Processes for the formulation of pneumococcal polysaccharides for conjugation to a carrier protein
A polysaccharide technology for Streptococcus pneumoniae and Streptococcus, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as the increase in the prevalence of pneumococcus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Embodiment 1: Preparation of Streptococcus pneumoniae capsular polysaccharide
[0141] Methods of culturing pneumococci are well known in the art. See, eg, Chase, 1967, Methods of Immunology and Immunochemistry 1:52. Methods of preparing pneumococcal capsular polysaccharides are also well known in the art. See eg European Patent No. EP 0 497 524 B1. The method described below generally follows that described in European Patent No. EP 0497 524 B1 and is generally applicable to all pneumococcal serotypes unless specifically modified.
[0142] Isolates of pneumococcal serotypes 3, 8, 12F were obtained from the University of Pennsylvania (Dr. Robert Austrian). Isolates of pneumococcal serotypes 15A, 16F, 23A, 24F, 35B were obtained from Merck Culture Collection. Isolates of pneumococcal serotypes 23B and 31 were obtained from the Centers for Disease Control and Prevention (Atlanta, GA). Isolates of pneumococcal serotype 17F were obtained from the FDA Office of Biologic...
Embodiment 2
[0149] Example 2: General conjugation method
[0150] Polysaccharide size reduction and oxidation
[0151] The purified pneumococcal capsular polysaccharide powder was dissolved in water and subjected to 0.45 micron filtration. Polysaccharides were homogenized to reduce polysaccharide molecular weight unless otherwise stated. Homogenization pressure and number of passes through the homogenizer were controlled to serotype specific targets (150-1000 bar; 4-7 passes).
[0152] Size-reduced polysaccharides were subjected to 0.2 micron filtration, concentrated and diafiltered with distilled water using 5 kDa or 10 kDa NMWCO tangential flow ultrafiltration membranes.
[0153] The polysaccharide solution was then adjusted to 22 °C and pH 5 with sodium acetate buffer to minimize polysaccharide size reduction due to activation.
[0154] Purified polysaccharides were prepared for conjugation, ie, activation, using sodium metaperiodate oxidation (see Anderson et al., 1986, J. Immunol....
Embodiment 3
[0164] Example 3: Acid hydrolysis of polysaccharides from serotypes 12F, 23A, 24F and 31
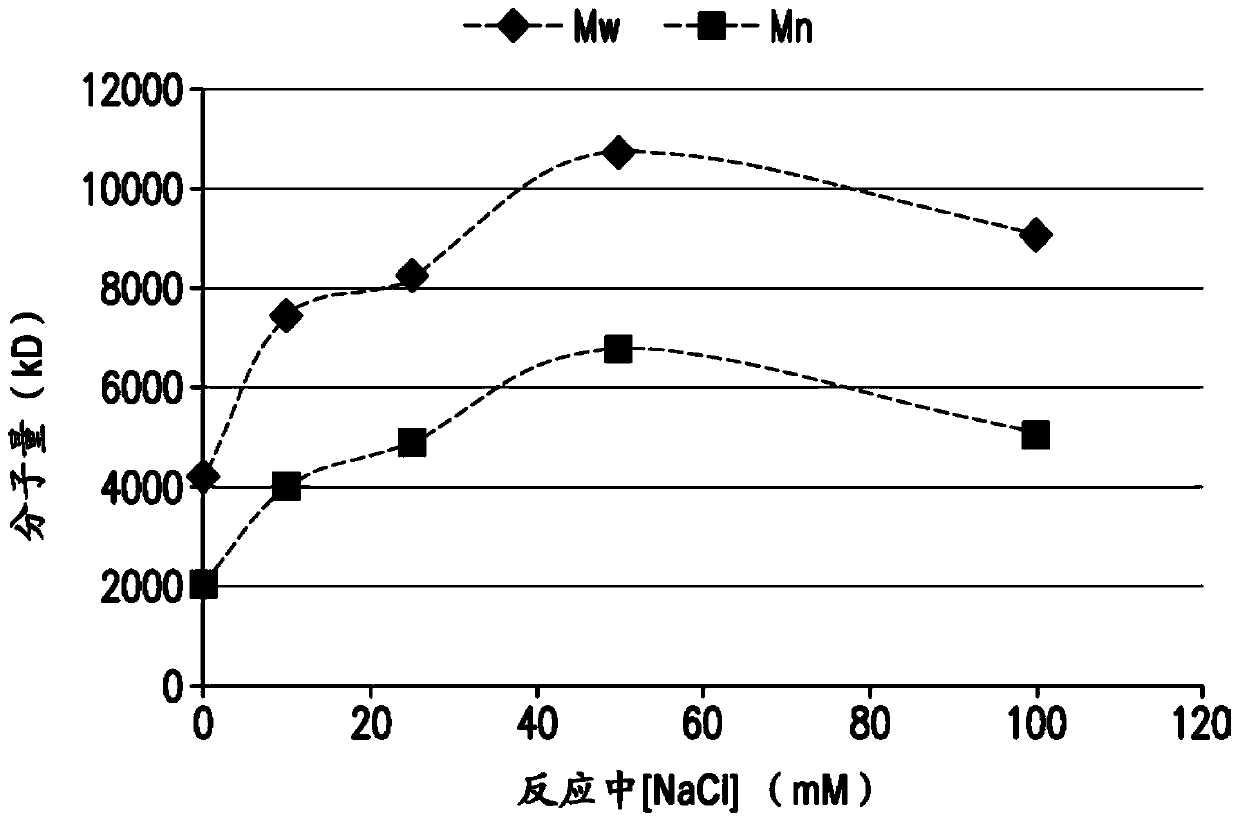
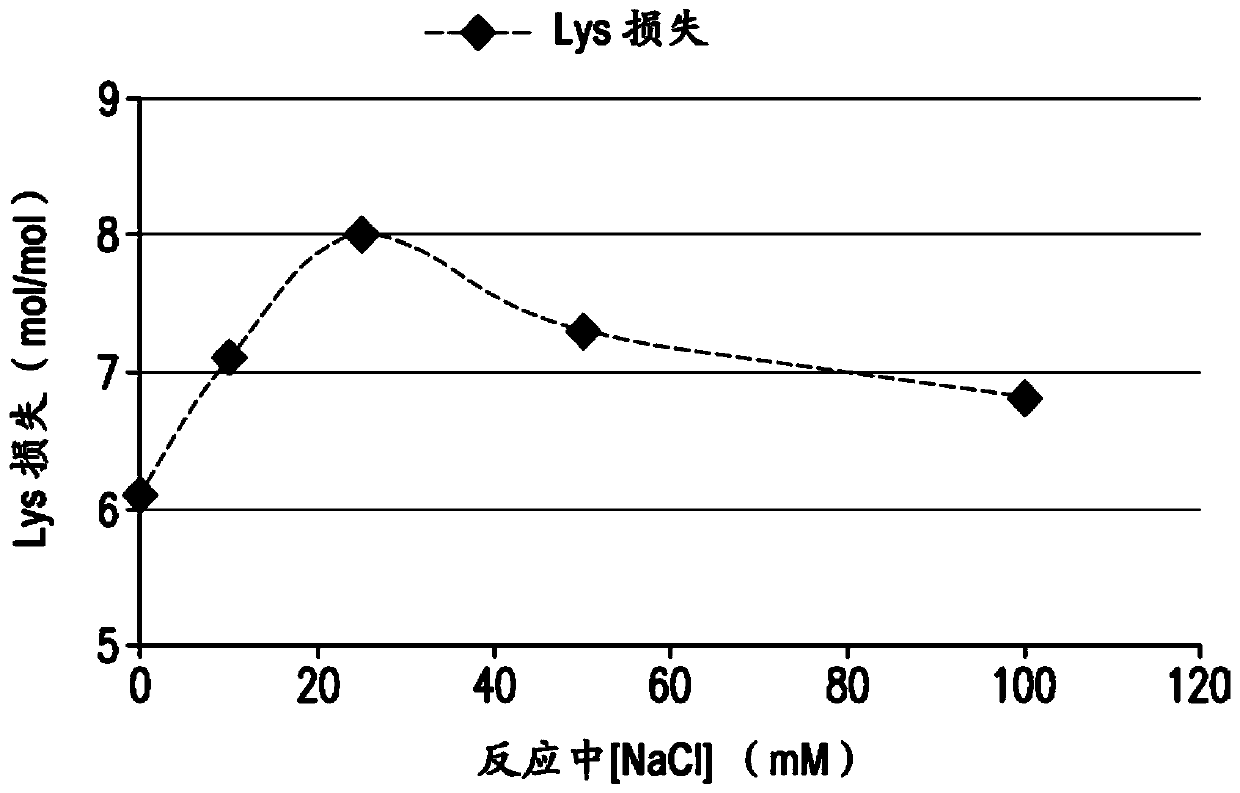
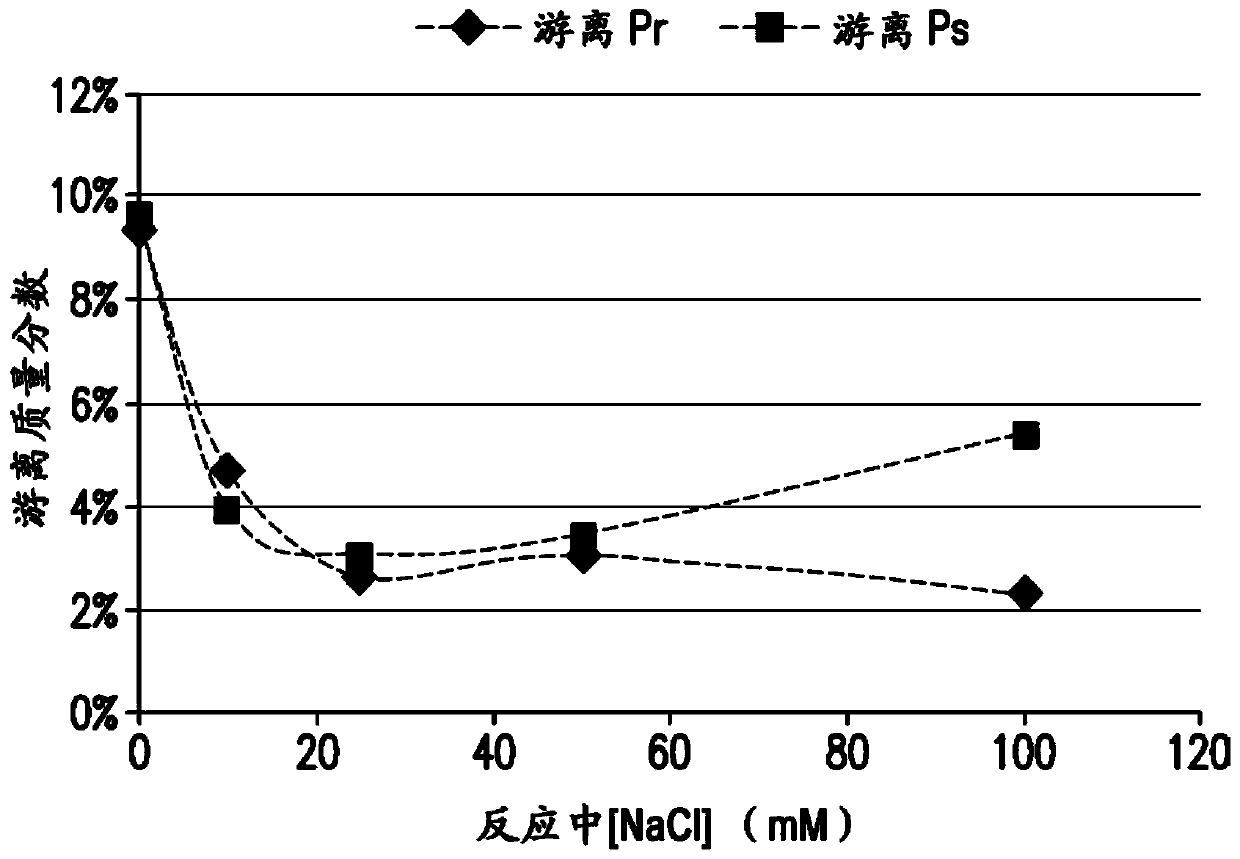
[0165] Conjugation of pneumococcal polysaccharides to proteins by reductive amination in aprotic solvents such as DMSO has been previously described. Activated polysaccharides (Ps) and proteins (Pr) are typically lyophilized, resuspended in DMSO, then mixed and incubated with sodium cyanoborohydride and sodium borohydride to achieve conjugation. The polysaccharide can be mechanically reduced in size (eg, by homogenization) prior to oxidation to reduce the Ps molecular weight and provide a consistent Ps size for conjugation. For many pneumococcal serotypes, conjugation of mechanically reduced size and oxidized Ps produced conjugates meeting the targeted properties of size, lysine consumption, free polysaccharide and free protein. However, for some serotypes, it was found difficult to achieve the target conjugation properties using this process even after optimizing the process parameters...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com