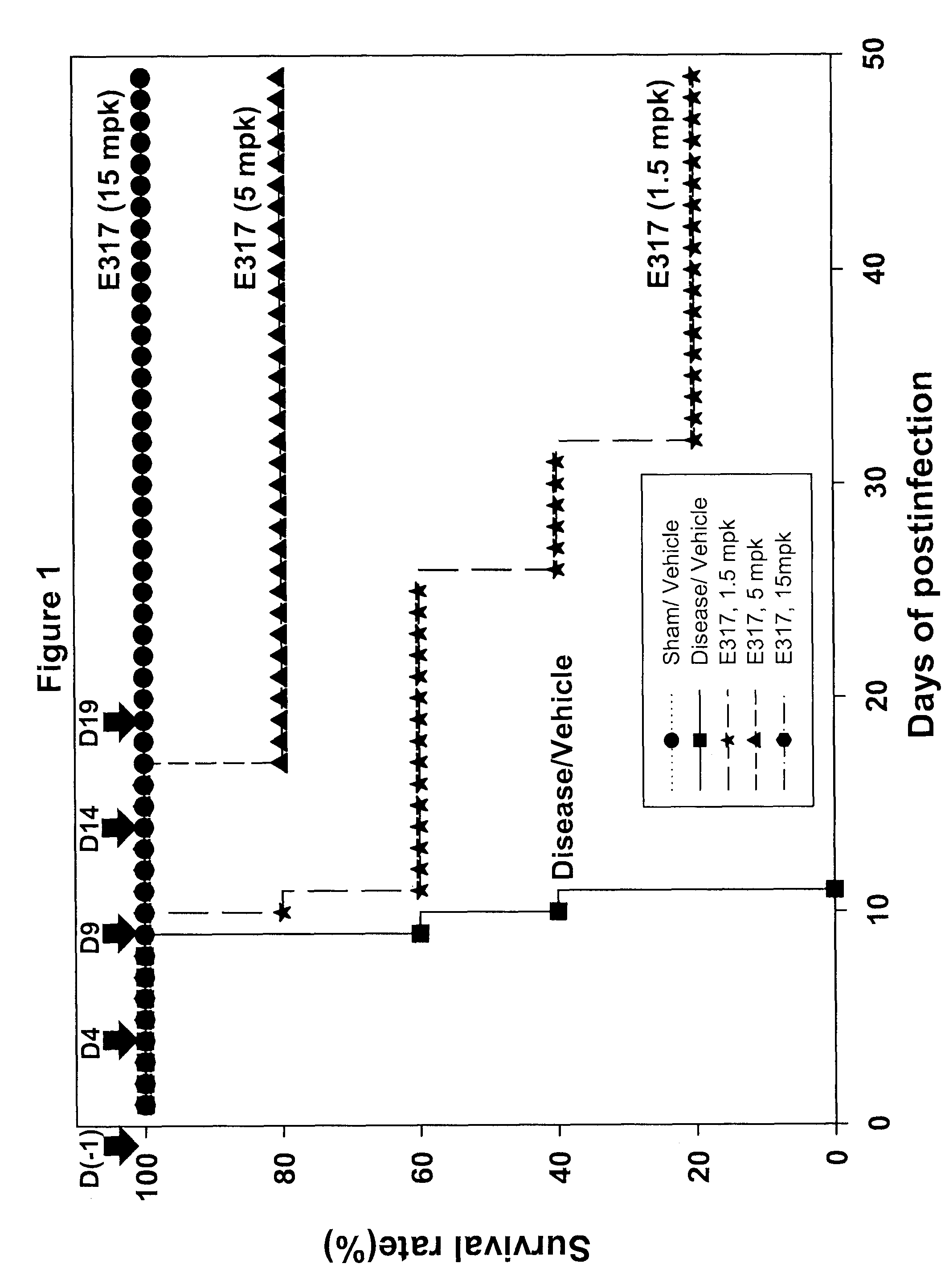
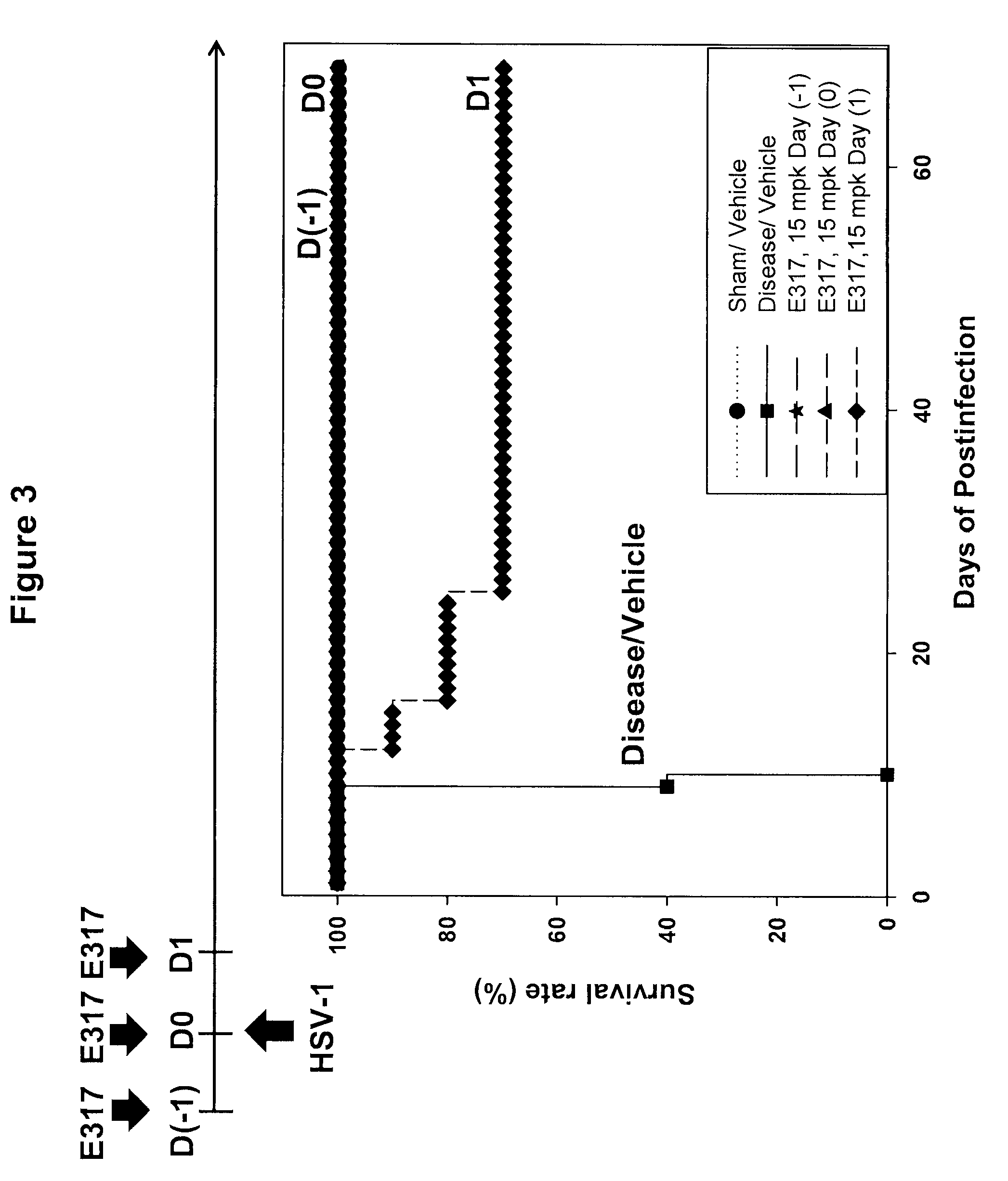
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Hsv infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The herpes simplex virus, also known as HSV, is an infection that causes herpes. Herpes can appear in various parts of the body, most commonly on the genitals or mouth. There are two types of the herpes simplex virus.
Use of valid target of periplaneta americana
The invention provides the application of the effective fraction of a periplaneta Americana in preparing drugs for inhibiting herpesvirus (HSV) infection and preventing and treating oral ulcer and / or genital herpes. The effective fraction of the periplaneta americana is obtained by extracting fresh or dry periplaneta Americana belonging to the blattidae family with ethanol aqueous solution, purifying with macroporous adsorption resin column, and refining by eluting with ethanol solution. The effective fraction of the periplaneta Americana has distinct HSV inhibitory activity, and can be processed into medicinal preparations or health products for inhibiting HSV infection and preventing and treating related diseases or symptoms.
Owner:ZHEJIANG UNIV
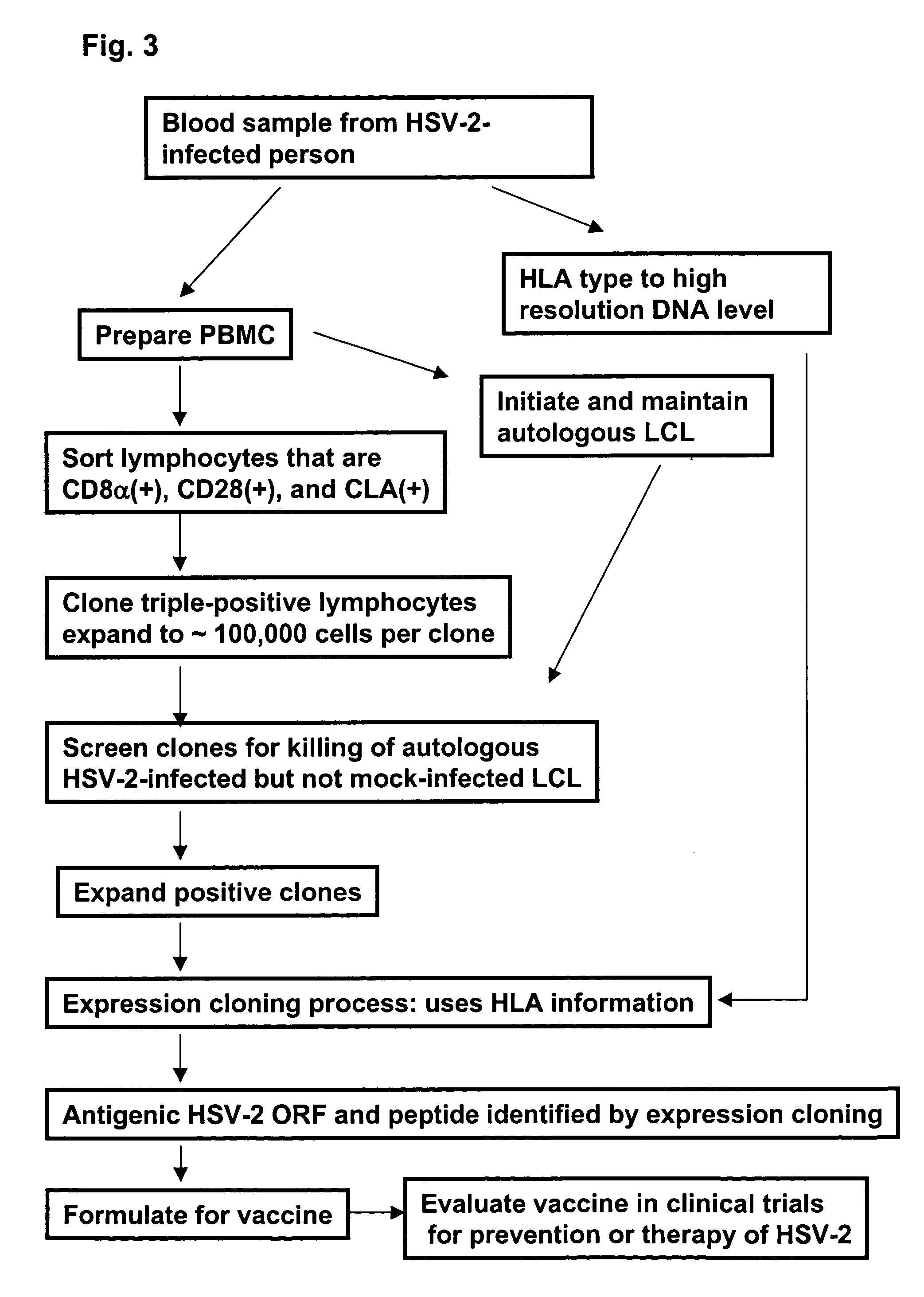
Rapid, efficient purification of HSV-specific T-lymphocytes and HSV antigens identified via same
InactiveUS7078041B2Increase secretionIncrease productionPeptide/protein ingredientsViral antigen ingredientsT-Cell SpecificityT lymphocyte
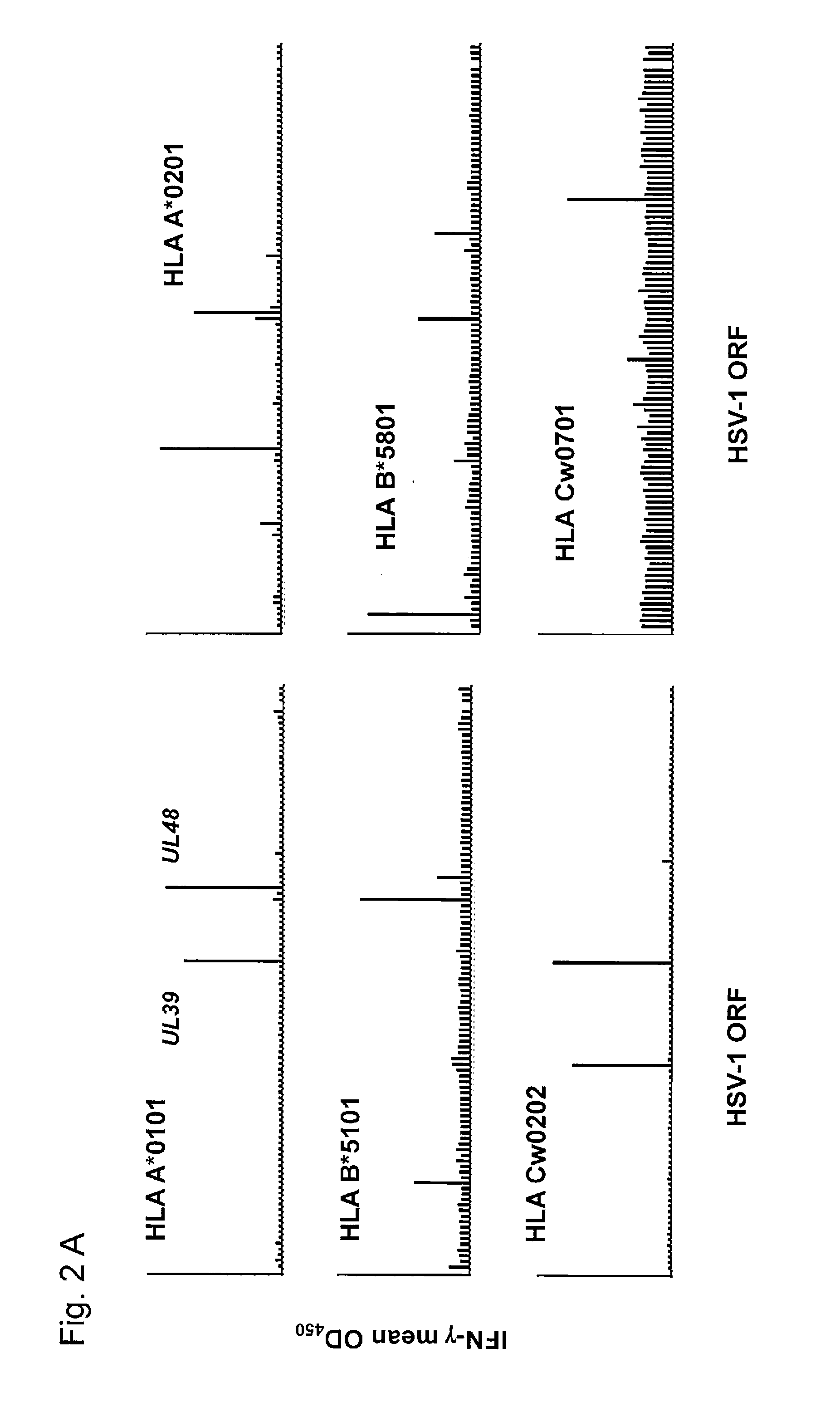
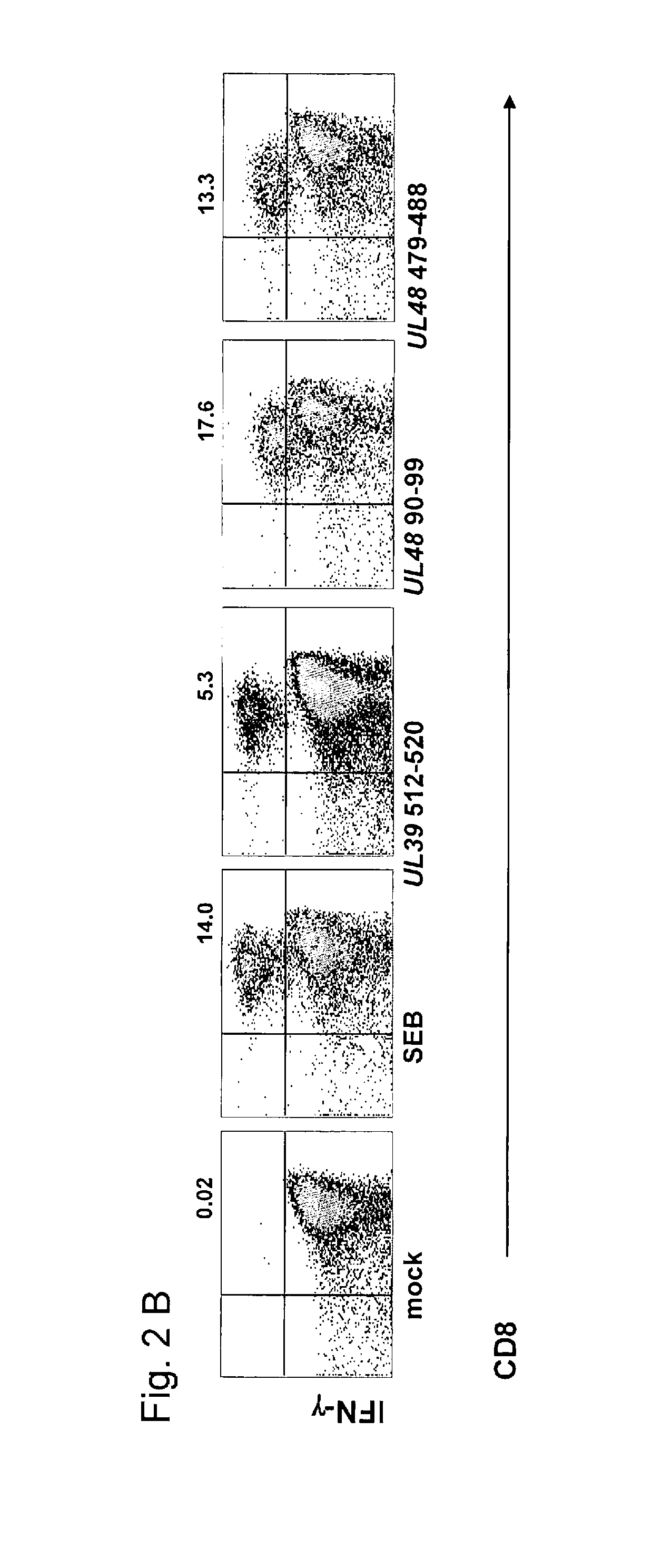
Described is a method of identifying an immunologically active antigen of a virus that attacks skin, as well as a method of enriching a population of lymphocytes for T lymphocytes that are specific to a virus that attacks skin. Also provided are HSV antigens and epitopes that are useful for the prevention and treatment of HSV infection that have been identified via the methods of the invention. T-cells having specificity for antigens of the invention have demonstrated cytotoxic activity against cells loaded with virally-encoded peptide epitopes, and in many cases, against cells infected with HSV. The identification of immunogenic antigens responsible for T-cell specificity provides improved anti-viral therapeutic and prophylactic strategies. Compositions containing antigens or polynucleotides encoding antigens of the invention provide effectively targeted vaccines for prevention and treatment of HSV infection.
Owner:UNIV OF WASHINGTON
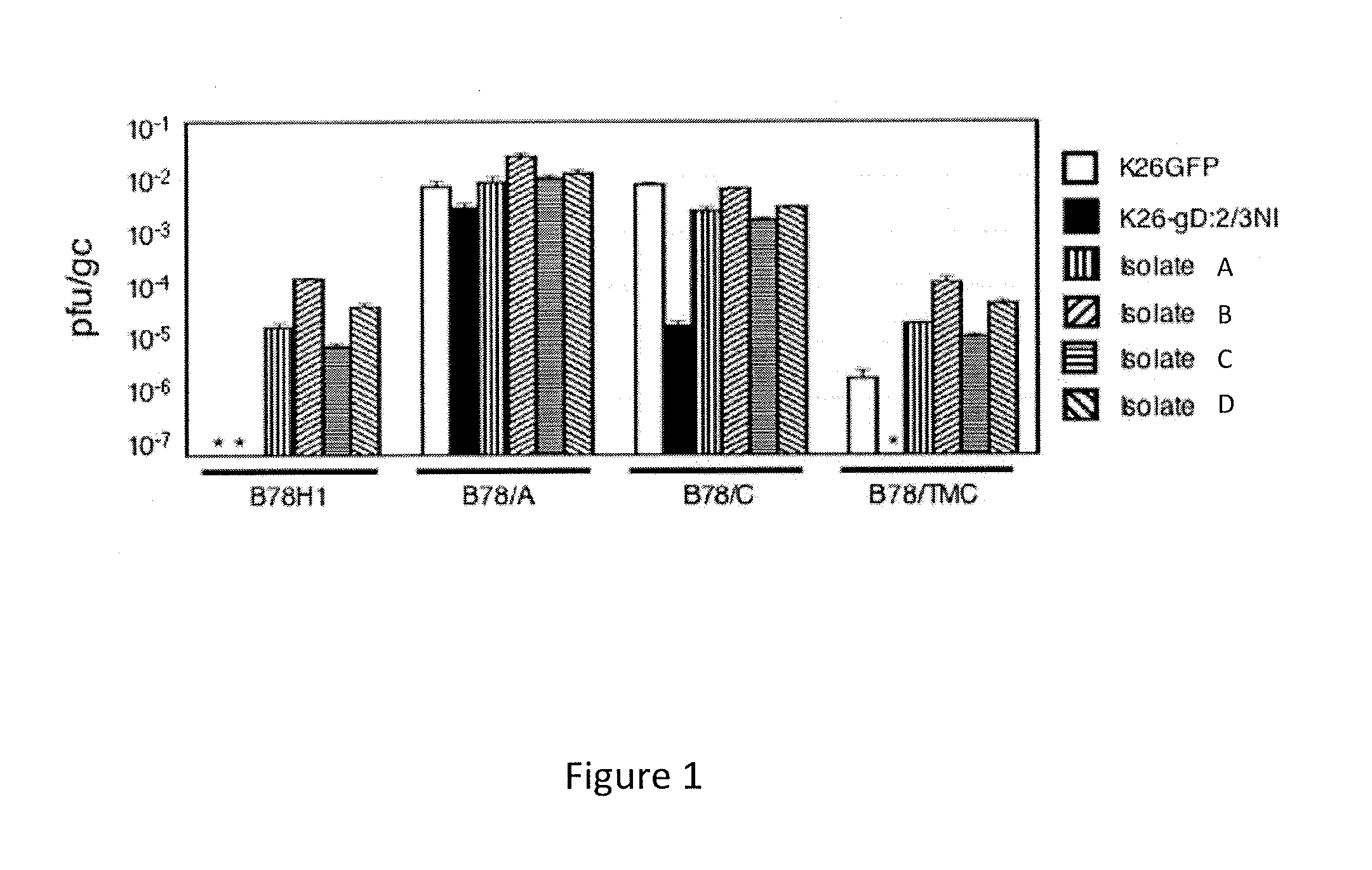
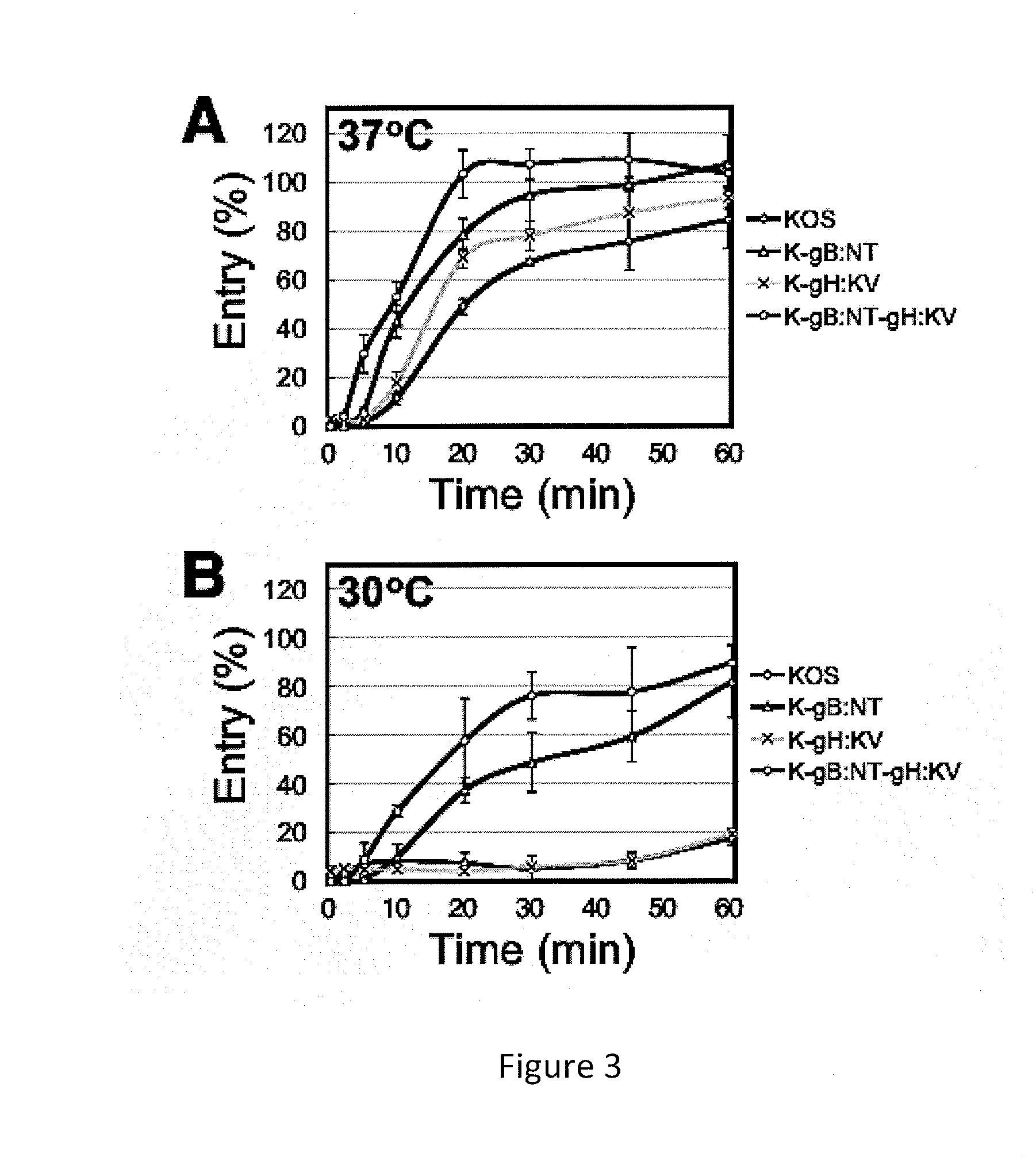
Identification of mutations in herpes simplex virus envelope glycoproteins that enable or enhance vector retargeting to novel non-hsv receptors
ActiveUS20130096186A1Improve efficiencyVectorsGenetic material ingredientsDna encodingMembrane glycoproteins
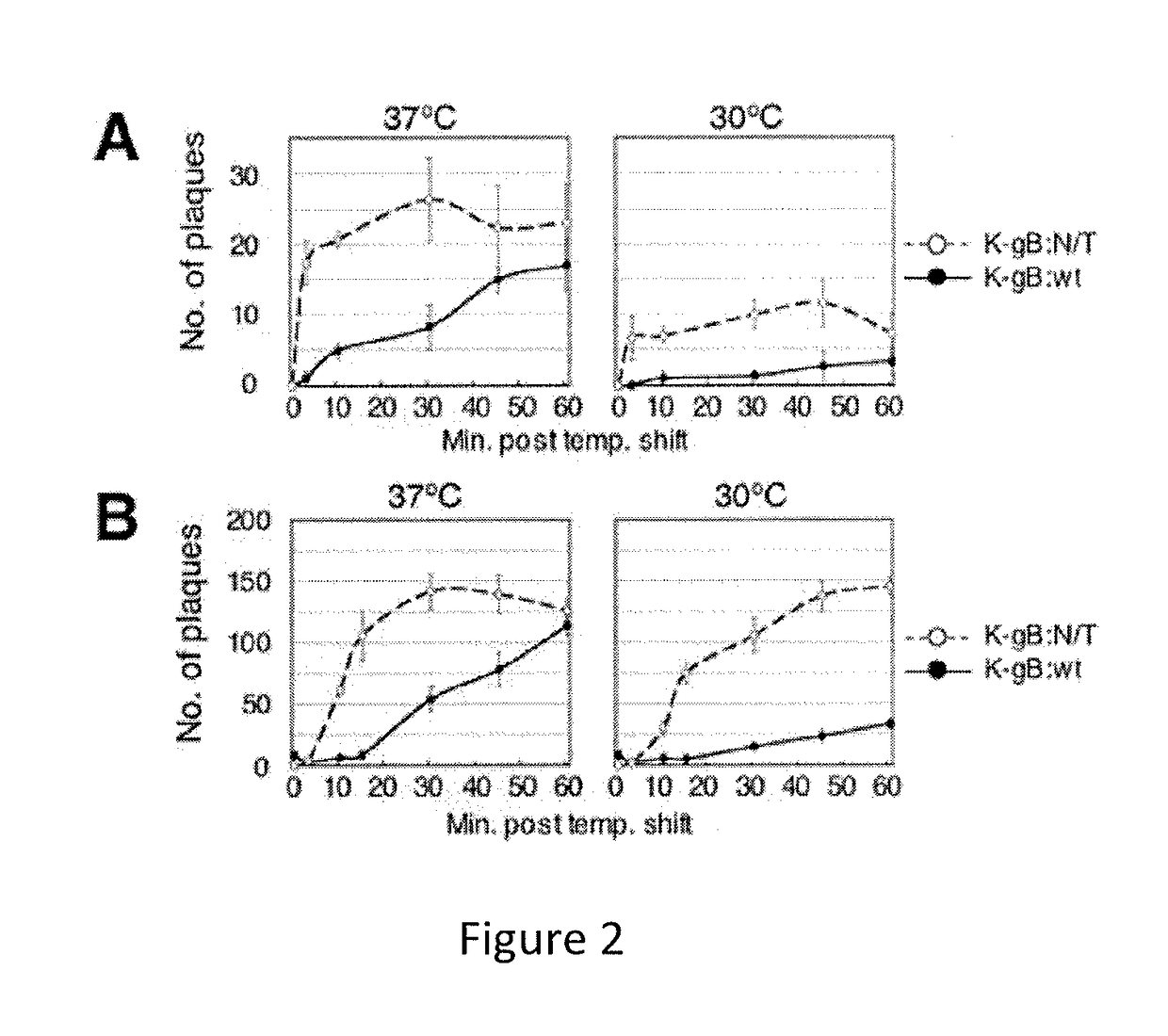
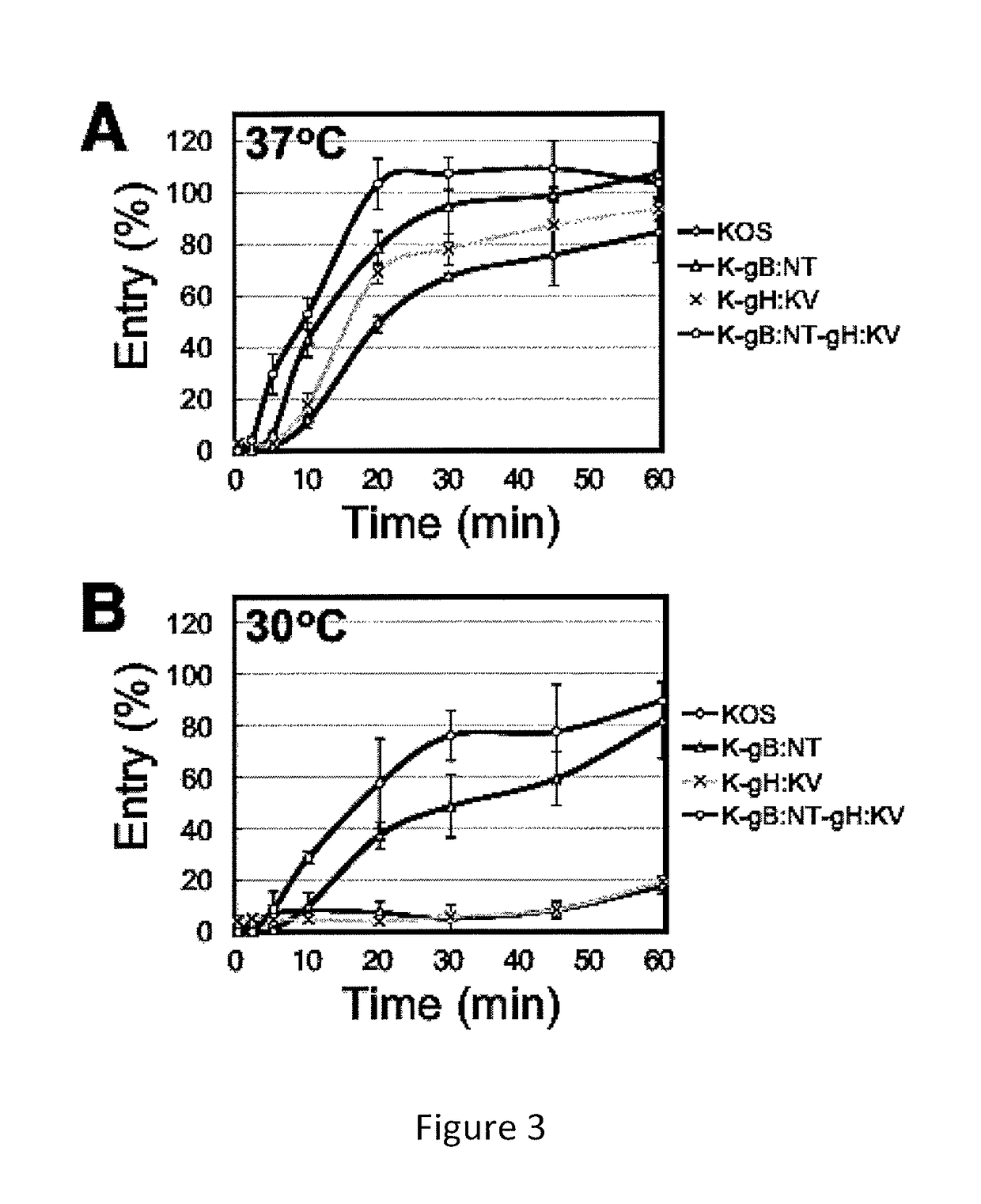
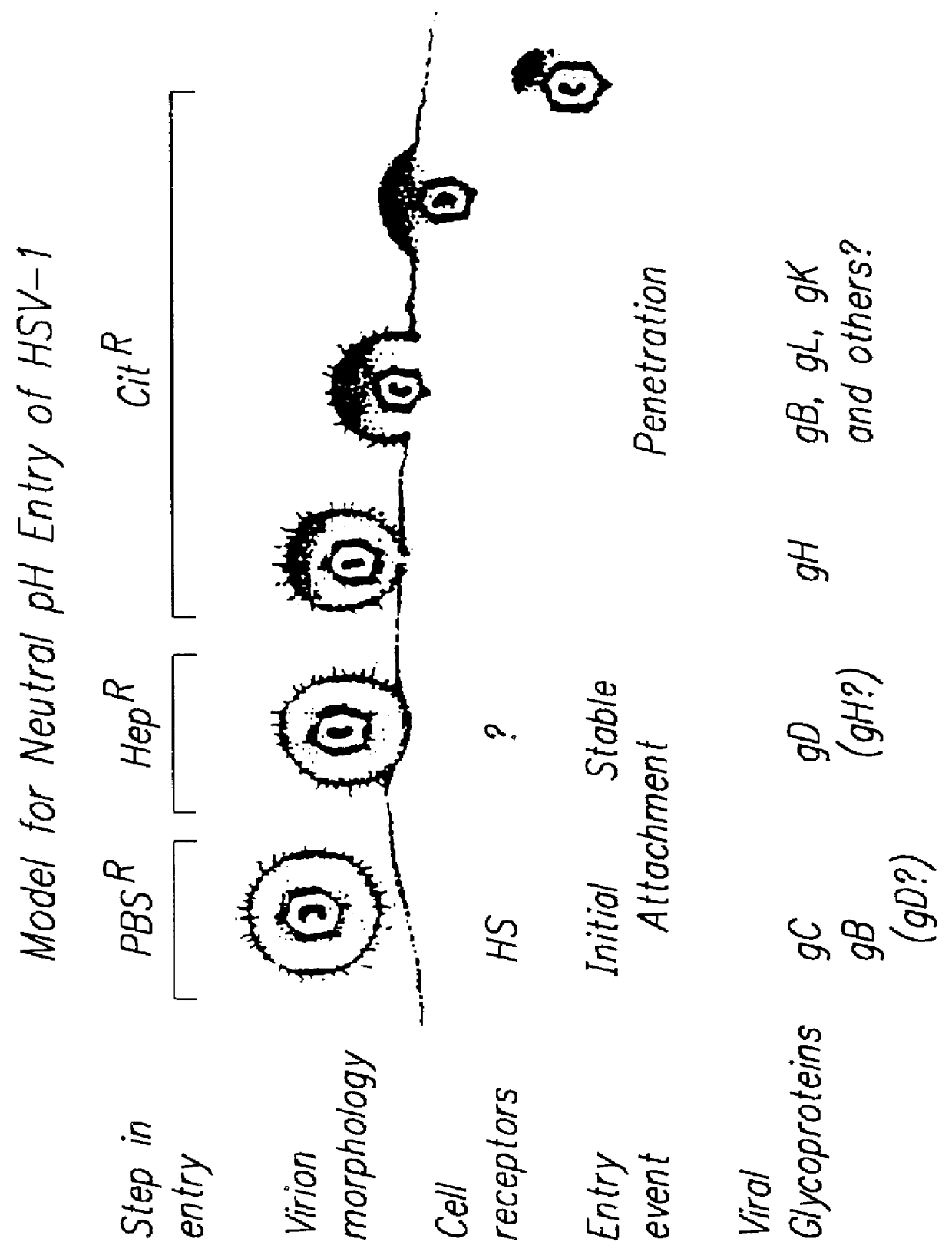
The invention provides modified HSV vectors that exhibit enhanced entry of cells, either through direct infection and / or lateral spread. In one aspect, HSV vectors of the present invention can directly infect cells through interaction with cell proteins other than typical mediators of HSV infection. In another aspect, the invention provides an HSV vector, which exhibits lateral spread in cells typically resistant to HSV lateral spread, such as cells lacking gD receptors. The invention further provides DNA encoding mutant forms of the HSV gB and gH glycoproteins, stocks of the inventive virus, and methods for effecting viral targeting and efficient entry of cells. The invention also pertains to the use of the inventive vectors for treating cancers.
Owner:UNIVERSITY OF PITTSBURGH
Rapid, efficient purification of HSV-specific T-lymphocytes and HSV antigens identified via same
Described is a method of identifying an immunologically active antigen of a virus that attacks skin, as well as a method of enriching a population of lymphocytes for T lymphocytes that are specific to a virus that attacks skin. Also provided are HSV antigens and epitopes that are useful for the prevention and treatment of HSV infection that have been identified via the methods of the invention. T-cells having specificity for antigens of the invention have demonstrated cytotoxic activity against cells loaded with virally-encoded peptide epitopes, and in many cases, against cells infected with HSV. The identification of immunogenic antigens responsible for T-cell specificity provides improved anti-viral therapeutic and prophylactic strategies. Compositions containing antigens or polynucleotides encoding antigens of the invention provide effectively targeted vaccines for prevention and treatment of HSV infection.
Owner:UNIV OF WASHINGTON
Methods of use for hsv-1 and hsv-2 vaccines
ActiveUS20090246227A1Reduce decreaseSymptoms improvedViral antigen ingredientsVirus peptidesRecurrent herpes simplexHsv infection
This invention provides methods of treating, suppressing, inhibiting, reducing an incidence, reducing the pathogenesis of, ameliorating the symptoms of, or ameliorating the secondary symptoms of a primary or recurring Herpes Simplex Virus (HSV) infection, or prolonging the latency to a relapse of an HSV infection, and disorders and symptoms associated with same and inducing an anti-HSV immune response in a subject comprising the step of contacting the subject with a composition comprising a mutant HSV strain comprising an inactivating mutation in a Us8 gene, followed by a second contacting with the composition.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Anti-herpes simplex virus antibodies and methods of use thereof
The invention provides antibodies and polypeptides that specifically bind to the glycoprotein D of herpes simplex virus (HSV) and use of the antibodies and polypeptides for treating or diagnosing HSV infections.
Owner:DEV CENT FOR BIOTECHNOLOGY
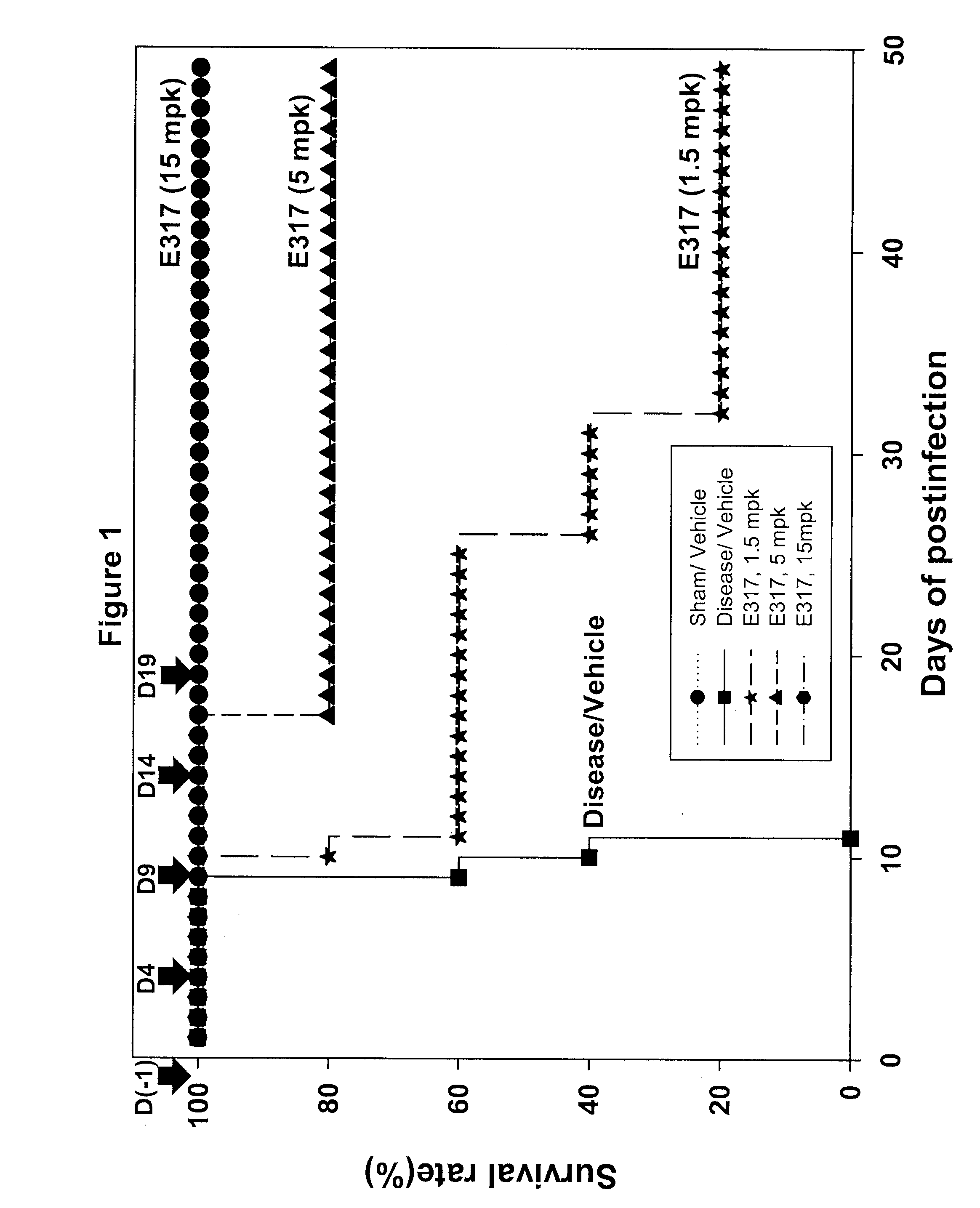
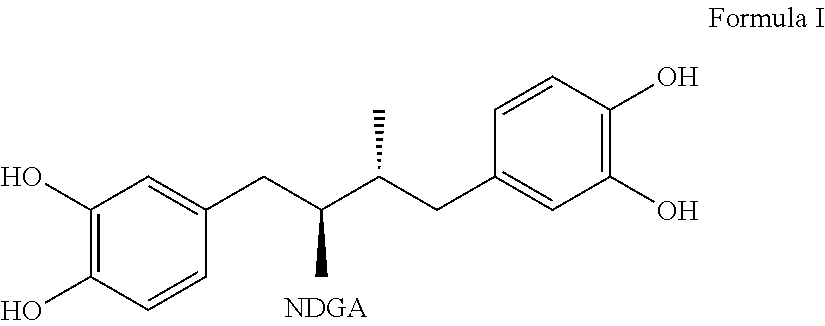
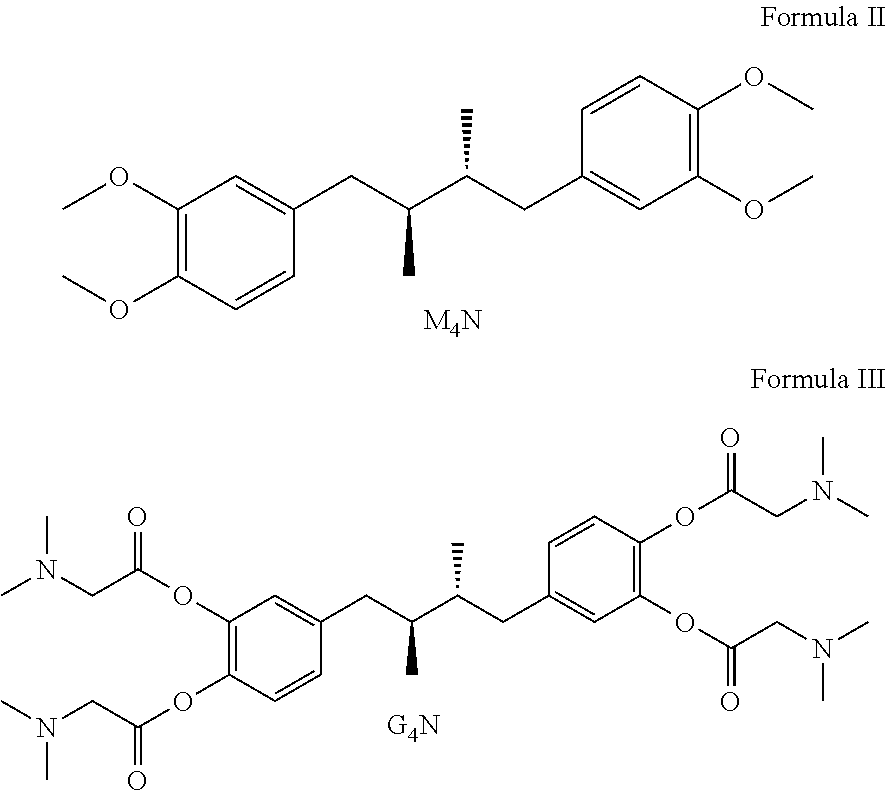
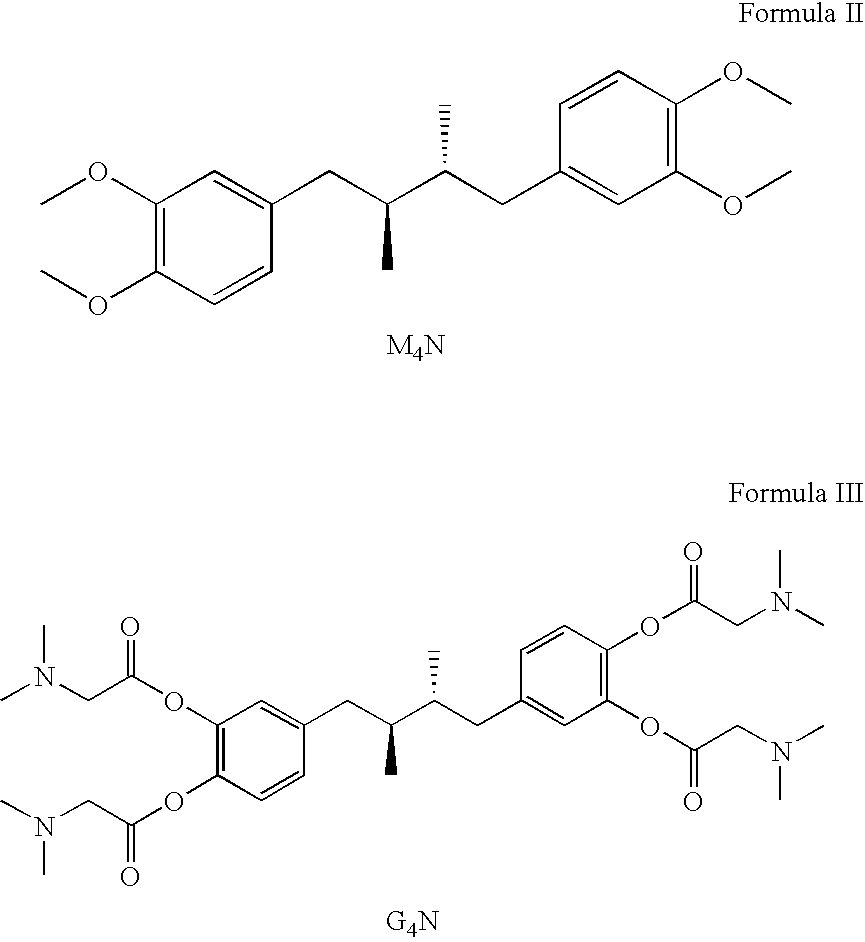
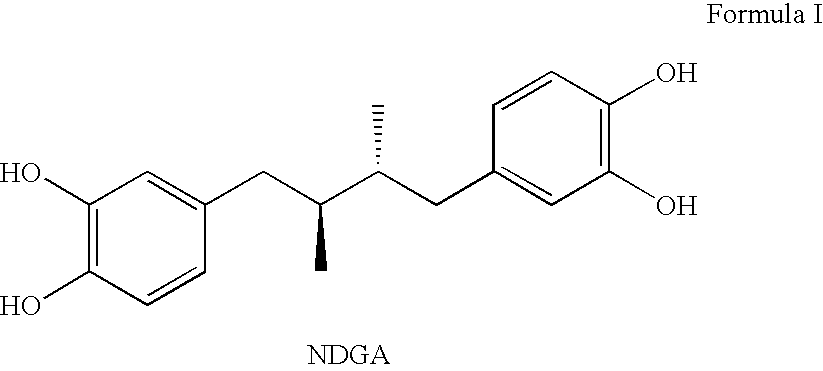
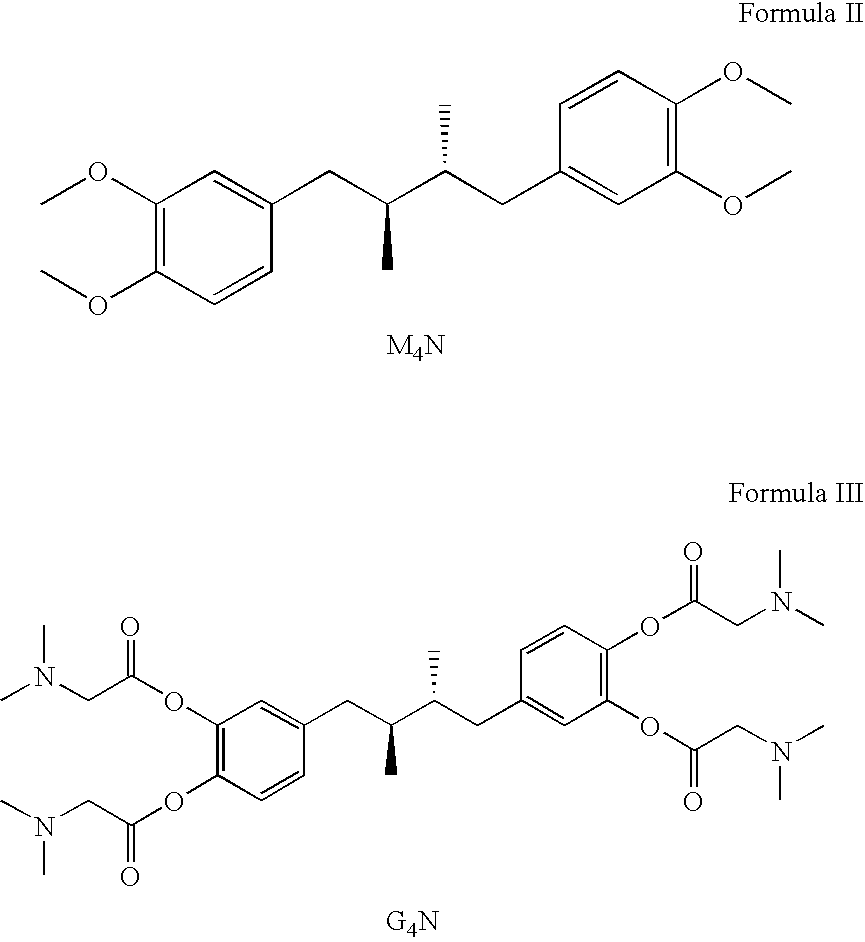
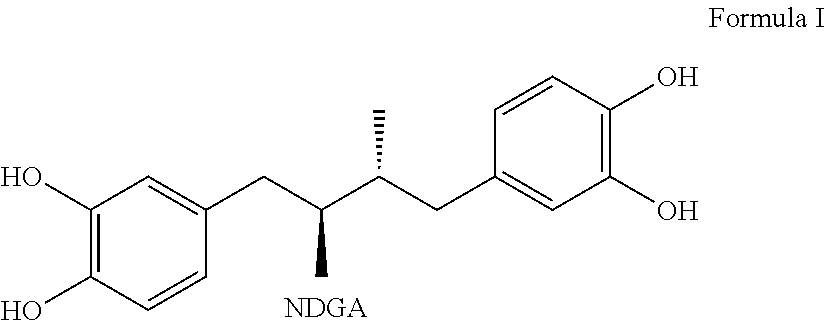
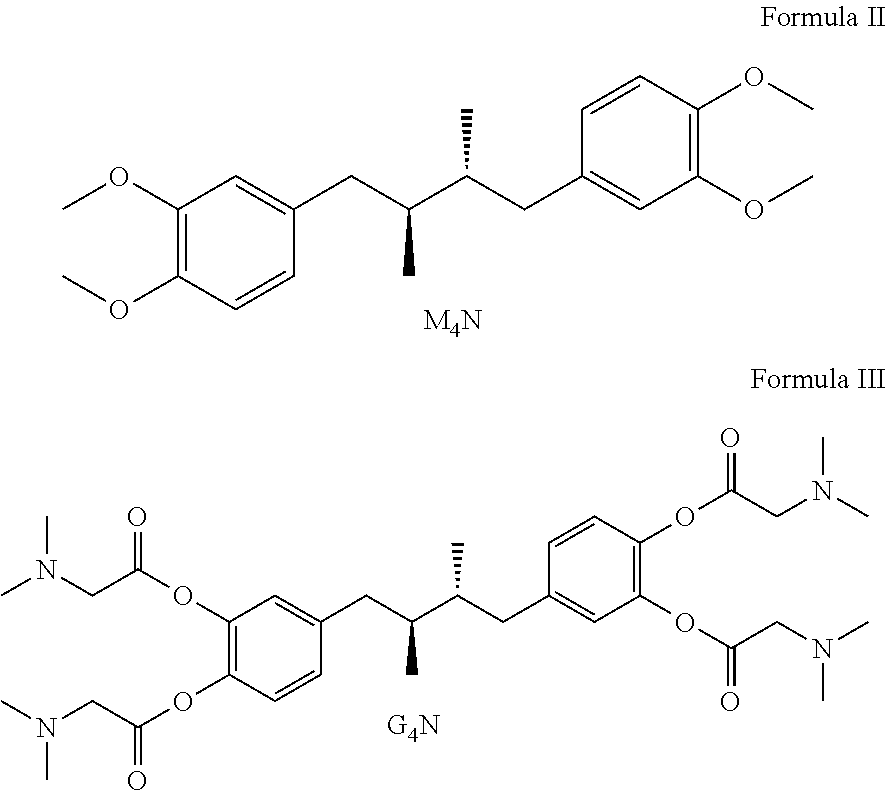
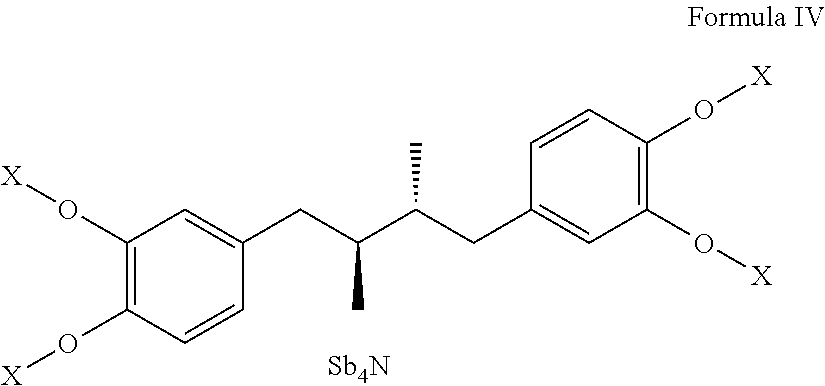
Tetra-substituted NDGA derivatives via ether bonds and carbamate bonds and their synthesis and pharmaceutical use
Disclosed are nordihydroguaiaretic acid derivative compounds including various end groups bonded by a carbon atom or heteroatom though a side chain bonded to the respective hydroxy residue O groups by an ether bond or a carbamate bond, pharmaceutical compositions, methods of making them, and methods of using them and kits including them for the treatment of diseases and disorders, in particular, diseases resulting from or associated with a virus infection, such as HIV infection, HPV infection, or HSV infection, an inflammatory disease, such as various types of arthritis and inflammatory bowel diseases, a metabolic disease, such as diabetes, a vascular disease, such as hypertension and macular degeneration, or a proliferative disease, such as diverse types of cancers.
Owner:ERIMOS PHARMA
Hsv-1 epitopes and methods for using same
The invention provides HSV antigens and epitopes that are useful for the prevention and treatment of HSV infection. T-cells having specificity for antigens of the invention have demonstrated cytotoxic activity against cells loaded with virally-encoded peptide epitopes, and in many cases, against cells infected with HSV. The identification of immunogenic antigens responsible for T-cell specificity provides improved anti-viral therapeutic and prophylactic strategies. Compositions containing antigens or polynucleotides encoding antigens of the invention provide effectively targeted vaccines for prevention and treatment of HSV infection.
Owner:UNIV OF WASHINGTON
Identification of mutations in herpes simplex virus envelope glycoproteins that enable or enhance vector retargeting to novel non-HSV receptors
The invention provides modified HSV vectors that exhibit enhanced entry of cells, either through direct infection and / or lateral spread. In one aspect, HSV vectors of the present invention can directly infect cells through interaction with cell proteins other than typical mediators of HSV infection. In another aspect, the invention provides an HSV vector, which exhibits lateral spread in cells typically resistant to HSV lateral spread, such as cells lacking gD receptors. The invention further provides DNA encoding mutant forms of the HSV gB and gH glycoproteins, stocks of the inventive virus, and methods for effecting viral targeting and efficient entry of cells. The invention also pertains to the use of the inventive vectors for treating cancers.
Owner:UNIVERSITY OF PITTSBURGH
Hsv-1 and hsv-2 vaccines and methods of use thereof
ActiveUS20110177125A1Reduce morbidityReducing pathogenesisSenses disorderNervous disorderVaccinationGenital ulcer
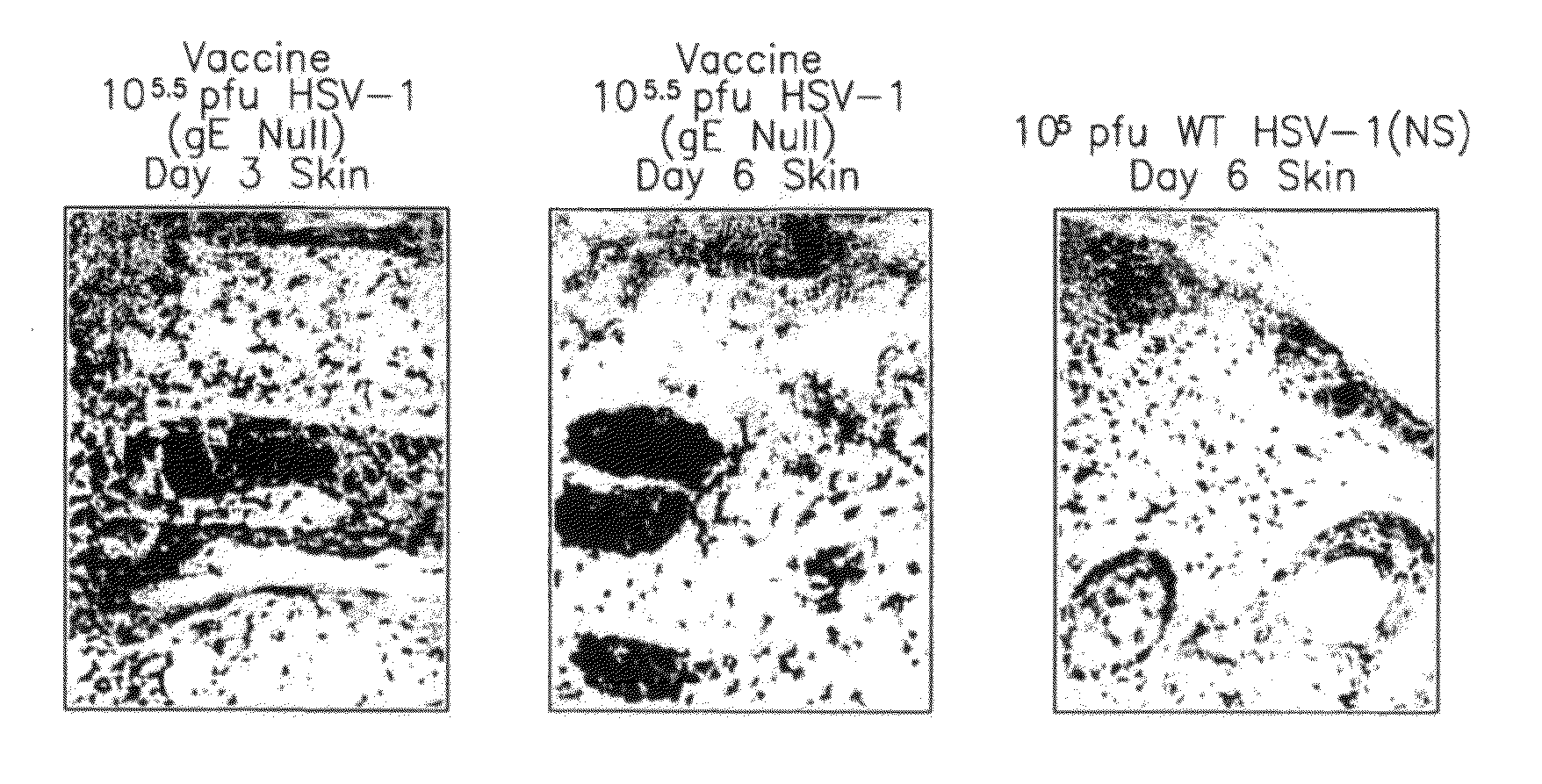
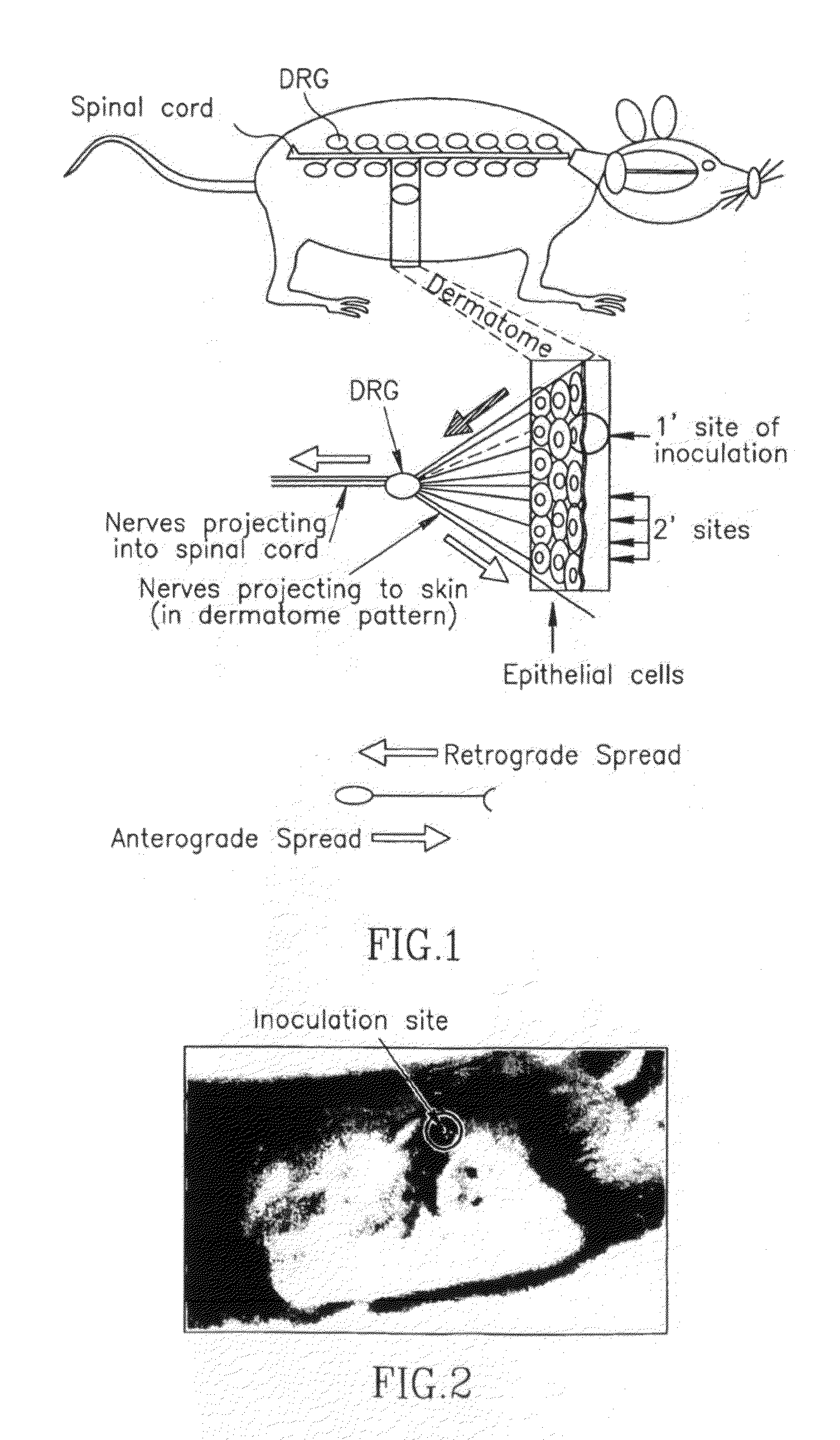
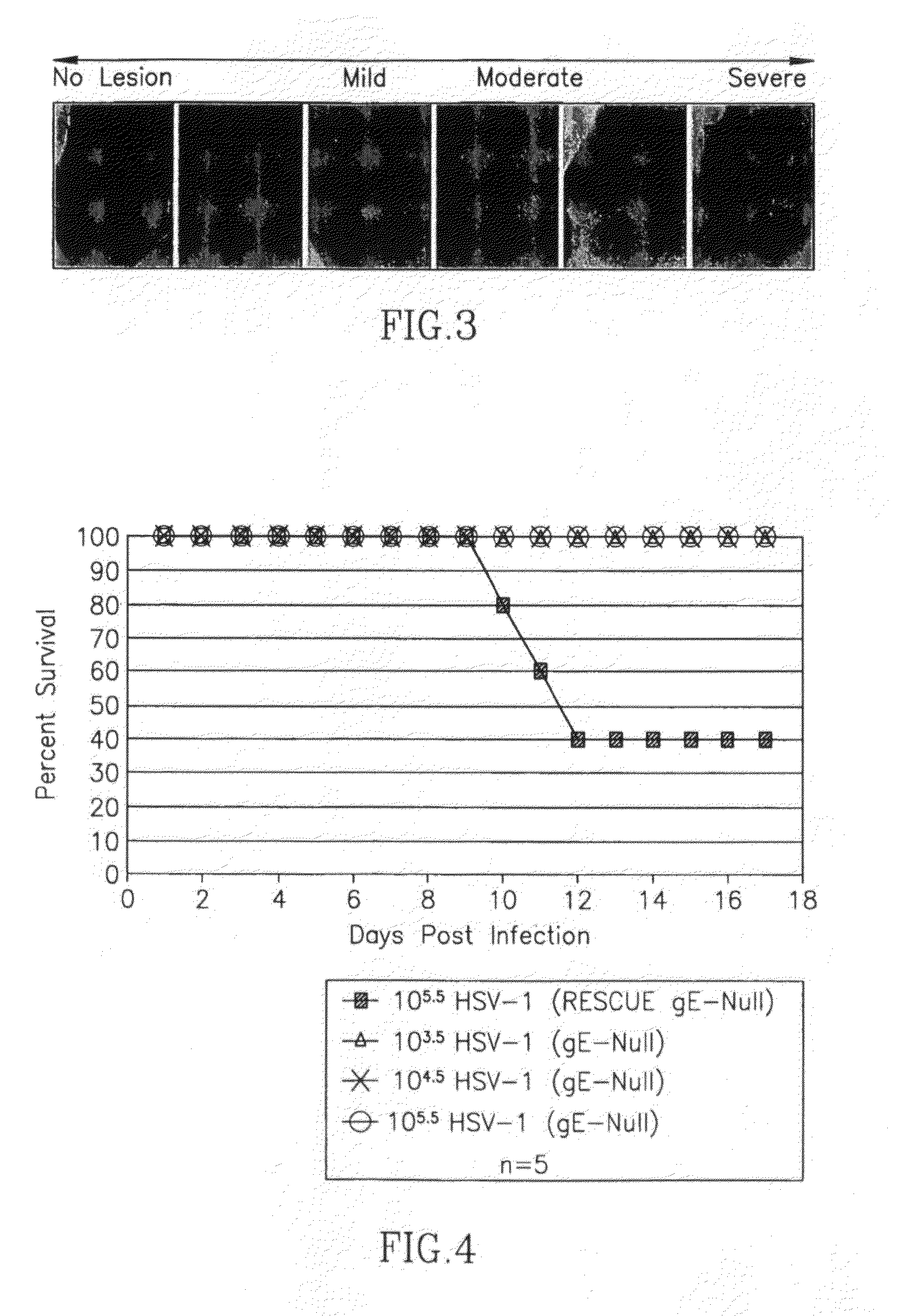
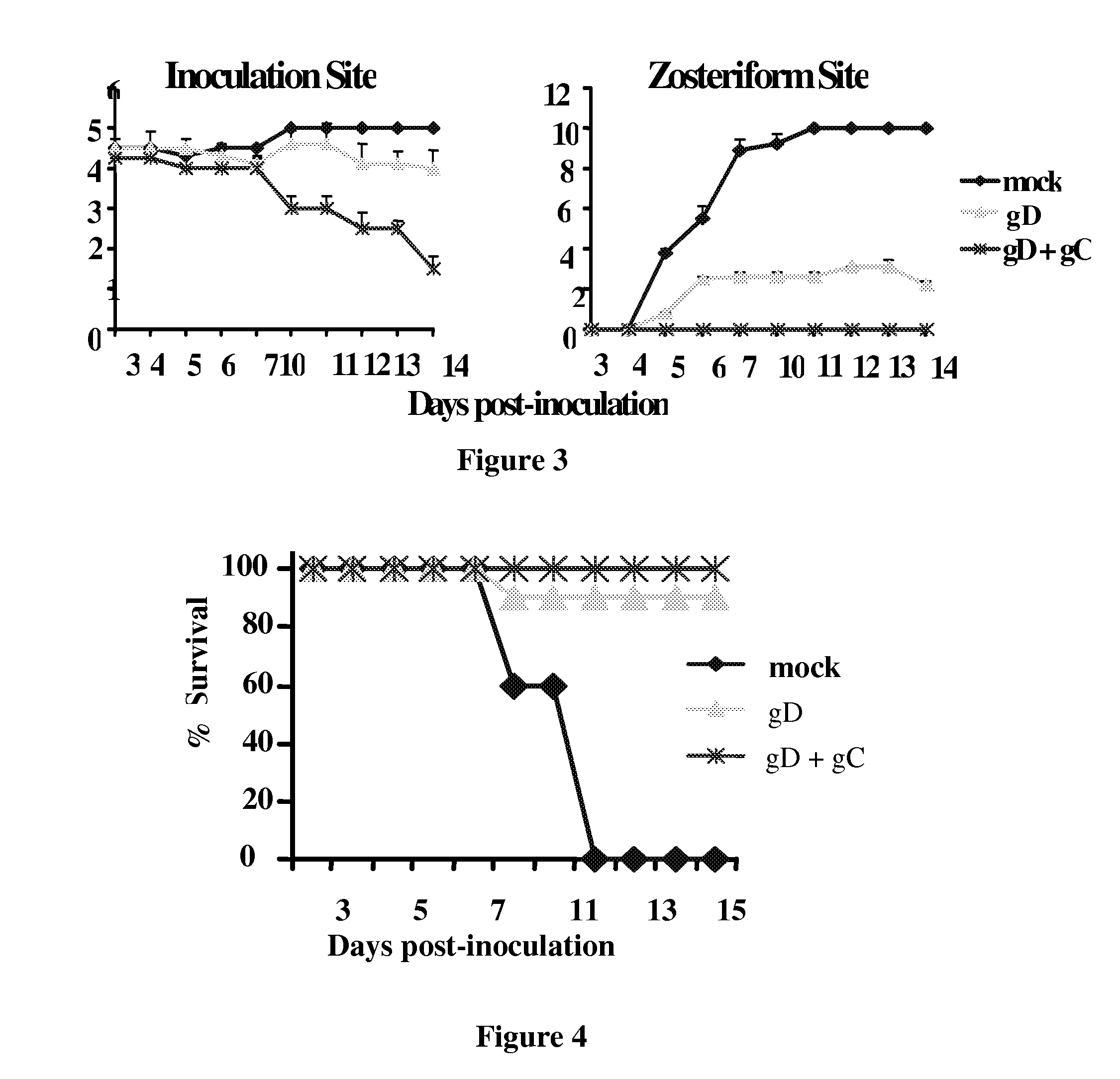
This invention provides methods of vaccinating a subject against a Herpes Simplex Virus (HSV) infection and disorders and symptoms associated with same, and impeding, inhibiting, reducing the incidence of, and suppressing HSV infection, neuronal viral spread, formation of zosteriform lesions, herpetic ocular disease, herpes-mediated encephalitis, and genital ulcer disease in a subject, comprising the step of contacting the subject with a mutant strain of the HSV, containing an inactivating mutation in a gene encoding a gE, gl, Us9, or other proteins.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
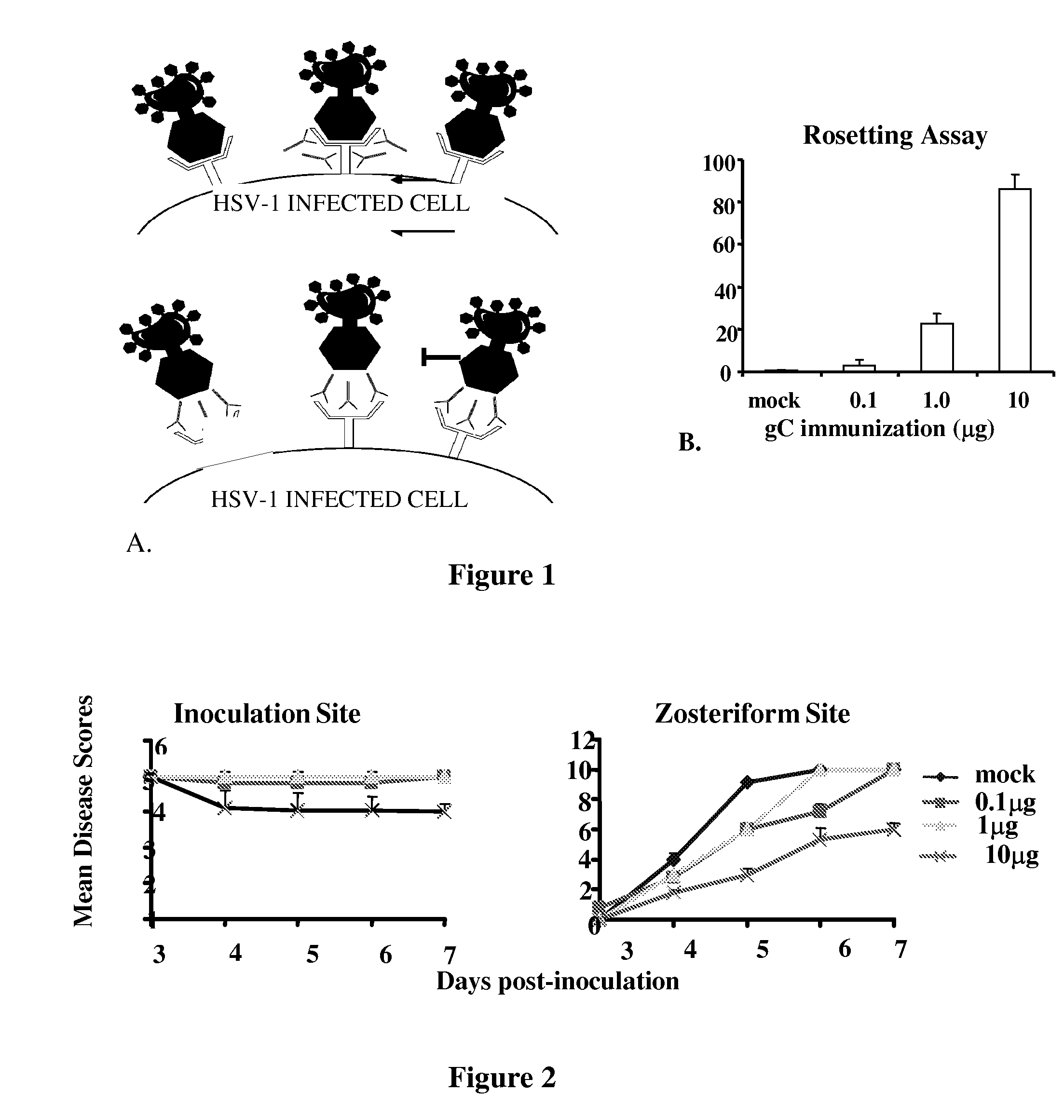
Herpes simplex virus combined subunit vaccines and methods of use thereof
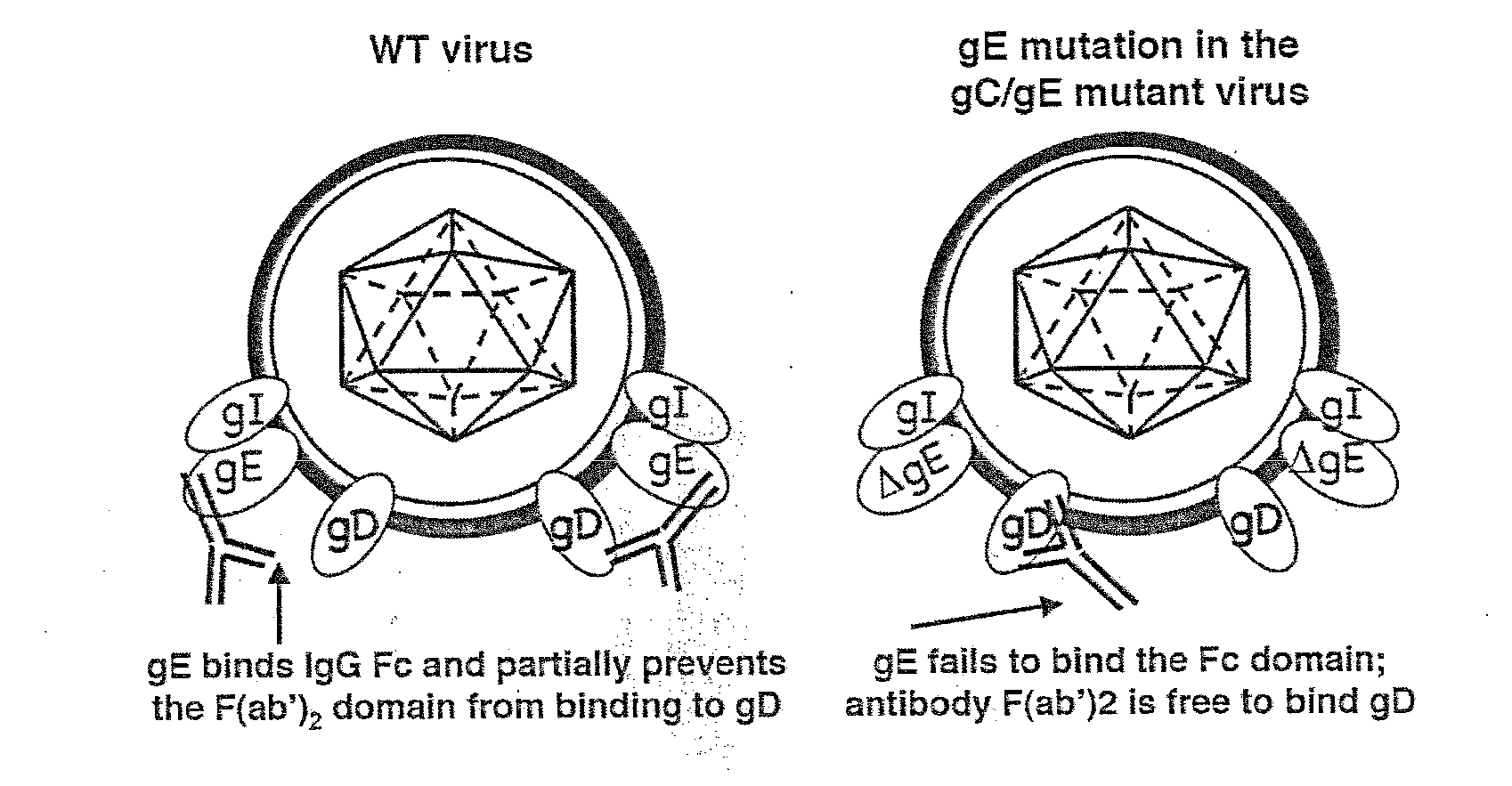
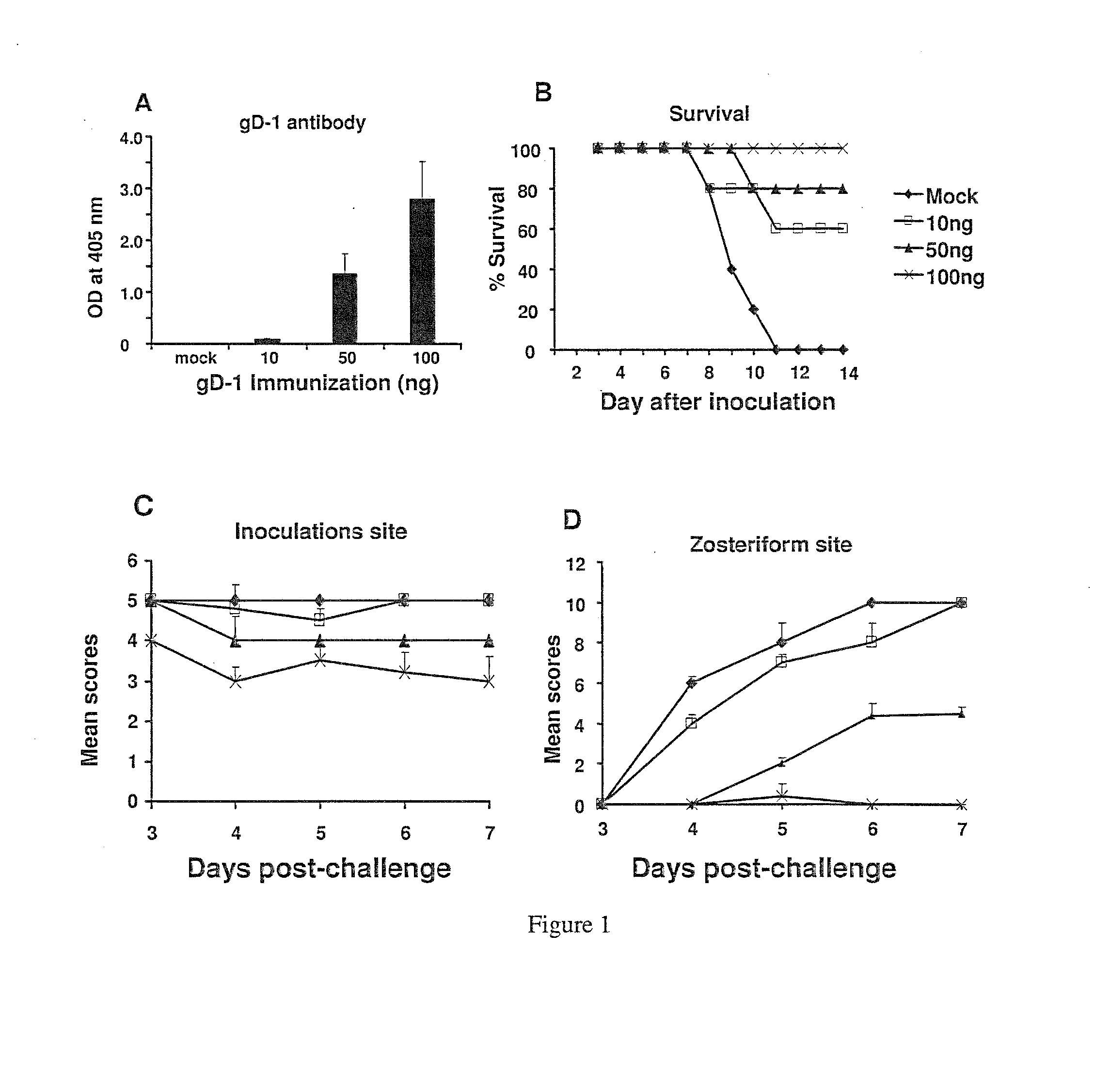
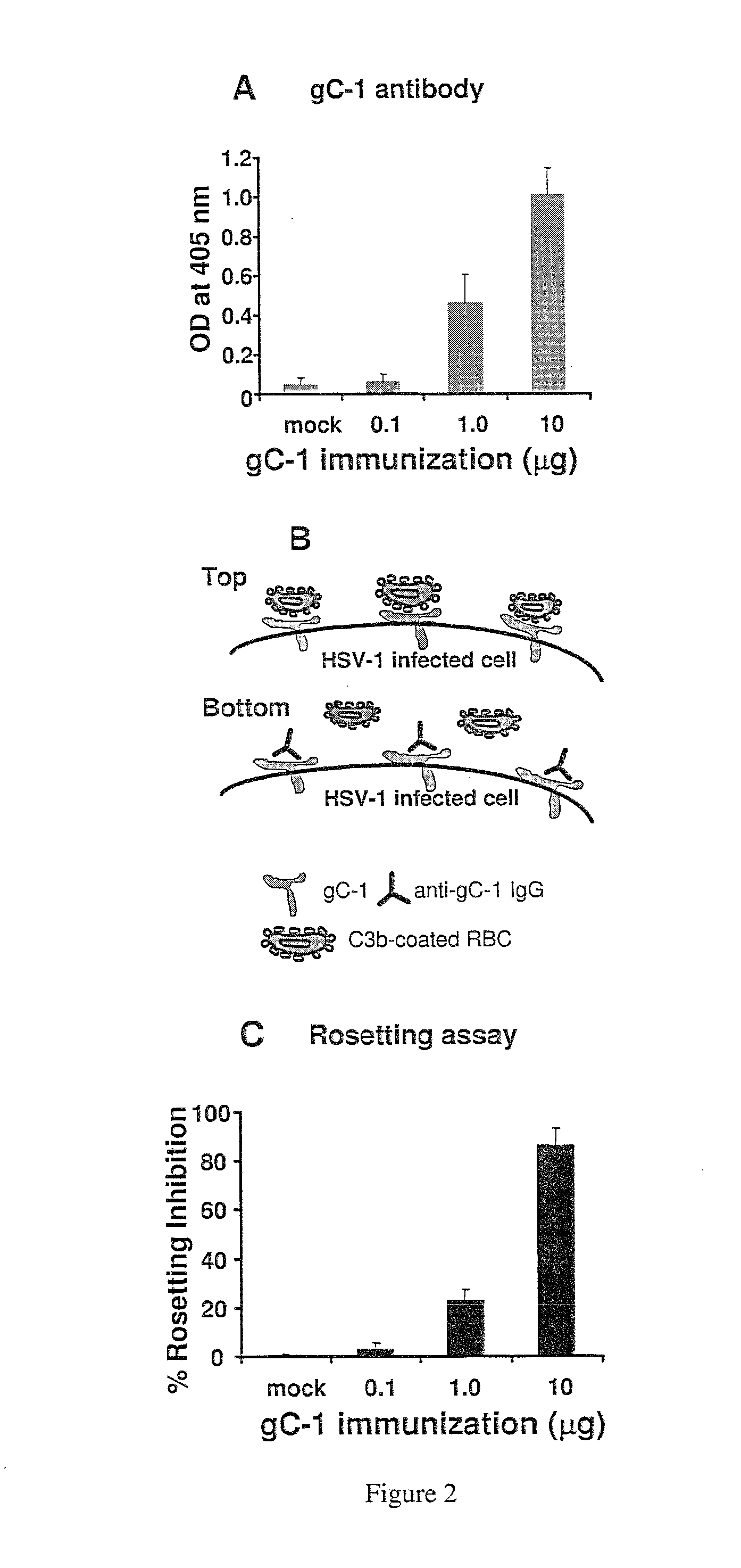
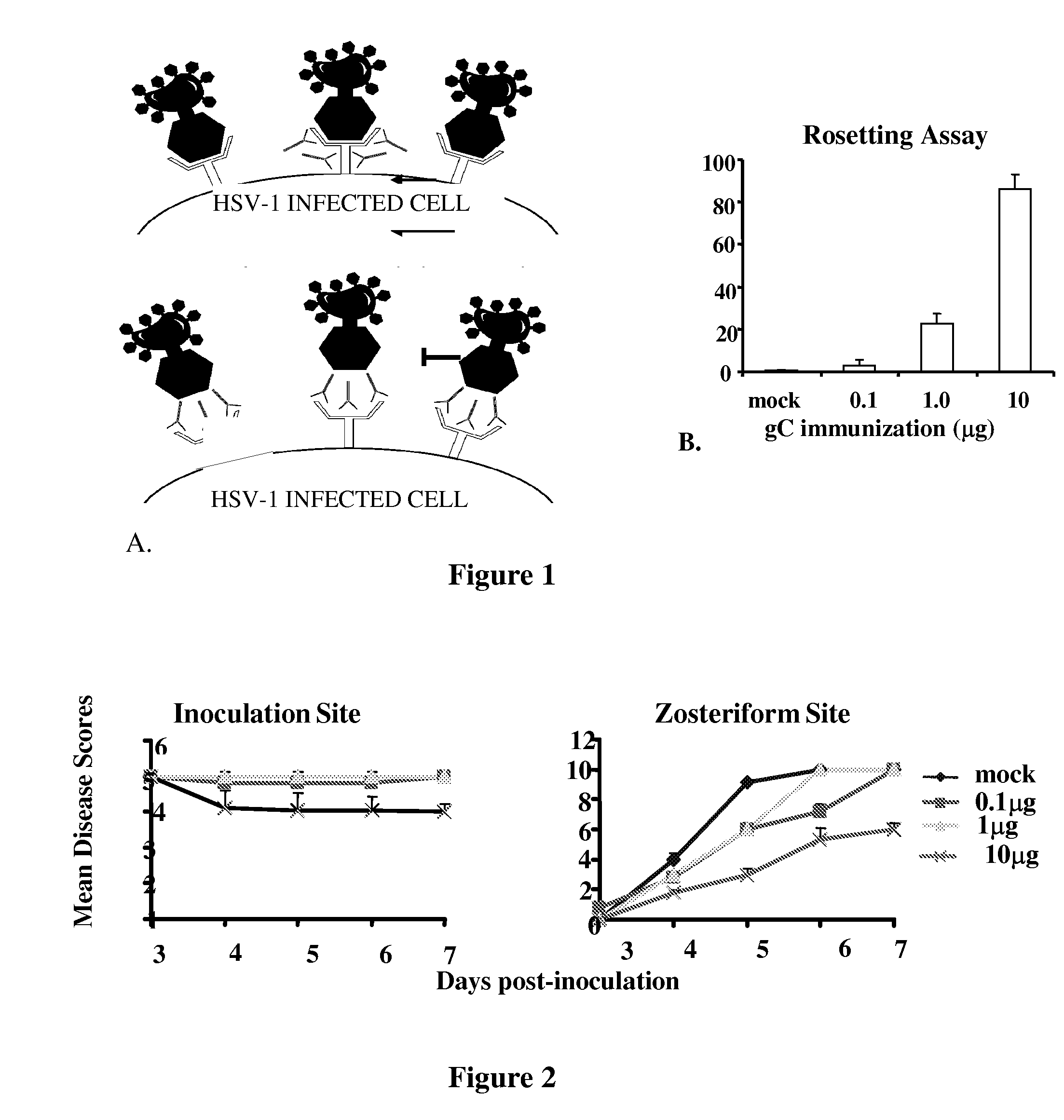
This invention provides immunogenic compositions comprising two or three recombinant Herpes Simplex Virus (HSV) proteins selected from a gD protein, a gC protein and a gE protein; and methods of impeding immune evasion by HSV, inducing an anti-HSV immune response, and treating, suppressing, inhibiting, and / or reducing an incidence of an HSV infection or a symptom or manifestation thereof, comprising administration of a vaccine of the present invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Herpes simplex virus combined subunit vaccines and methods of use thereof
ActiveUS20090148467A1Reduce morbidityViral antigen ingredientsVirus peptidesImmune EvasionsHsv infection
This invention provides vaccines comprising two or more recombinant Herpes Simplex Virus (HSV) proteins selected from a gD protein, a gC protein and a gE protein; and methods of impeding immune evasion by HSV thereby vaccinating a subject against HSV and treating, suppressing, inhibiting, and / or reducing an incidence of an HSV infection or a symptom or manifestation thereof, comprising administration of a vaccine of the present invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Polynucleotide herpes virus vaccine
InactiveUS7094767B2Avoid infectionAmeliorate HSV-related diseaseSugar derivativesGenetic material ingredientsMammalSeroconversion
Genes encoding herpes simplex virus type 2 (HSV-2) proteins were cloned into eukaryotic expression vectors to express the encoded proteins in mammalian muscle cells in vivo. Animals were immunized by injection of these DNA constructs, termed polynucleotide vaccines or PNV, into their muscles. In a DNA titration, it was found that a single immunization of ≧0.5 μg of (one) PNV, gave >90% seroconversion by ten weeks post immunization. Immune antisera neutralized both HSV-2 and HSV-1 in cell culture. When animals were challenged with HSV-2, significant (p<0.001) protection from lethal infection was achieved following PNV vaccination. DNA constructs may be full-length, truncated and / or mutated forms and may be delivered along or in combination in order to optimize immunization and protection from HSV infection.
Owner:MERCK SHARP & DOHME CORP
Hsv-1 epitopes and methods for using same
InactiveUS20100203073A1Increase secretionIncrease productionVirusesAntibody mimetics/scaffoldsEpitopeT-Cell Specificity
The invention provides HSV antigens and epitopes that are useful for the prevention and treatment of HSV infection. T-cells having specificity for antigens of the invention have demonstrated cytotoxic activity against cells loaded with virally-encoded peptide epitopes, and in many cases, against cells infected with HSV. The identification of immunogenic antigens responsible for T-cell specificity provides improved anti-viral therapeutic and prophylactic strategies. Compositions containing antigens or polynucleotides encoding antigens of the invention provide effectively targeted vaccines for prevention and treatment of HSV infection.
Owner:UNIV OF WASHINGTON
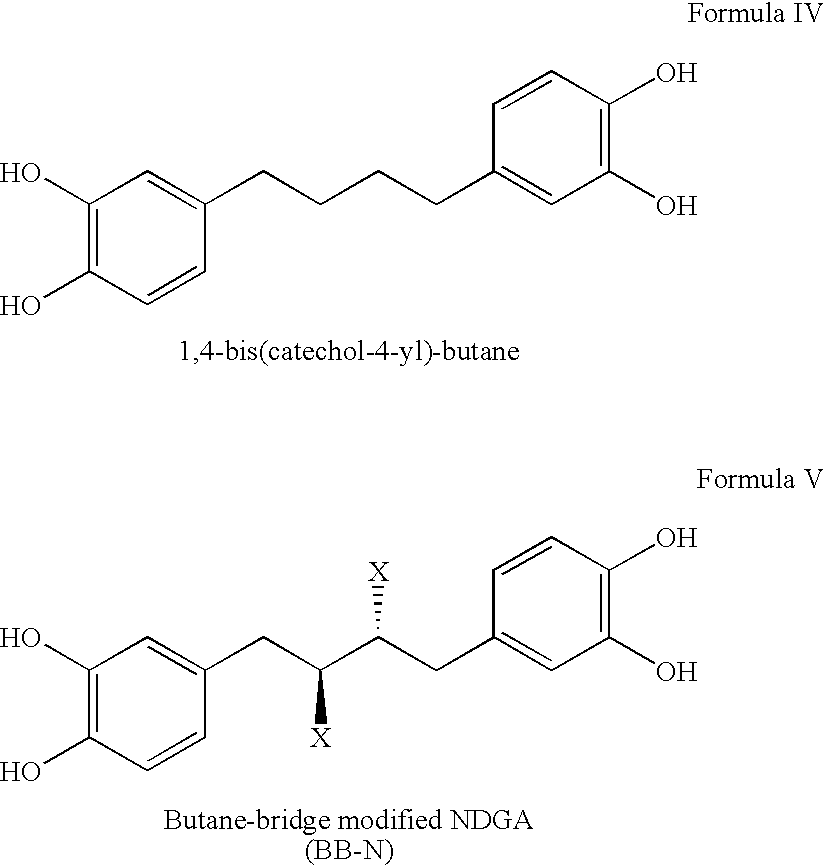
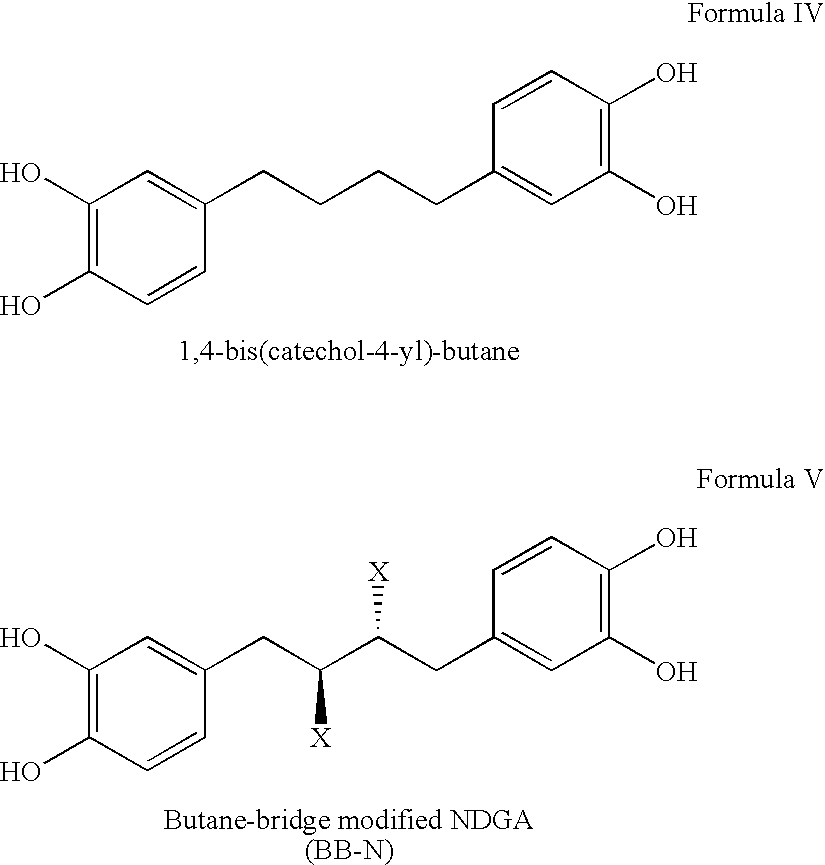
Tetra-O-substituted butane-bridge modified NDGA derivatives, their synthesis and pharmaceutical use
The present invention relates to nordihydroguaiaretic acid derivative compounds, namely, butane bridge modified nordihydroguaiaretic acid (NDGA) compounds and butane bridge modified tetra-O-substituted NDGA compounds, pharmaceutical compositions containing them, methods of making them, and methods of using them and kits including them for the treatment of diseases and disorders, in particular, diseases resulting from or associated with a virus infection, such as HIV infection, HPV infection, or HSV infection, an inflammatory disease, such as various types of arthritis and inflammatory bowel diseases, metabolic diseases, such as diabetes and hypertension, or a proliferative disease, such as diverse types of cancers.
Owner:ERIMOS PHARMA
Method of treating subject infected with Neisseria gonorrhea
InactiveUS7094809B2Reducing cytopathic effectAvoid spreadingAntibacterial agentsBiocideVaricella-zoster virus infectionCytomegalovirus infections
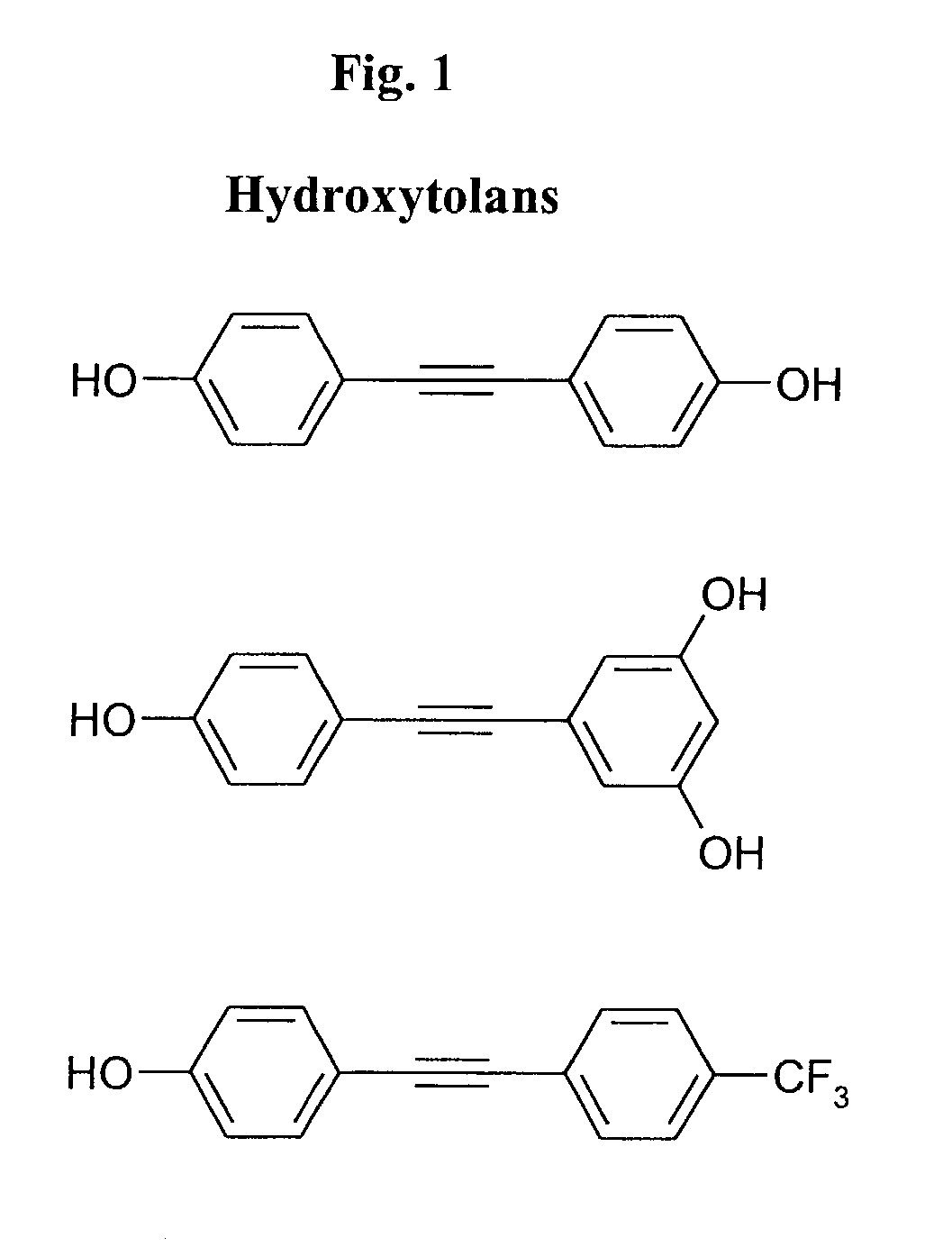
The present invention provides a method of inhibiting the formation of infectious herpes virus particles, particularly infectious herpes simplex virus (HSV) particles, in a host cell. The method involves administering an effective amount of a hydroxylated tolan, particularly a polyhydroxylated tolan, to a herpes virus infected host cell. The present invention also provides a method of treating a herpes virus infection, particularly an HSV infection. The method comprises administering a topical composition comprising a therapeutically effective amount of a hydroxylated tolan to a herpes virus-infected site. The present invention also relates to a topical composition for treating a herpes virus infection selected from the group consisting of an HSV infection, a cytomegalovirus infection, and a varicella zoster virus infection. The present invention also provides a method of treating a subject infected with Neisseria gonorrhea.
Owner:NORTHEASTERN OHIO UNIVERSITIES COLLEGE OF MEDICINE +1
Tetra-o-substituted butane-bridge modified ndga derivatives, their synthesis and pharmaceutical use
The present invention relates to nordihydroguaiaretic acid derivative compounds, namely, butane bridge modified nordihydroguaiaretic acid (NDGA) compounds and butane bridge modified tetra-O-substituted NDGA compounds, pharmaceutical compositions containing them, methods of making them, and methods of using them and kits including them for the treatment of diseases and disorders, in particular, diseases resulting from or associated with a virus infection, such as HIV infection, HPV infection, or HSV infection, an inflammatory disease, such as various types of arthritis and inflammatory bowel diseases, metabolic diseases, such as diabetes and hypertension, or a proliferative disease, such as diverse types of cancers.
Owner:ERIMOS PHARMA
Herpes simplex virus combined subunit vaccines and methods of use thereof
ActiveUS8057804B2Reduce morbidityViral antigen ingredientsVirus peptidesImmune EvasionsHsv infection
This invention provides vaccines comprising two or more recombinant Herpes Simplex Virus (HSV) proteins selected from a gD protein, a gC protein and a gE protein; and methods of impeding immune evasion by HSV thereby vaccinating a subject against HSV and treating, suppressing, inhibiting, and / or reducing an incidence of an HSV infection or a symptom or manifestation thereof, comprising administration of a vaccine of the present invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and methods for identifying and testing therapeutics against HSV infection
The invention provides compositions a methods of identifying and testing therapeutics against HSV infection, and in particular, compositions comprising receptors which enable cell specific entry of HSV. The invention also provides a novel DNA sequence that encodes a protein B5T74 that confers the ability of herpes simplex virus (HSV) to infect and replicate in otherwise non-permissive cells. Also provided are vectors comprising the isolated nucleic acids encoding HSV receptors in host suitable for expression of the nucleic acids encoding the HSV receptors, fragments of the HSV receptors, or homologs of the HSV receptor. Further provided is a porcine cell system which expresses a herpes simplex virus receptor, but does not express endogenous, HSV entry receptors.
Owner:MICHIGAN UNIV OF THE RGT
Recombinant aviadenovirus type 4 fiber2 protein as well as preparation method and application thereof
ActiveCN112142830AImprove solubilityImproving immunogenicityBacteriaViral antigen ingredientsAntigen epitopeHighly pathogenic
The invention discloses a recombinant fowl adenovirus type 4 (FAdV-4) fiber2 protein as well as a preparation method and application thereof, and belongs to the technical field of gene engineering. The recombinant protein is obtained by removing 274 amino acid residues at the N end of the fowl adenovirus type 4 fiber2 protein and then recombining with the 21-55th amino acid sequence of the fowl adenovirus type 4 hexon protein; and can be used for preparing the avian adenovirus 4-type genetic engineering subunit vaccine. Aiming at the defects of poor solubility and immunogenicity of prokaryoticexpression natural fiber2 protein, the fiber2 protein coding gene is subjected to series modification, and compared with the unmodified fiber2 protein, the recombinant protein has the advantages thatthe gene coding sequence of 274 amino acid residues at the N end of the fiber2 protein is removed, and the gene coding sequences of two important antigen epitopes of the hexon protein are fused; so that the solubility and the immunogenicity are obviously improved, so that the subunit vaccine which is controllable in quality, safe and effective and can prevent highly pathogenic FAdV-4 virus infection can be prepared, and complete protection can be obtained by once immunization.
Owner:YANGTZE UNIVERSITY +1
Herpes simplex virus ICP4 is an inhibitor of apoptosis
InactiveUS6723511B2Peptide/protein ingredientsGenetic material ingredientsInhibitor of apoptosisViral Genes
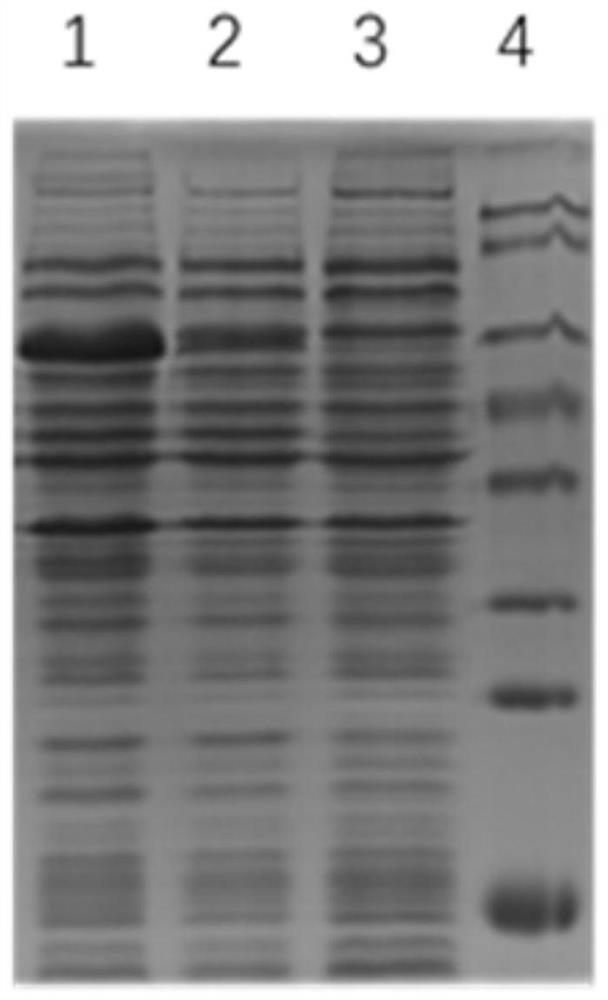
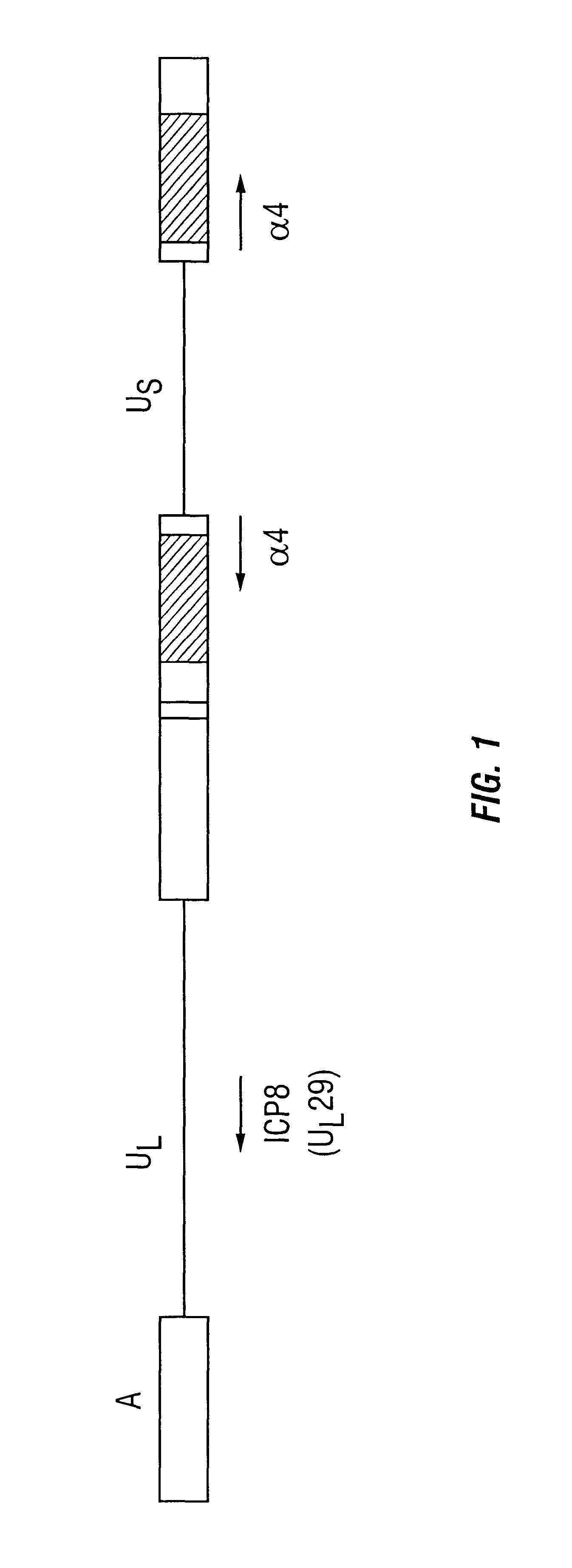
The ICP4 protein of herpes simplex virus plays an important role in the transactivation of viral genes. The present invention discloses that ICP4 also has the ability to inhibit apoptosis. This function appears to reside in functional domain distinct from the transactivating function, as indicated by studies using temperature sensitive mutants of ICP4 that transactivating function at elevated temperatures. Also disclosed are methods for inhibition of apoptosis using ICP4 or an ICP4 encoding gene, such as an alpha4 gene, methods of inhibiting ICP4's apoptosis-inhibiting function, and methods for the production of recombinant proteins and treatment of HSV infections.
Owner:ARCH DEVMENT
Tetra-substituted ndga derivatives via ether bonds and carbamate bonds and their synthesis and pharmaceutical use
Disclosed are nordihydroguaiaretic acid derivative compounds including various end groups bonded by a carbon atom or heteroatom though a side chain bonded to the respective hydroxy residue O groups by an ether bond or a carbamate bond, pharmaceutical compositions, methods of making them, and methods of using them and kits including them for the treatment of diseases and disorders, in particular, diseases resulting from or associated with a virus infection, such as HIV infection, HPV infection, or HSV infection, an inflammatory disease, such as various types of arthritis and inflammatory bowel diseases, a metabolic disease, such as diabetes, a vascular disease, such as hypertension and macular degeneration, or a proliferative disease, such as diverse types of cancers.
Owner:ERIMOS PHARMA
Viral prophylaxis treatment methods and pre-exposure prophylaxis kits
ActiveUS20200397791A1Improve bioavailabilityMinimal protein bindingAntiviralsTamponsPre-exposure prophylaxisSerum reaction
The present disclosure provides compositions and methods for the prevention of HSV infection in an HSV seronegative individual and for the treatment and prevention of recurrence in an HSV seropositive individual.
Owner:ELIAN LLC
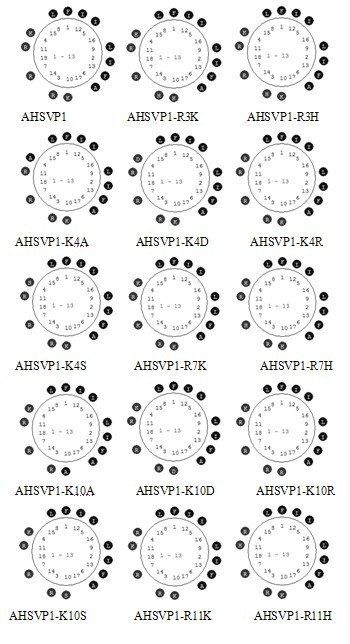
Anti-herpes simplex virus (HSV) active polypeptide of Tibetan Pi scorpion and application thereof
InactiveCN102382839AEffective treatmentThe effect is obviousPeptide/protein ingredientsPharmaceutical delivery mechanismChemical synthesisMedicine
The invention discloses an anti-HSV active polypeptide of Tibetan Pi scorpion and application thereof. According to the invention, through the methods of molecular biology and chemical synthesis, the anti-HSV active polypeptide of Tibetan Pi scorpion, AHSVP1, is obtained; the antiviral activity of AHSVP1 to HSV is determined by using the method of plaque array. The invention also discloses externally used anti-HSV gel which is prepared from 0.5 wt % of the medicine AHSVP1 and 1.5 wt % of a carboxymethylcellulose matrix. The gel provided in the invention is used for anti-HSV infection; the products of the gel are stable and are suitable for storing and transporting. A production process for the gel has the advantages of simple and reasonable design, low production cost and suitability for mass industrial production.
Owner:WUHAN UNIV
Hsv-1 and hsv-2 vaccines and methods of use thereof
ActiveUS20120114695A1Reducing pathogenesisImprove latencyViral antigen ingredientsVirus peptidesRecurrent herpes simplexHsv infection
This invention provides methods of treating, suppressing, inhibiting, reducing an incidence, reducing the pathogenesis of, ameliorating the symptoms of, or ameliorating the secondary symptoms of a primary or recurring Herpes Simplex Virus (HSV) infection, or prolonging the latency to a relapse of an HSV infection, and disorders and symptoms associated with same and inducing an anti-HSV immune response in a subject comprising the step of contacting the subject with a composition comprising a mutant HSV strain comprising an inactivating mutation in a Us8 gene, followed by a second contacting with the composition.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Application of germacrone in preparing HSV (Herpes Simplex Virus) resisting medicine
InactiveCN103330700AGood activity against herpes simplex virusOrganic active ingredientsAntiviralsHuman useRabies
The invention relates to an application of germacrone in preparing an HSV (Herpes Simplex Virus) resisting medicine. By taking HSV-1 (Herpes Simplex Virus type 1) and HSV-II (Herpes Simplex Virus type 2) strains and Vero (Rabies Purified Vaccine for Human Use) cells as experimental subjects, the HSV resisting activity of the germacrone is researched. The result shows that the germacrone has the good HSV resisting activity. As the germacrone is used for preparing the HSV treating / preventing medicine, an important development and application prospect for preventing and treating diseases caused by the HSV infection is achieved.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Preparation method of trace biological sample DNA template and kit for detecting HSV infection of eye
Owner:PEKING UNIV THIRD HOSPITAL
Viral prophylaxis treatment methods and pre-exposure prophylaxis kits
ActiveUS10052329B2Reduce frequencyReduce riskPharmaceutical delivery mechanismAntiviralsPre-exposure prophylaxisVirus
The present disclosure provides compositions and methods for the prevention of HSV infection in an HSV seronegative individual.
Owner:ELIAN LLC
Herpes simplex virus combined subunit vaccines and methods of use thereof
ActiveUS20090098162A1Suppressing and inhibiting and reducing incidenceSenses disorderNervous disorderHsv infectionSubunit vaccines
This invention provides vaccines comprising two or more recombinant Herpes Simplex Virus (HSV) proteins selected from a gD protein, a gC protein and a gE protein; and methods of vaccinating a subject against HSV and treating, impeding, inhibiting, reducing the incidence of, or suppressing an HSV infection or a symptom or manifestation thereof, comprising administration of a vaccine of the present invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Anti-herpes simplex virus antibodies and methods of use thereof
The invention provides antibodies and polypeptides that specifically bind to the glycoprotein D of herpes simplex virus (HSV) and use of the antibodies and polypeptides for treating or diagnosing HSV infections.
Owner:DEV CENT FOR BIOTECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com