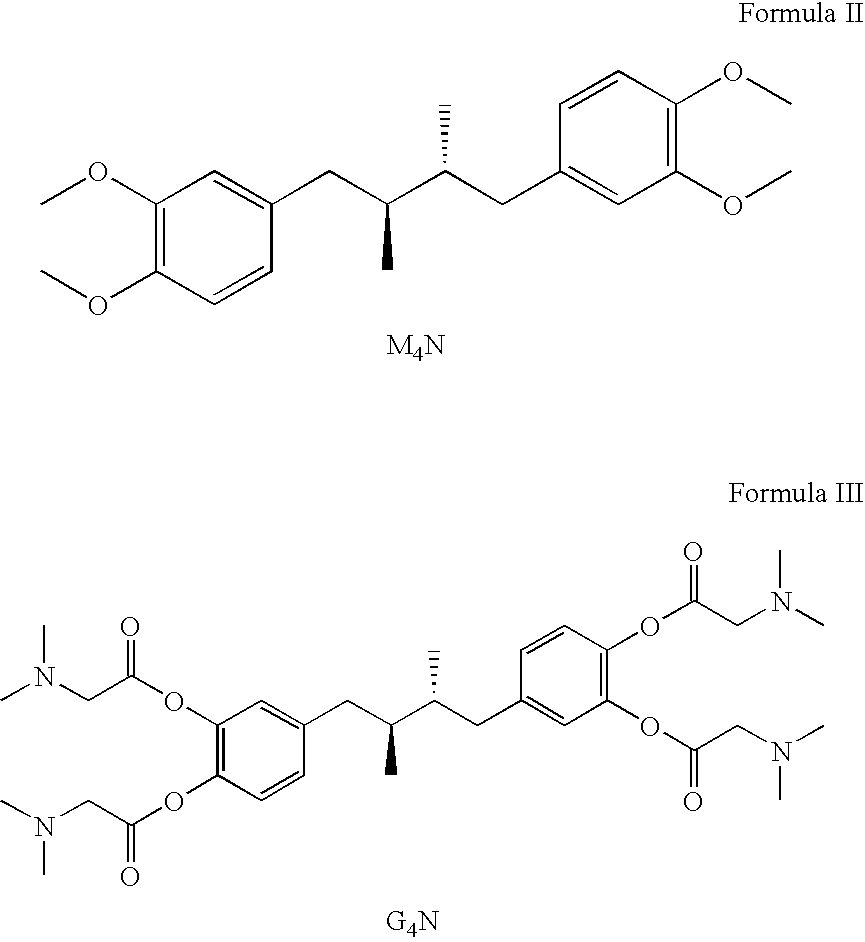
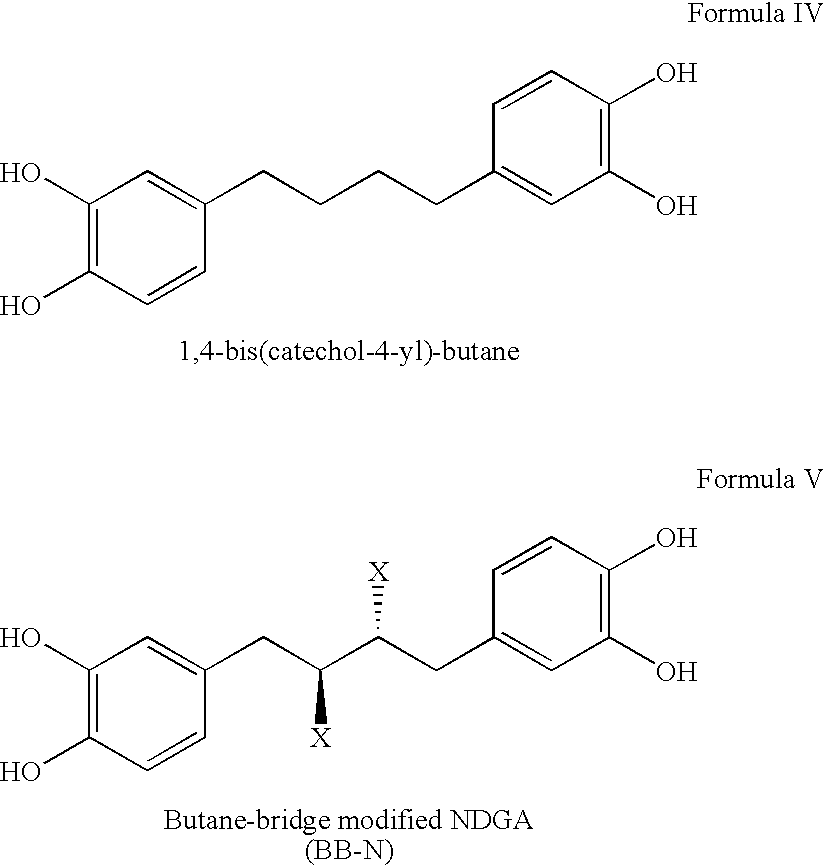
Tetra-O-substituted butane-bridge modified NDGA derivatives, their synthesis and pharmaceutical use
a technology of tetra-o-substituted butane bridge and derivatives, applied in the field of nordihydroguaiaretic acid derivatives, can solve the problems of poor water solubility, limited application for certain drug action studies, and unstable stability, and achieves a relatively short half-life in aqueous solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 1,4-bis-(3,4-methoxyphenyl)butane (C20H26O4, FW=330.42) “Compound A”
[0141]
[0142]The compound was synthesized using a modification of Nakamura, et al.20 involving coupling of 1,4-diiodobutane with Grignard reagent derived from 3,4dimethoxybromobenzene in 43% yield. m.p. 93-94° C. (literature21 m.p. 91-92° C.). HPLC purity: 99.25%.
[0143]1HNMR (CDCl3, 300 MHz): δ=1.58-1.68 (m, 4H), 2.55-2.65 (m, 4H), 3.85 (s, 6H), 3.86 (s, 6H), 6.69 (s, 2H), 6.70 (dd, J=7.9, 1.6 Hz, 2H), 6.78 (d, J=7.9 Hz, 2H) ppm, consistent with the structure.
[0144]13CNMR (CDCl3, 75 MHz): δ=31.1, 35.3, 55.7, 55.9, 111.2, 111.7, 120.1, 135.2, 147.0, 148.7 ppm; consistent with the structure.
[0145]Analysis: calculated for C20H26O4, C: 72.70, H: 7.95; found C: 72.51, H: 7.82.
[0146]MS (EI), m / e=331 (M+1); consistent with C20H26O4
example 2
Synthesis of 1,4-bis-(3,4-hydroxyphenyl)butane (alternatively named 1,4-bis(catechol-4-yl)-butane) [C16H18O4, FW=274.31) “Compound B”
[0147]
[0148]This compound was synthesized by cleavage of the aromatic methoxy group of the product of Example 1, (1,4-bis-(3,4-methoxyphenyl) butane), using boron tribromide22 in quantitative yield. The crude product was found to be pure by TLC, so it was used for the preparation of derivatives without further purification. Analytical sample was purified by a flash silica gel chromatographic column using dichloromethane and methanol (95:5, V / V) as eluant.
[0149]m.p 140-142° C. HPLC purity: 98.5%.
[0150]1H NMR (DMSO-d6, 300 MHz): δ=1.60 (t, J=6.5 Hz, 4H, 2 CH2), 2.65 (t, J=6.5 Hz, 4H, 2 CH2), 6.65-6.85 (m, 6H, 6 Ar—H), 9.56 (brs, 4H, 40H) ppm; consistent with the structure.
[0151]13C NMR (DMSO-d6, 75 MHz): δ=31.5, 36.3, 114.5, 115.8, 120.1, 136.5, 143.5, 145.5 ppm, consistent with the structure.
[0152]MS (EI), m / e=275 (M+1), consistent with C16H18O4.
[0153]A...
example 3
Synthesis of 1,4-bis-(3,4-ethoxyphenyl)butane (C24H34O4, FW=386.52) “Compound C”
[0154]
[0155]To a solution of 1,4-bis-(3,4-hydroxyphenyl)butane from Example 2 (700 mg, 2.56 mmol) in acetone (26 mL) was added potassium carbonate (2.39 g, 16.9 mmol, 6.6 equivalents) and iodoethane (2.40 g, 15.4 mmol, 6.0 equivalents) and the mixture heated to reflux for 24 hours. TLC of the reaction indicated completion of the reaction. The reaction mixture was cooled to room temperature, filtered and the solids were washed with acetone. The combined filtrate was concentrated under reduced pressure. The residue was dissolved in ethyl acetate (100 mL) and washed with water (50 mL). The aqueous layer was re-extracted with ethyl acetate (50 mL). The combined organic extracts were dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the crude product (1.06 g), which was crystallized from ethyl acetate -isopropanol (1:1, 6 mL) to give a pure crystalline product (700 mg, 70% yield).
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com