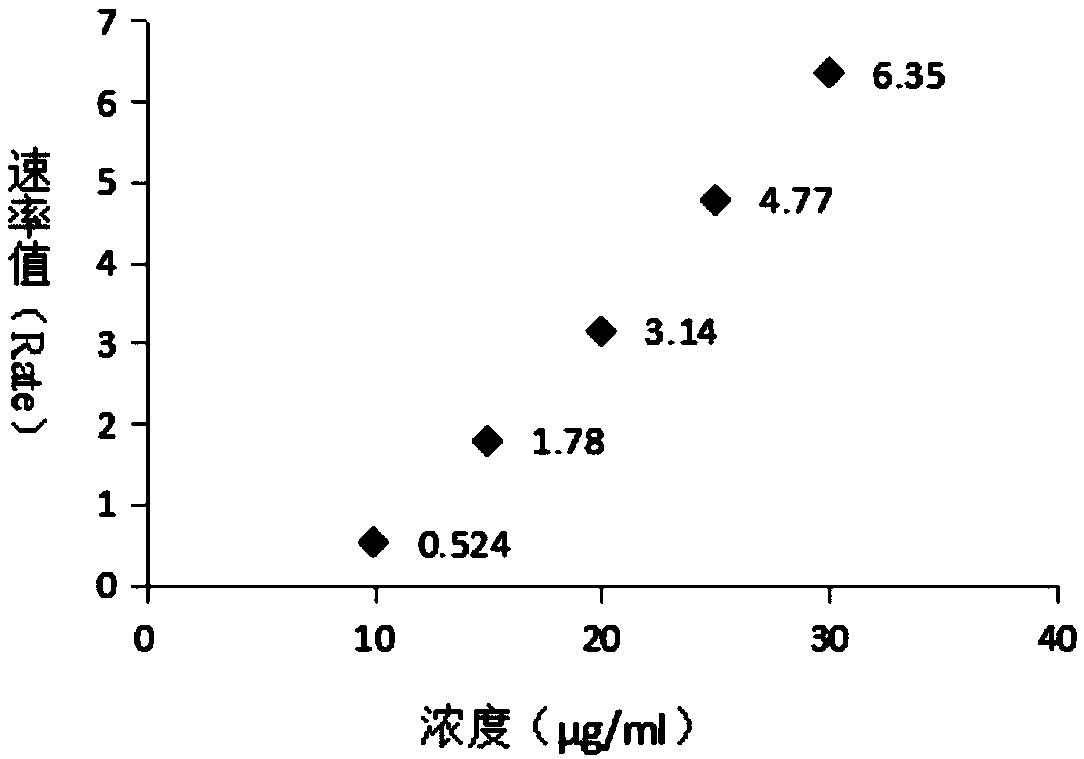
The invention relates to a polysaccharide protein conjugate and an identification method thereof, in particular to a polysaccharide protein conjugate vaccine made from the polysaccharide protein conjugate, and an identification method thereof. A rate scattering turbidimetry is a technical means established based on an immunochemical method, and can measure scattered-light intensity changes causedby antigen-antibody complex aggregate particles suspended in a small cup, and a reaction rate value is acquired within tens seconds; complex aggregate particles are formed through the antigen-antibodyreaction, and have different rates according to different concentrations of antigens and / or antibodies. The identification method has strong specificity and sensitivity, and the detection time is shortened; the antigens directly contact with the antibodies, and accordingly, low-concentration sample detection is benefited; the identification method can be applied to identification of stock solutions and / or semi-finished products and / or finished products in the process of vaccine production.
Owner:复星安特金(成都)生物制药有限公司
Zika virus E protein conjugate vaccine and preparation method thereof
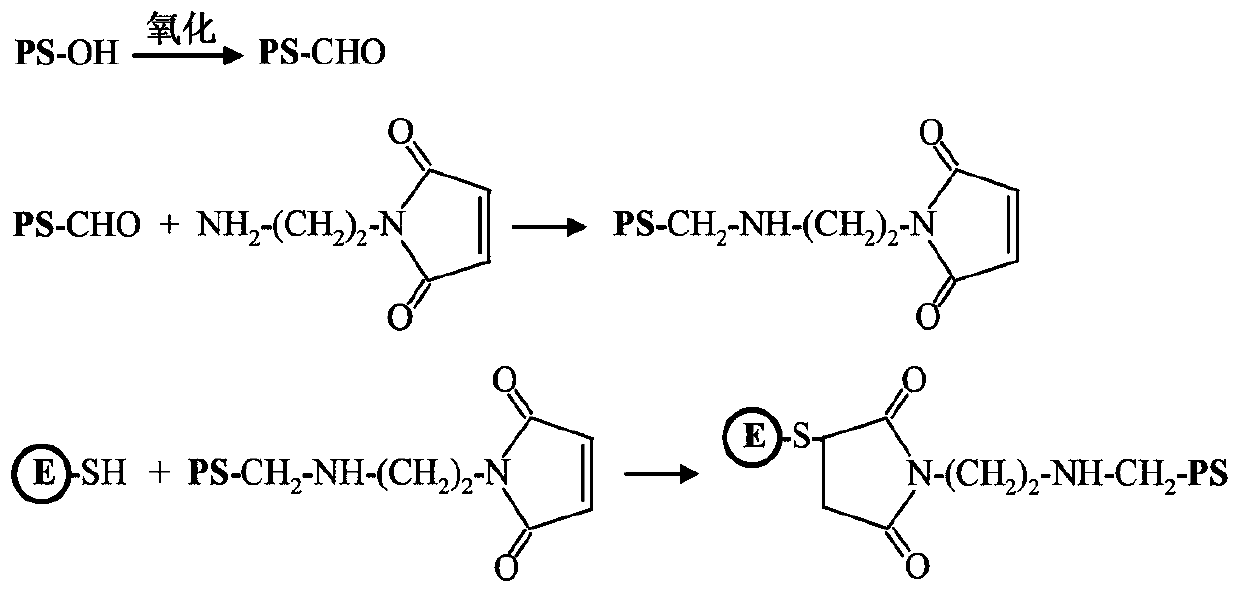
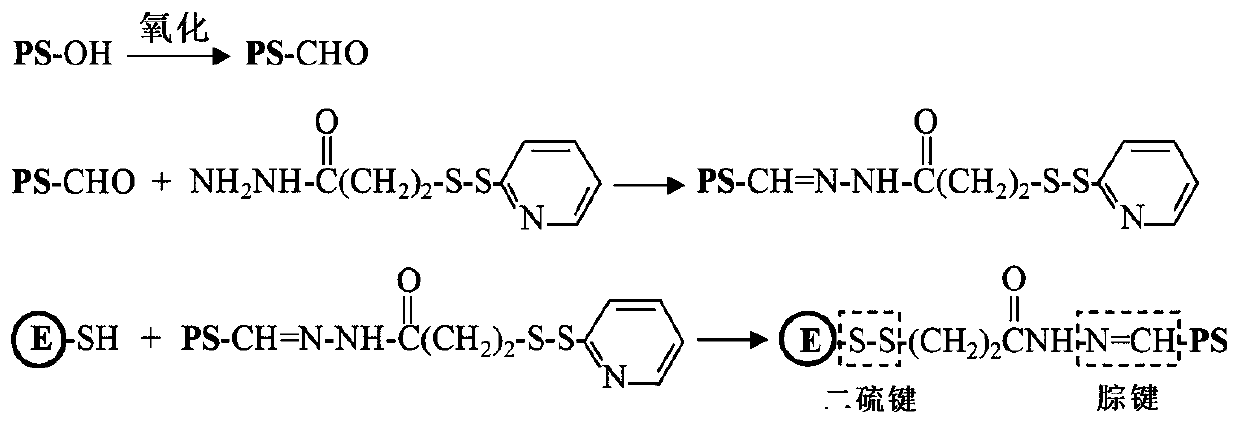
ActiveCN111514286AInnovativeOriginalSsRNA viruses positive-senseViral antigen ingredientsZika virusChemical compound
The invention discloses a Zika virus E protein conjugate vaccine and a preparation method thereof. The preparation method comprises the following specific steps of: 1) oxidizing an ortho-position hydroxyl group of [beta]-glucan into an aldehyde group, so as to obtain oxidized [beta]-glucan; 2) adding a connecting bridge compound, enabling the connecting bridge compound to react with the aldehyde group in the oxidized [beta]-glucan to obtain [beta]-glucan containing a connecting bridge; and 3) enabling Zika virus E protein to react with the [beta]-glucan containing the connecting bridge, and carrying out purification to obtain the Zika virus E protein conjugate vaccine. The vaccine can be induced to generate a high-level cellullar immunologic response and a body fluid immunologic response.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com