Novel pneumococcal conjugate vaccine and preparation method thereof
A pneumococcal conjugate vaccine technology, which can be used in medical preparations containing active ingredients, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as restricting the development of pneumococcal conjugate vaccines and inconclusive PPV23 efficacy and effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
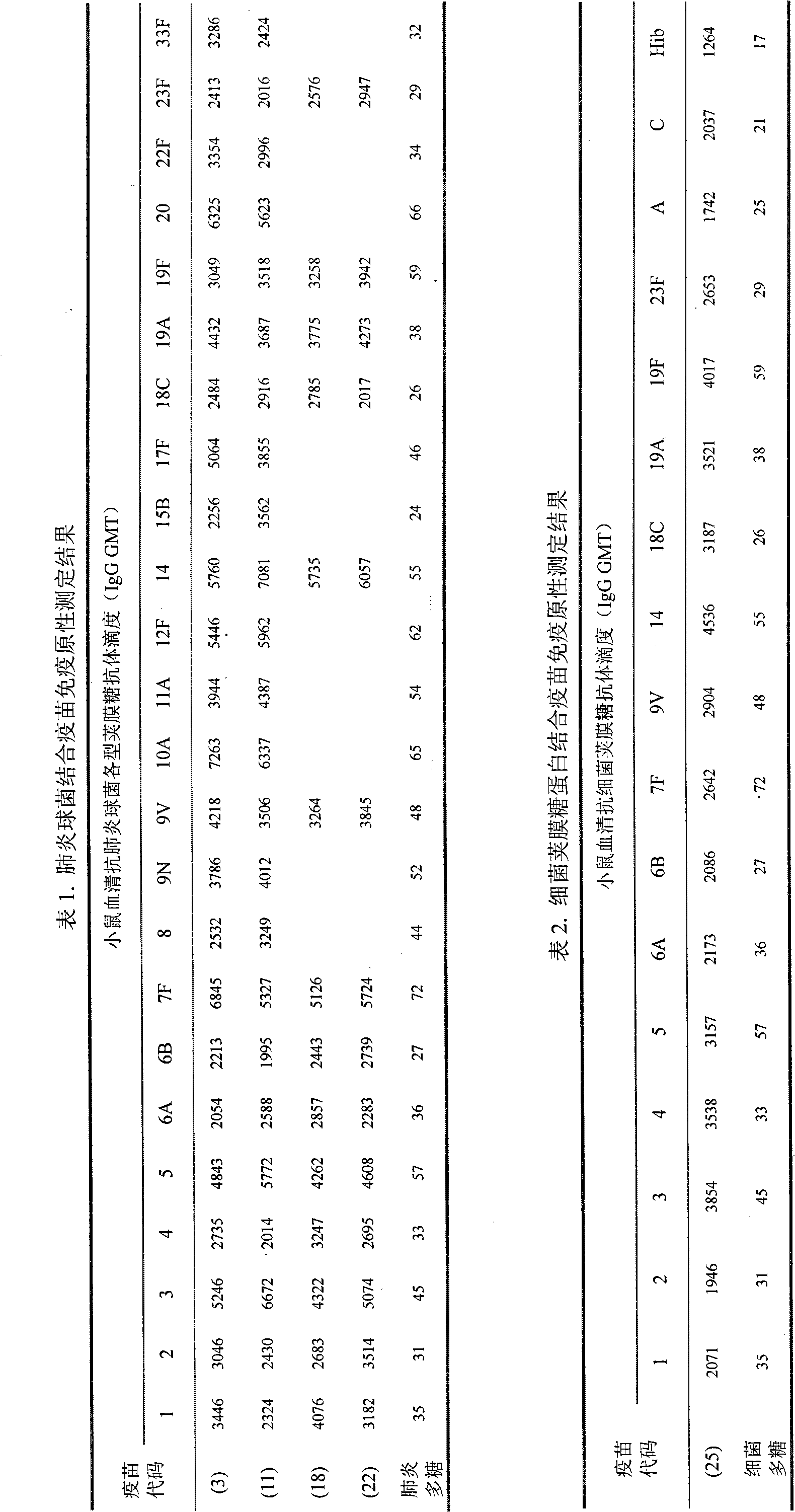
[0077] Example 1 Fermentation culture of pneumococcus and preparation of capsular polysaccharide
[0078] Open the cryopreserved pneumococcal freeze-dried strains, redissolve with physiological sodium chloride solution, inoculate into 5-10% sheep blood agar slant medium, place in 5-8% CO 2 In the environment, cultivate at 35-37°C for 16-24 hours. Transfer to pneumococcal liquid basal medium, and cultivate at 35-37°C for 10-18 hours. Continue to transfer to pneumococcal liquid basal medium for enrichment culture, and cultivate at 35-37°C for 10-18 hours. Inoculate the enriched pneumococcal liquid culture into the fermenter with liquid basal medium according to the inoculum amount of 5-10%, stir and ventilate, cultivate at 35-37°C for 8-12 hours, and control the pH at 6.0 ~7.4. During the fermentation period, the concentration of bacteria was monitored, and the purity of the culture and the morphological characteristics of the bacteria were checked under a microscope. At the...
Embodiment 2
[0082] Example 2 Preparation of Pneumococcal Capsular Oligosaccharides
[0083] The purified polysaccharide is dissolved in 0.2-1.0M acetic acid solution to a final concentration of 1-4mg / ml, and immersed in an oil bath or water bath at 70-100°C for 6-40 hours for hydrolysis. After hydrolysis, the pH was adjusted to 7.0, and dialyzed at 2-8°C. Purified by Sepharose 4FF chromatography column, eluted with 15mM NaCl solution, and collected oligosaccharides. Purified oligosaccharides were obtained by 0.22μm sterile filtration and stored below -20°C in liquid or lyophilized form. Purified oligosaccharides were tested according to the methods and standards stipulated in the pneumococcal conjugate vaccine manufacturing and testing regulations officially promulgated by the World Health Organization (WHO Technical Report Series, No.927, 2005).
[0084] Prepare pneumococcal serotypes 1, 2, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20 according to the ...
Embodiment 3
[0085] Example 3 Preparation of meningococcal capsular saccharide and Haemophilus influenzae type b capsular saccharide
[0086] According to the method of Chinese patent 200710007045.1, the capsular polysaccharides of group A, group C, group W135 and group Y meningococcus and the capsular polysaccharide of Haemophilus influenzae type b were prepared.
[0087] Then according to the method of Example 2 of the present invention, the capsular polysaccharides of group A, group C, group W135 and group Y meningococcus and the capsular oligosaccharide of Haemophilus influenzae type b were prepared.
[0088] Manufacture and test regulations (WHO Technical Report Series, No.658, 1980) and Haemophilus influenzae type b conjugate vaccines (WHO Technical Report Series, No.897, 2000) stipulated methods and standards to test the purified polysaccharides and oligosaccharides.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com