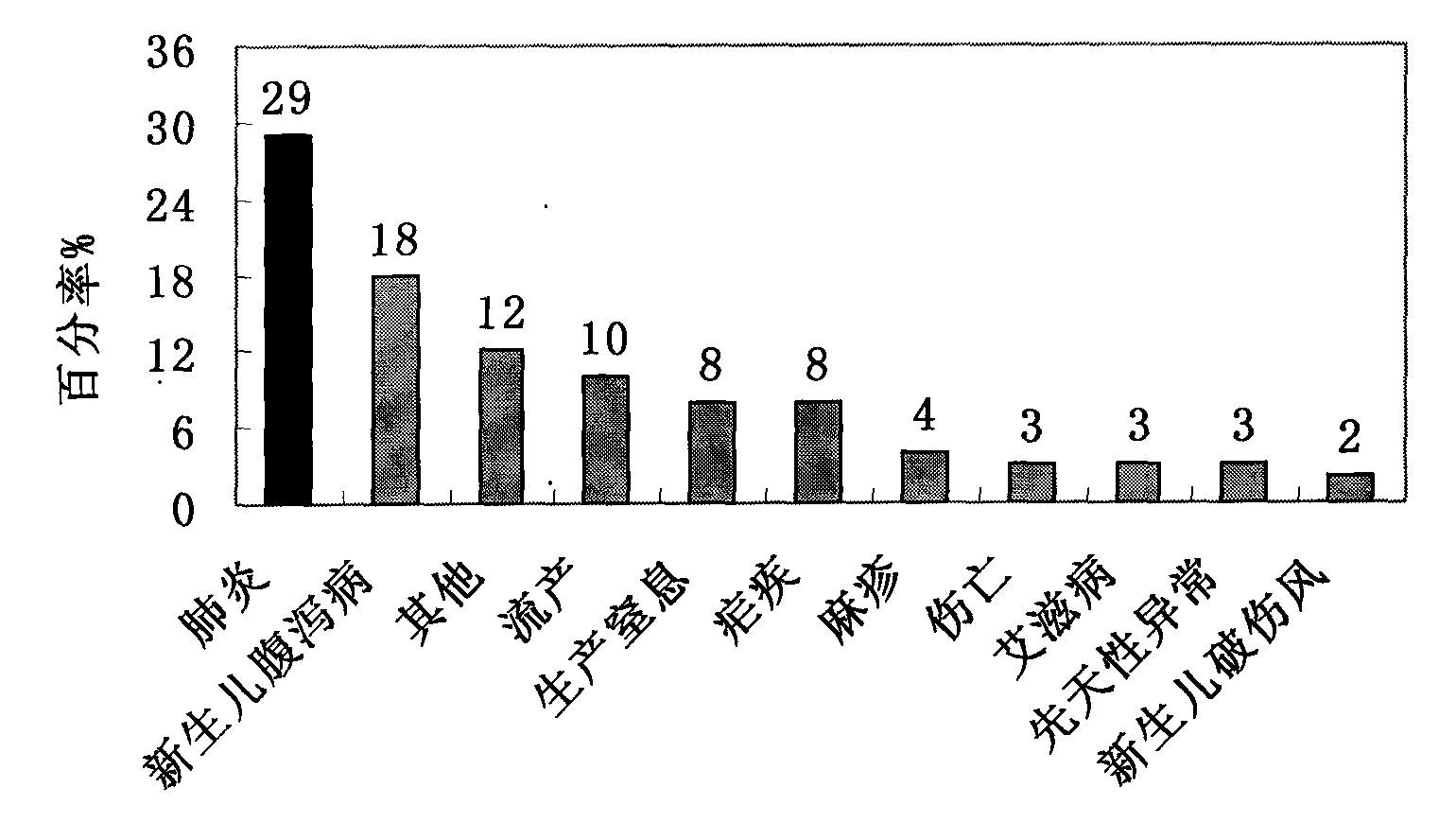
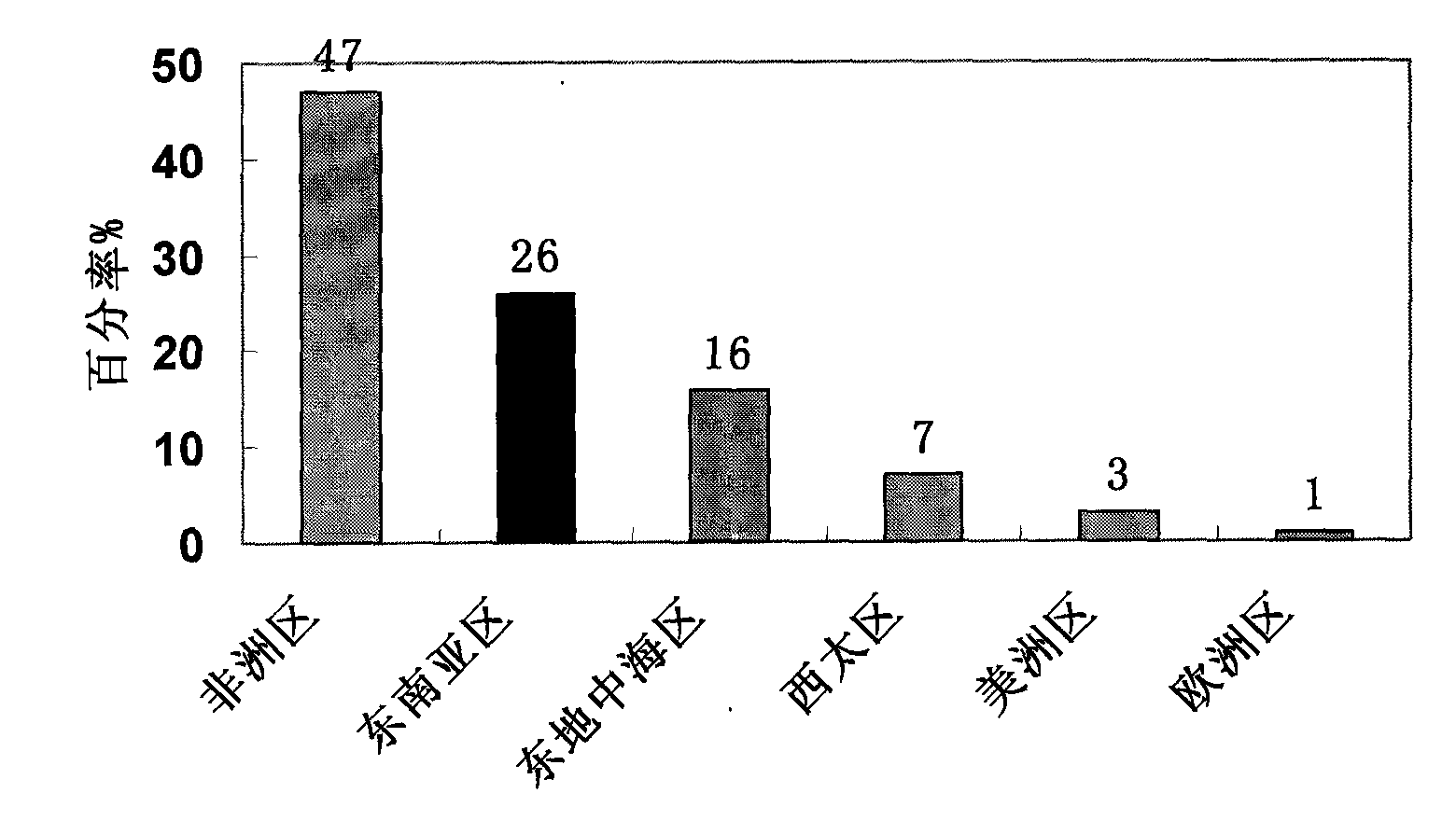
High-efficiency 14-valent pneumococcal conjugate vaccine
A pneumococcal and combined vaccine technology, applied in the field of immunology, can solve the problems of low-affinity antibodies, lack of immune memory and immune enhancement effects, and prone to immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the cultivation of 14 kinds of serotypes of pneumococcus
[0038] Considering factors such as the prevailing bacterial type in China, the susceptible bacterial type of children and the elderly, the drug-resistant bacterial type, and cross-protection, 14 serotypes (1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F and 23F) pneumococcal culture;
[0039] (1) Bacteria: 1, 2, 4, 5, 6A, 6B, 7F, 9N, 9V, 14, 18C, 19A, 19F, and 23F, a total of 14 serotype strains, which can be purchased from China Pharmaceutical and Biological Products Inspection Institute (China Medical Bacteria Preservation Management Center), the deposit numbers are 31401, 31402, 31404, 31405, 31406, 31409, 31414, 31419, 31423, 31426, 31451, 31456, 31457 and 31468. It can be identified by fluorescent labeling first, first incubated with serotype-specific primary antibody from rabbit, and then incubated with Rhodamine Red-X-labeled goat anti-rabbit IgG (secondary antibody), and the normal resul...
Embodiment 2
[0043] Example 2: Purification of pneumococcal capsular polysaccharide and its quality identification
[0044] 1. Purification of pneumococcal capsular polysaccharide:
[0045] (1) The sterilized bacterial liquid is centrifuged with a centrifuge to remove the bacterial cells to obtain a stock solution-crude polysaccharide, and the bacterial cells separated by centrifugation are sterilized with formaldehyde. (2) After obtaining the crude solution of polysaccharide aggregated from the stock solution, cetyltrimethylammonium bromide is added to precipitate the composite polysaccharide, and the polysaccharide is precipitated and centrifuged to aggregate the polysaccharide. (3) After the precipitation and centrifugation are completed to collect the complex polysaccharide, dissociation and purification of calcium chloride is carried out. Subsequently, 95% alcohol was added for nucleic acid removal. Afterwards, continue to add alcohol with a concentration of 95% to precipitate polys...
Embodiment 3
[0050] Embodiment 3: Carrier protein (influenza hemotropic protein D, tetanus toxoid protein and diphtheria toxin avirulent variant protein CRM 197 ) quality control
[0051] Using the safe and effective influenza hemotropic D protein, tetanus toxoid protein and diphtheria toxin non-toxic variant protein CRM that has been verified by clinical experiments 197 As the protein carrier of our conjugated vaccine, its characteristics and purity were checked by referring to the existing internationally recognized quality standards: Determination of CRM of influenza hemotropic protein D, tetanus toxoid protein and diphtheria toxin non-toxic variant protein by HPLC 197 The purity is more than 90% qualified; ensure that the amino acid sequence of each protein is correct; ensure that it is not produced in the same workshop as diphtheria toxoid; use SDS-PAGE analysis to determine the composition of the purified protein; the content of lipopolysaccharide is less than 8 % means that the qua...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com