Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
77 results about "Streptococcus pneumoniae conjugated" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
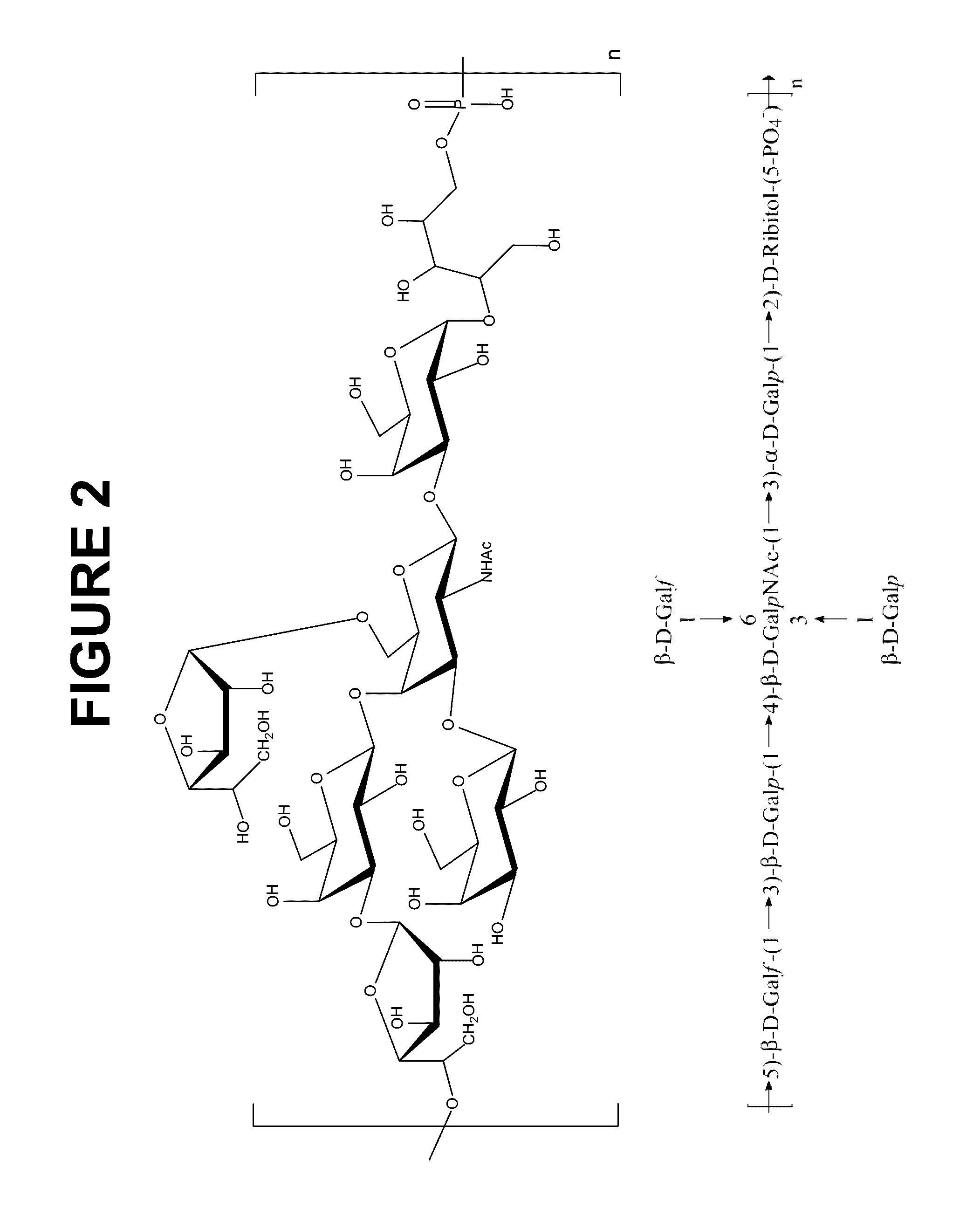
Pneumococcus polysaccharide conjugates for use as vaccine against tetanus an diphtheria
InactiveUS20030099672A1Induce protective immunityLoss of immunogenicityAntibacterial agentsBacterial antigen ingredientsDiphtheria vaccinationDiptheria toxoid
The invention relates to the use of a composition comprising n Streptococcus pneumoniae polysaccharides conjugated to the tetanus toxoid and p Streptococcus pneumoniae polysaccharides conjugated to the diphtheria toxoid, for manufacturing a vaccine which protects against Clostridium tetani and / or Corynebacterium diphtheriae infections in which: (1) n and p are other than 1, with p being, however, <=15, (2) 2<=n+p<=38, (3) the total amount of conjugated toxoid present in one vaccine dose is sufficient to induce protection against Clostridium tetani and / or Corynebacterium diphtheriae infections.
Owner:AVENTIS PASTUER LTD
Vaccine
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Immunogenic compositions comprising conjugated capsular saccharide antigens and uses thereof
ActiveUS9492559B2Antibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniae conjugatedPrevnar 13
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates) and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR®, SYNFLORIX® and / or PREVNAR 13®. The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
Protein-based Streptococcus pneumoniae vaccines
InactiveUS7504110B2Avoid infectionAntibacterial agentsBacterial antigen ingredientsCell membraneCell wall
The present invention is primarily directed to a method for preventing infection of a mammalian subject with S. pneumoniae, wherein said method comprises administering to said subject an effective amount of one or more S. pneumoniae cell wall and / or cell membrane proteins and / or immunogenically-active fragments, derivatives or modifications thereof, wherein said proteins are selected from a defined group of immunogenic proteins. The present invention further provides vaccine compositions containing said cell wall and / or cell membrane proteins.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Conjugation process of bacterial polysaccharides to carrier proteins
ActiveUS20120321660A1Size of it can retainAntibacterial agentsBacterial antigen ingredientsBacteroidesStreptococcus pneumoniae
Process for conjugation of bacterial saccharides including Streptococcus pneumoniae and Haemophilus influenzae saccharides by reductive amination are provided herein.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
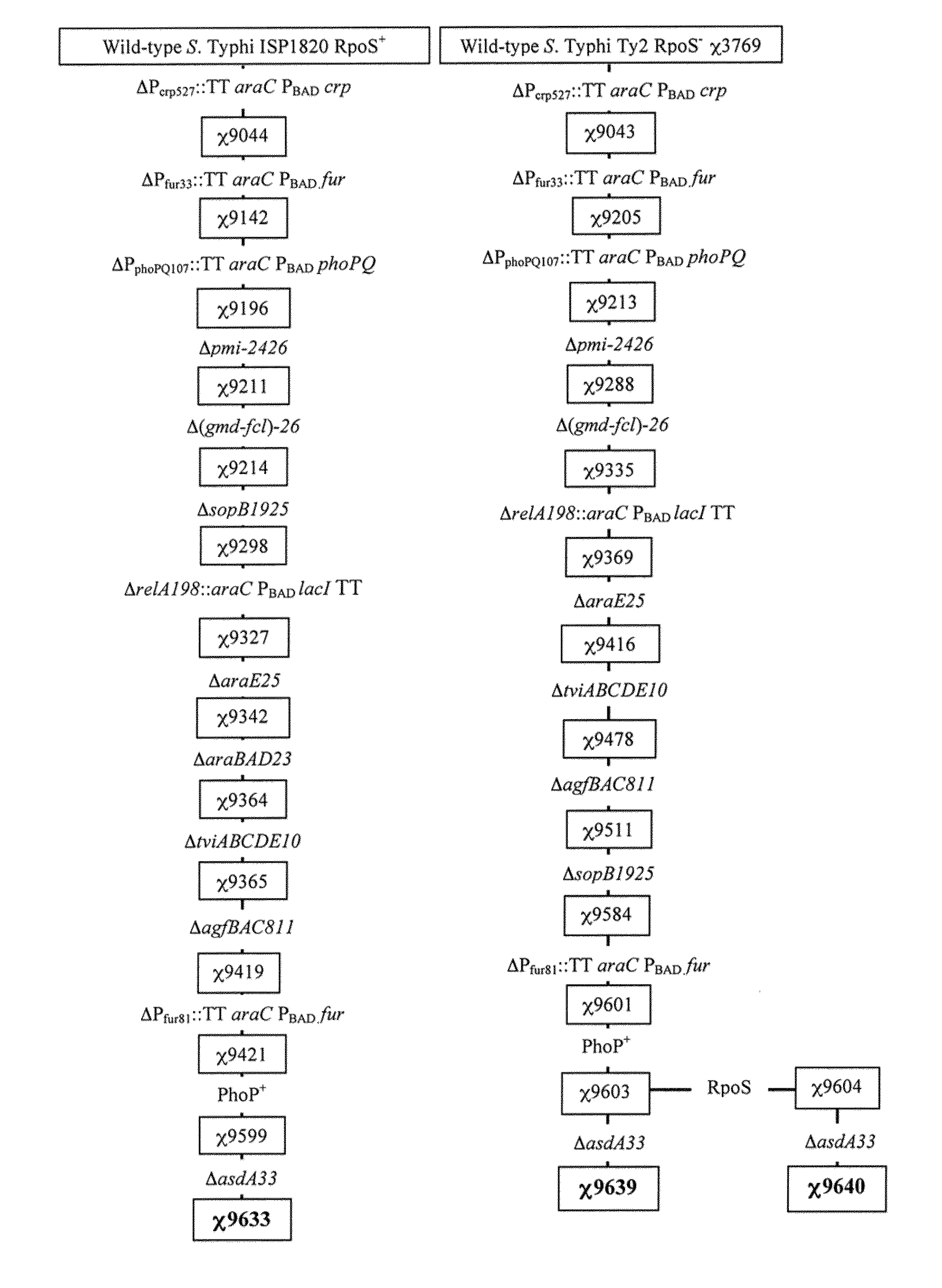
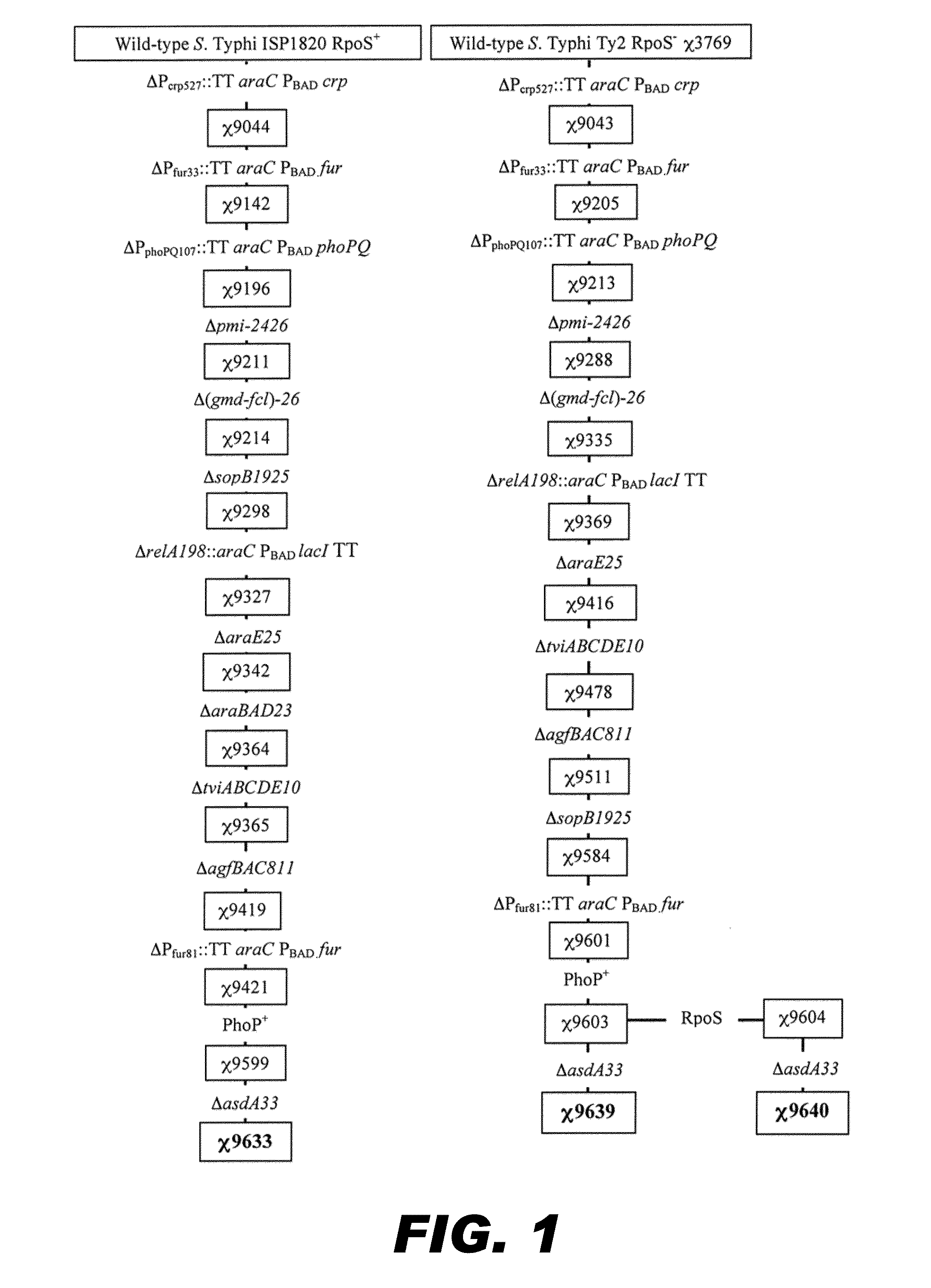
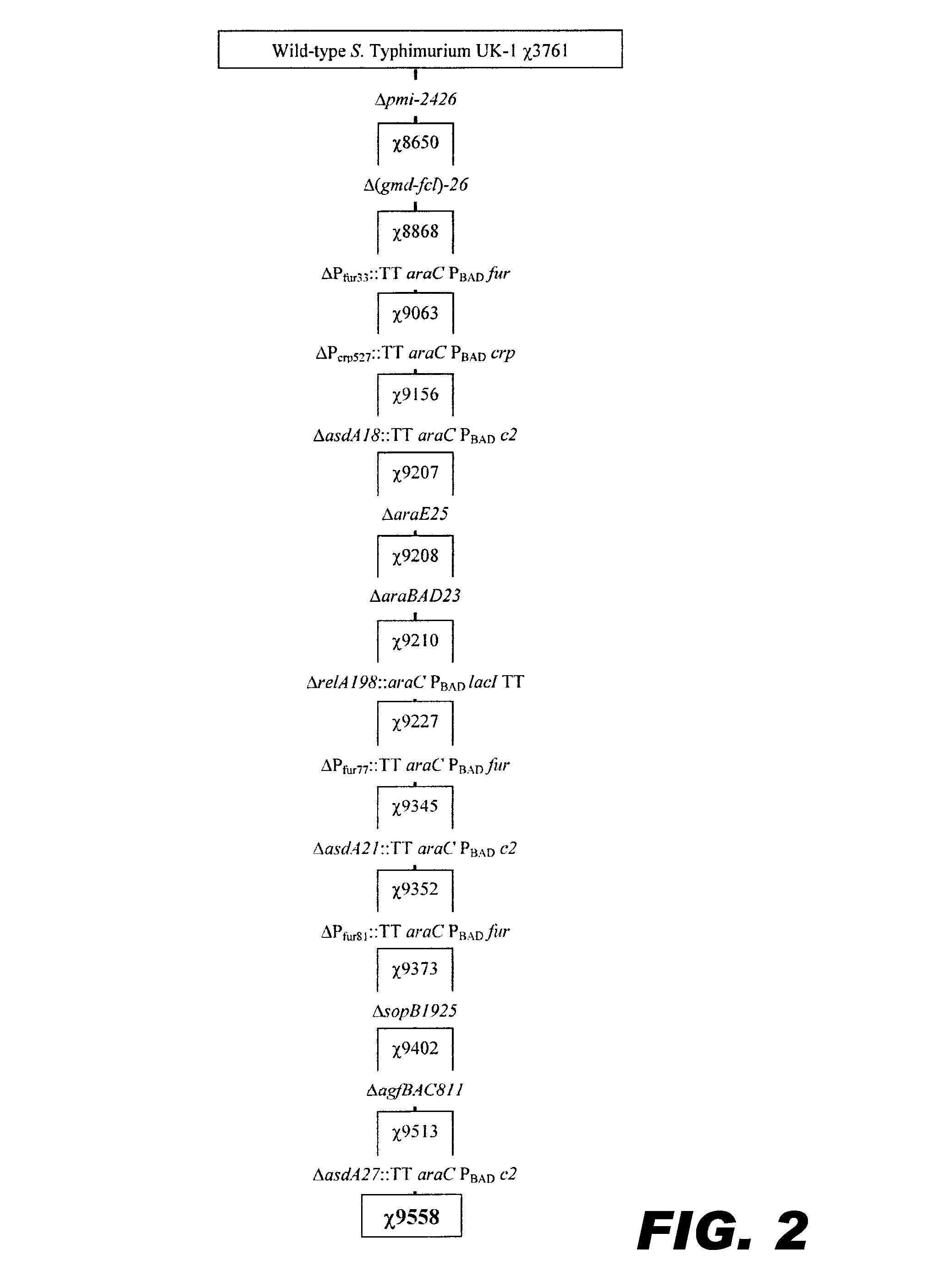
Recombinant bacterium capable of eliciting an immune response against Streptococcus pneumoniae
ActiveUS9050285B2Antibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniae conjugatedMicrobiology
The invention encompasses a recombinant bacterium capable of eliciting an immune response against Streptococcus pneumoniae, a vaccine comprising the bacterium, and methods of using the bacterium.
Owner:ARIZONA STATE UNIVERSITY
Preparation and application of recombinant plectasin
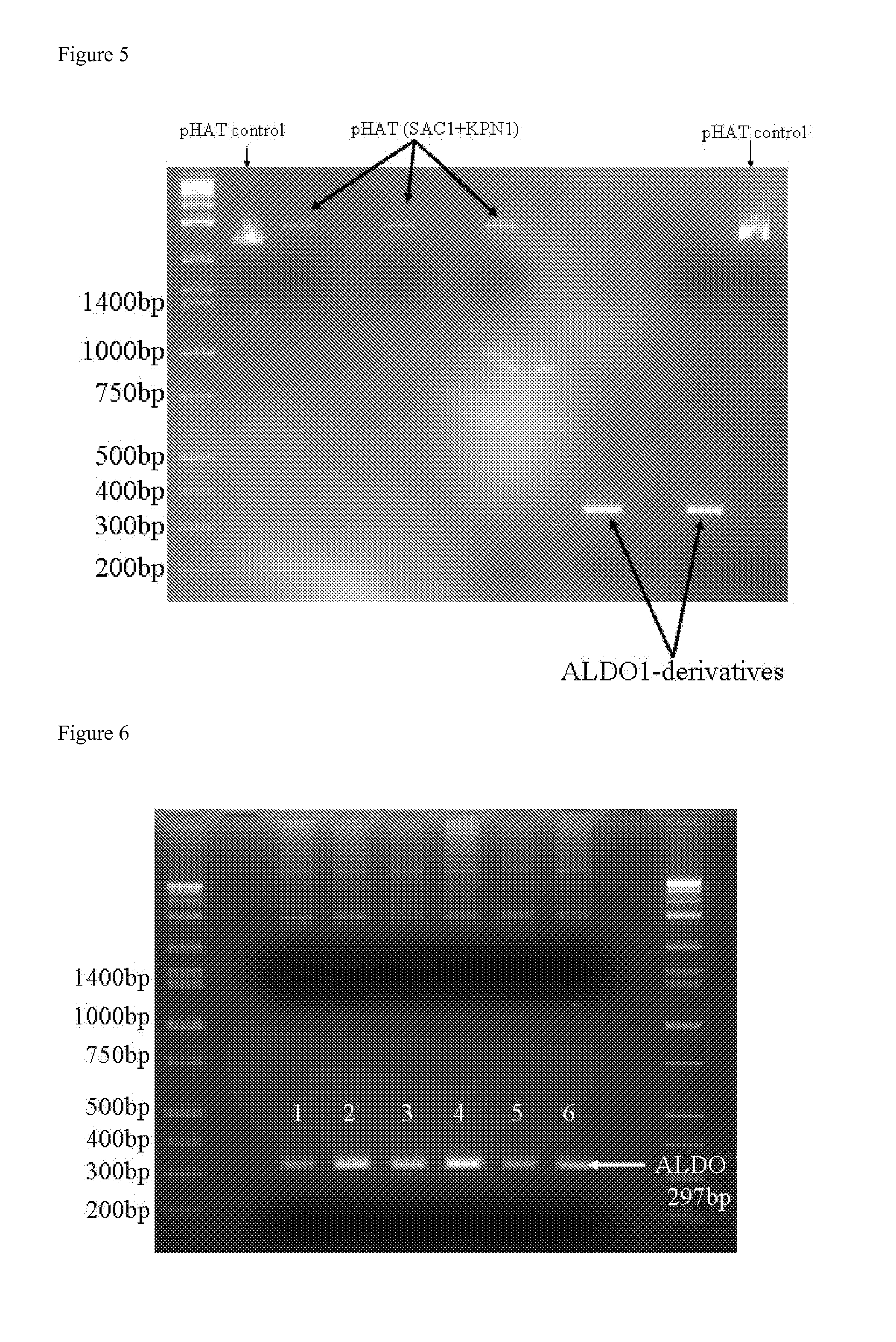
The invention discloses preparation and application of recombinant plectasin. The method comprises designing plectasin gene according to preferred codons of Pichia pastoris, wherein possible nucleotide sequences of the plectasin gene are expressed in SEQ ID NO. 1, constructing recombinant expression vectors pPICPlectasin and recombinant genetic engineering bacteria Pichia pastoris X33pPICPlectasin (CGMCC NO. 3564), carrying out a high density fermentation process on the recombinant genetic engineering bacteria Pichia pastoris having a high expression level, wherein a total protein concentration of supernate from the high density fermentation process is 729 microgrammes per milliliter, dialyzing and freeze-drying the supernate, and orderly carrying out a gel filtration chromatography treatment and a reversed phase high performance liquid chromatogram treatment on the freeze-dried supernate to obtain high purity recombinant plectasin. The high purity recombinant plectasin is not hemolytic, has favorable PH stability, heat stability and anti-pepsin activity, and can inhibit effectively the growth of gram-positive pathogen Streptococcus pneumonia, staphylococcus aureus and staphylococcus epidermidis. Therefore, the high purity recombinant plectasin can be utilized for treating and preventing gram-positive bacterium and especially streptococcus and has potential antimicrobial drug development values.
Owner:SHENZHEN SUNSMILE BIOTECH
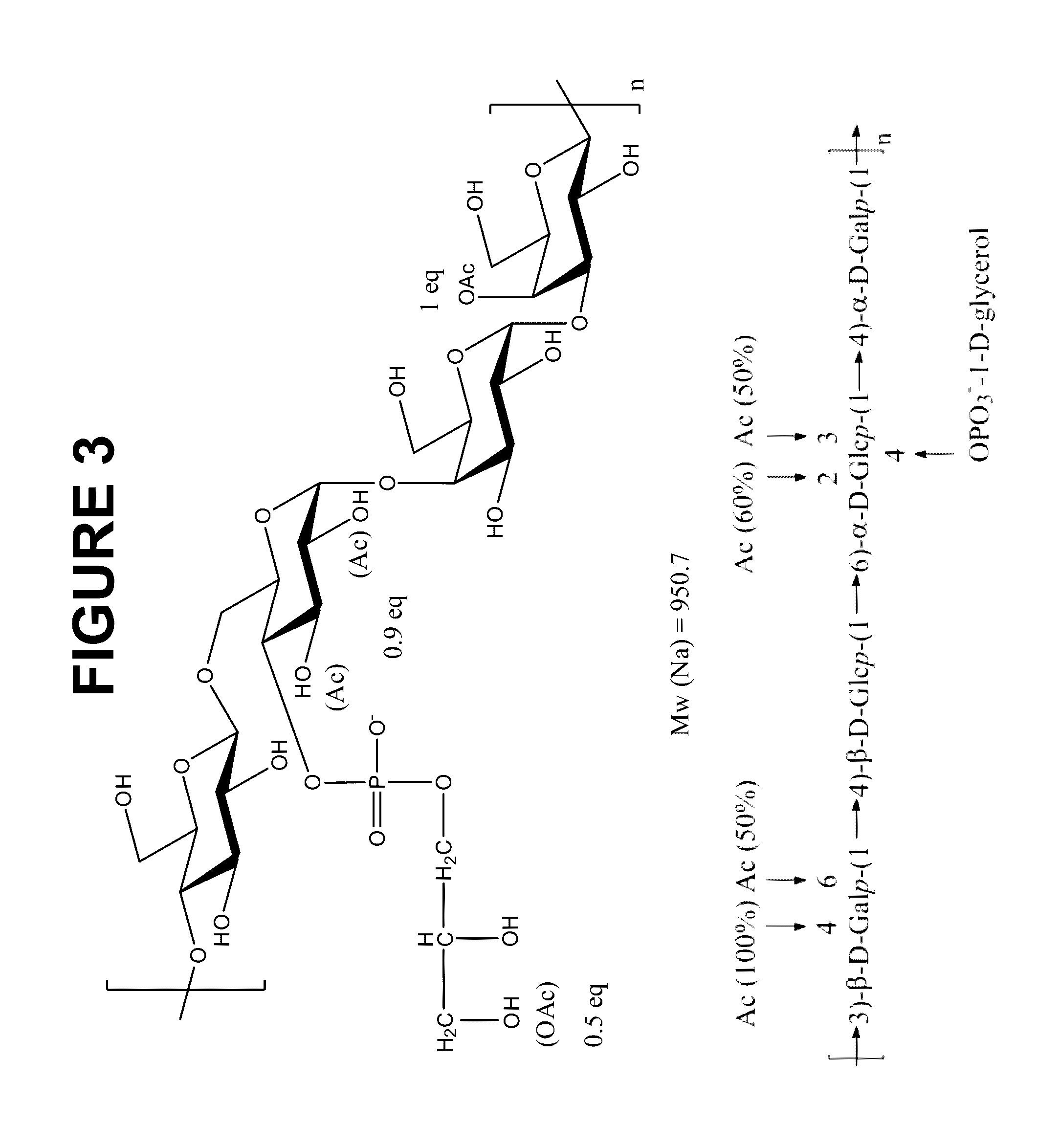
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
ActiveUS20190192648A1Improve stabilityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseCarrier protein
The invention is related to multivalent immunogenic compositions comprising more than one S. pneumoniae polysaccharide protein conjugates, wherein each of the conjugates comprises a polysaccharide from an S. pneumoniae serotype conjugated to a carrier protein, wherein the serotypes of S. pneumoniae are as defined herein. In some embodiments, at least one of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. In further embodiments, each of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. Also provided are methods for inducing a protective immune response in a human patient comprising administering the multivalent immunogenic compositions of the invention to the patient. The multivalent immunogenic compositions are useful for providing protection against S. pneumoniae infection and diseases caused by S. pneumoniae. The compositions of the invention are also useful as part of treatment regimes that provide complementary protection for patients that have been vaccinated with a multivalent vaccine indicated for the prevention of pneumococcal disease.
Owner:MERCK SHARP & DOHME LLC
Multivalent pneumococcal polysaccharide-protein conjugate composition
InactiveUS20150343076A1High activityAvoid problemsAntibacterial agentsBacterial antigen ingredientsAdjuvantStreptococcus pneumoniae conjugated
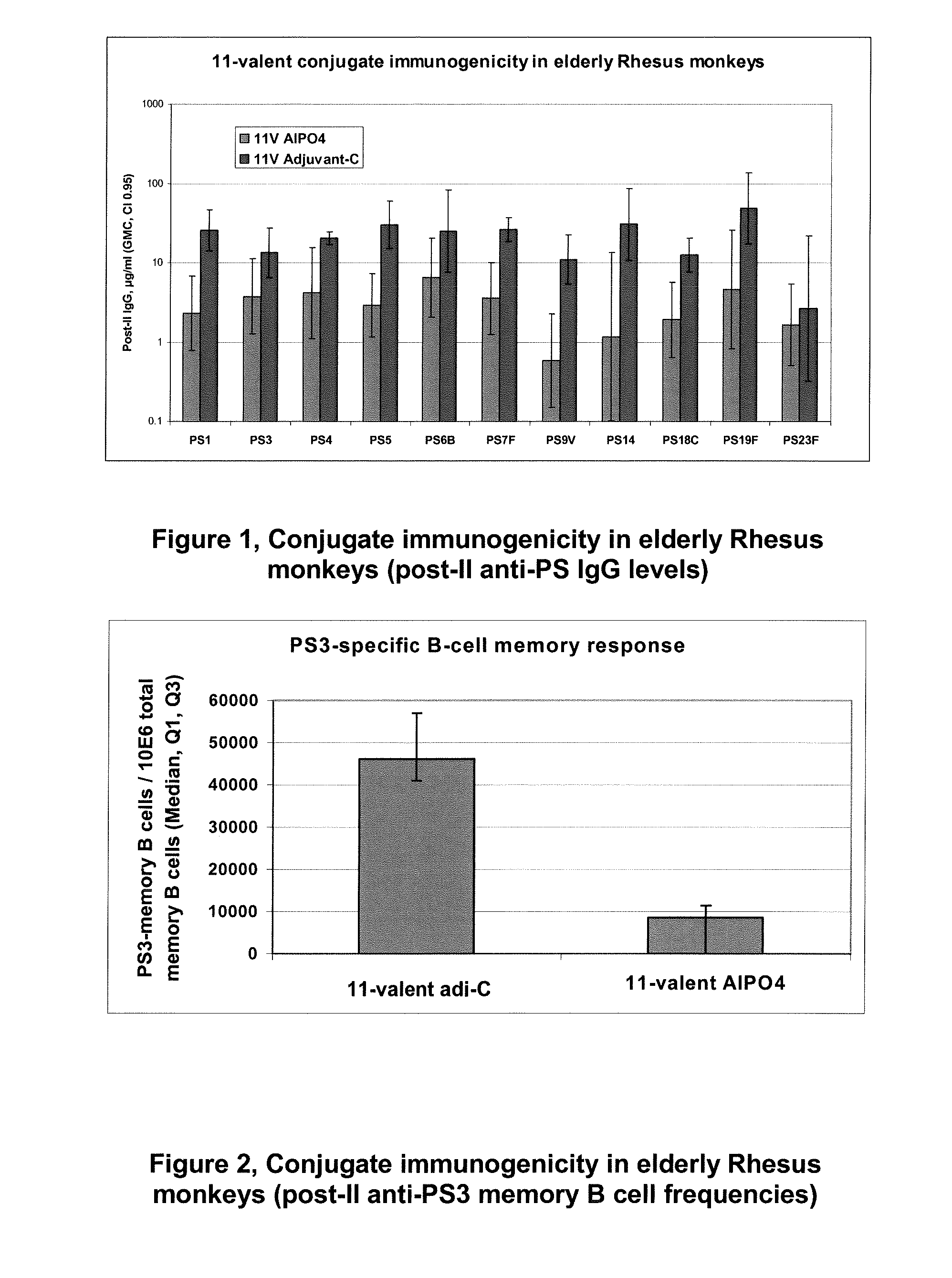
Provided is an immunogenic composition comprising 13 different polysaccharide-protein conjugates. Each of the conjugates comprises a capsular polysaccharide prepared from a different serotype Streptococcus pneumoniae conjugated to a carrier protein, that is, 12 serotypes selected from the group consisting of 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F and serotype 22F or 33F. The immunogenic composition formulated into a vaccine comprising an aluminum-based adjuvant increases coverage with respect to pneumococcal diseases in infants and children.
Owner:SK CHEM CO LTD
Separation of contaminants from Streptococcus pneumoniae polysaccharide by pH manipulation
ActiveUS7718791B2Soluble protein is effectively reduced.Bacterial antigen ingredientsBacteriaStreptococcus pneumoniaeLysis
A process for reducing the protein content and preserving the capsular polysaccharide content in a complex cellular Streptococcus pneumoniae lysate broth prior to purification is described. Utilizing pH reduction after cellular lysis has resulted in a purified polysaccharide that consistently meets the protein specification, and higher recovery yields of polysaccharide during the purification process.
Owner:WYETH LLC
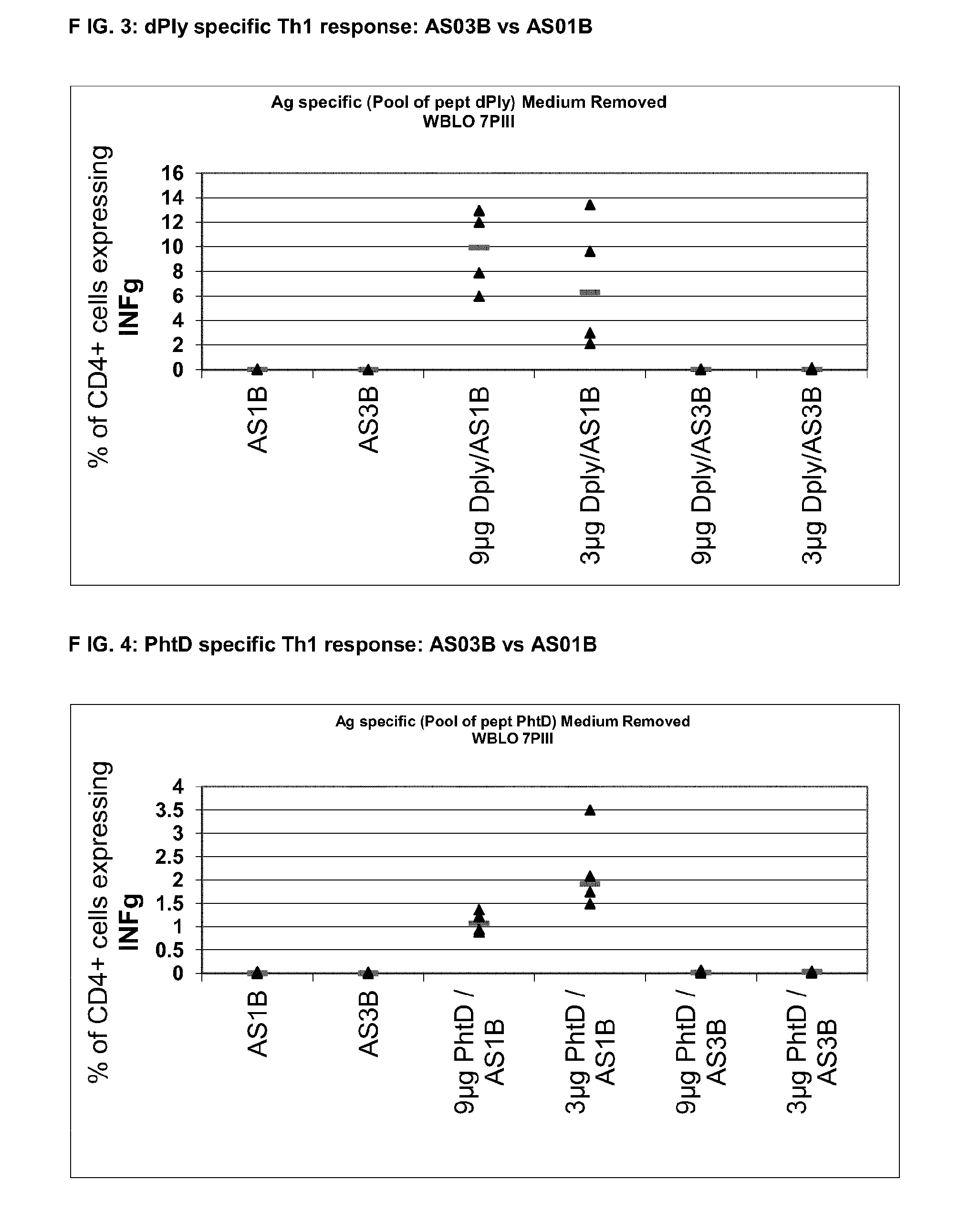
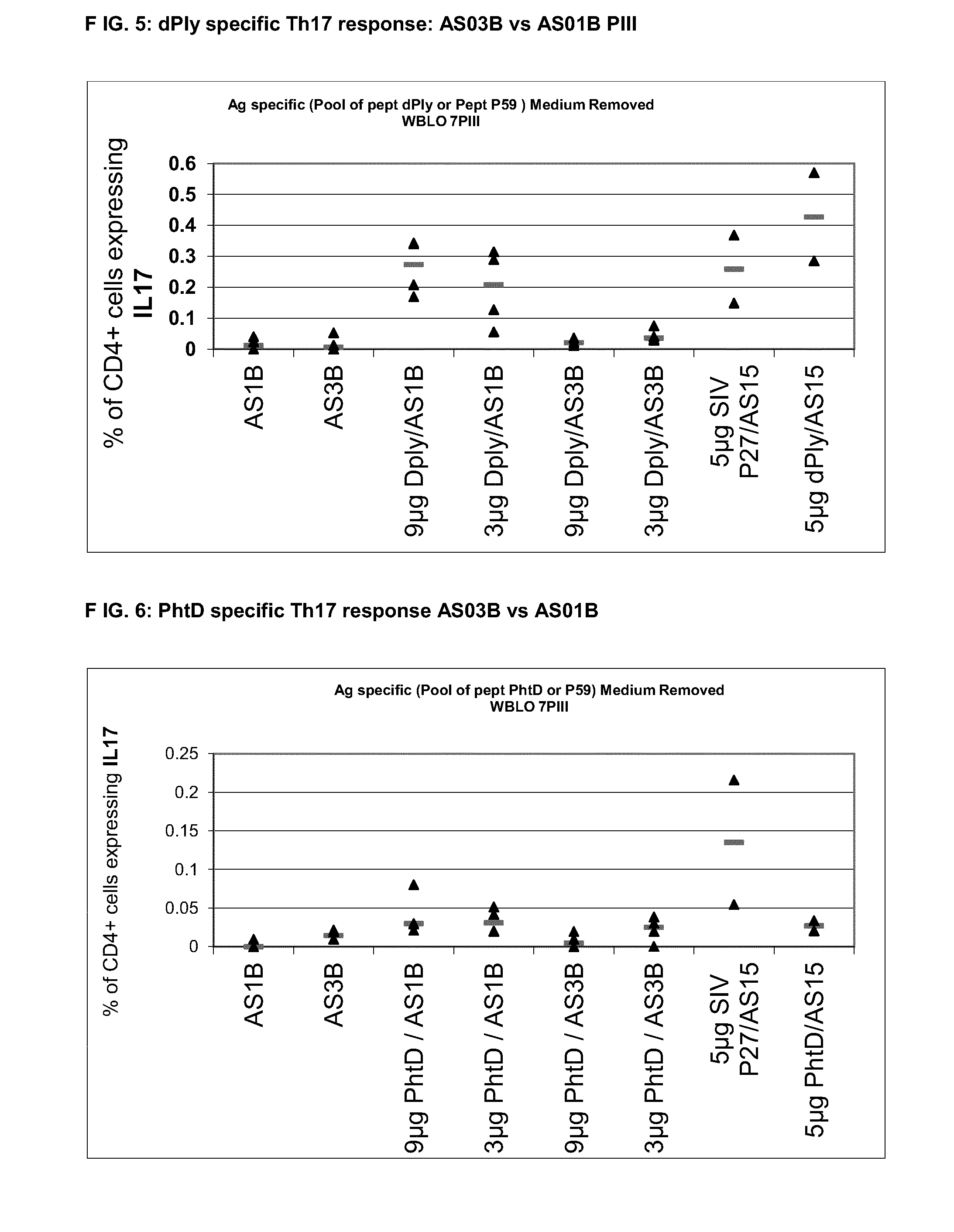
Vaccine against streptococcus pneumoniae
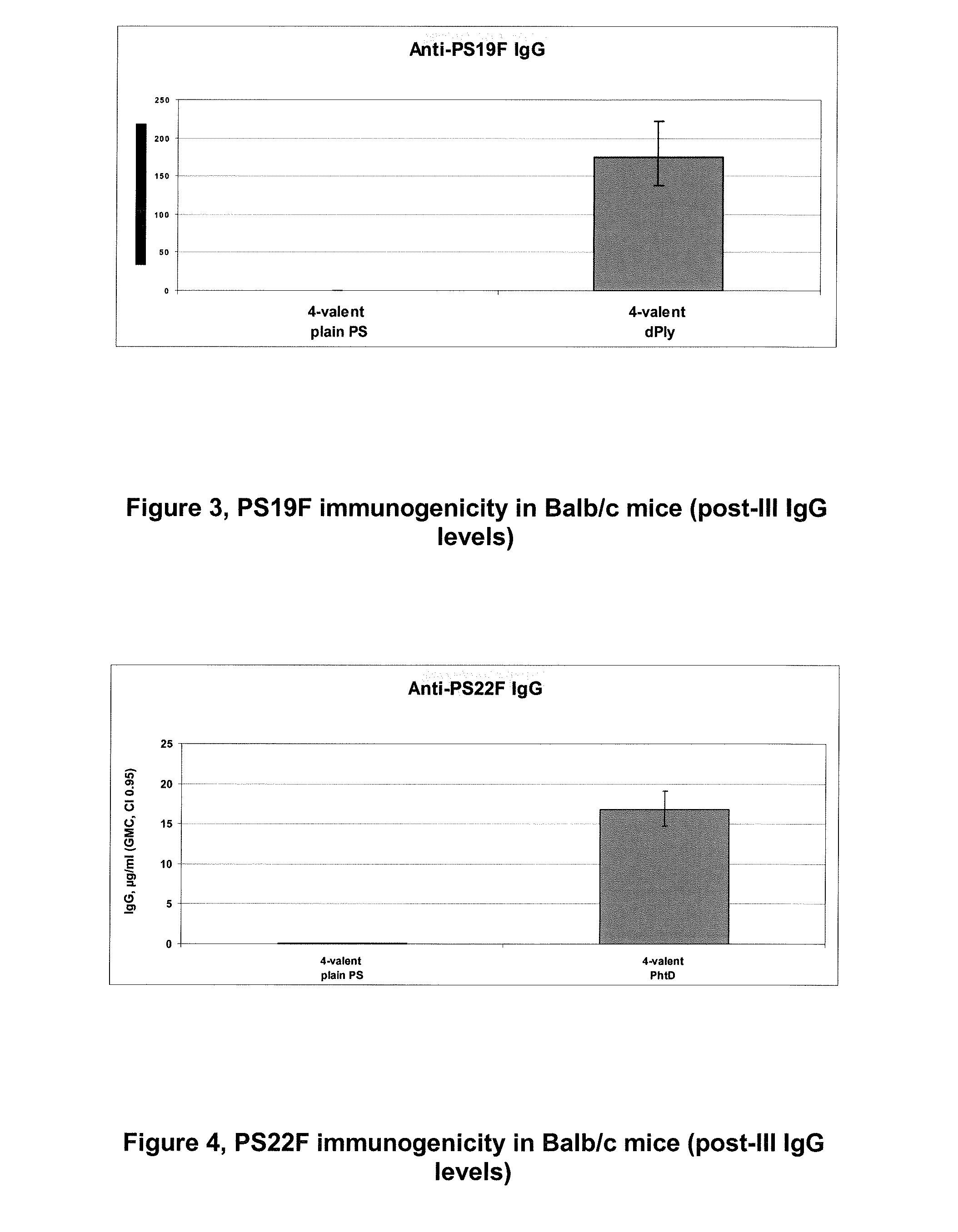
InactiveUS20140072622A1Maintain good propertiesRaise immunogenic responseAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeQS21
The present invention relates to improved immunogenic compositions and vaccines, methods for making them and their use in medicine. In particular the invention relates to immunogenic compositions of unconjugated Streptococcus pneumoniae proteins selected from: pneumolysin and member(s) of the Polyhistidine Triad family (e.g. PhtD), which comprise adjuvants comprising QS21 and monophosphoryl lipid A (MPL), and are presented in the form of a liposome.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
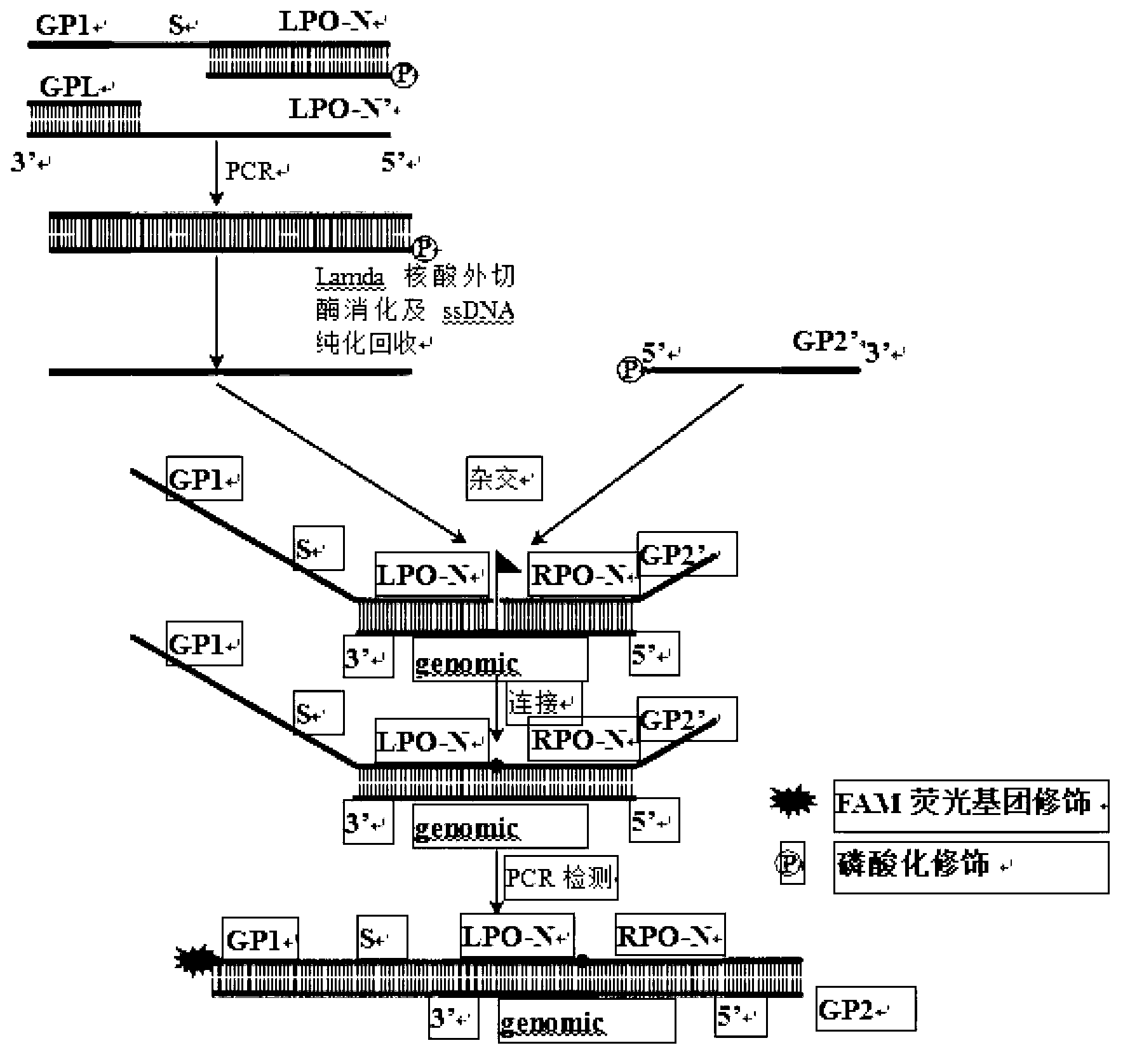
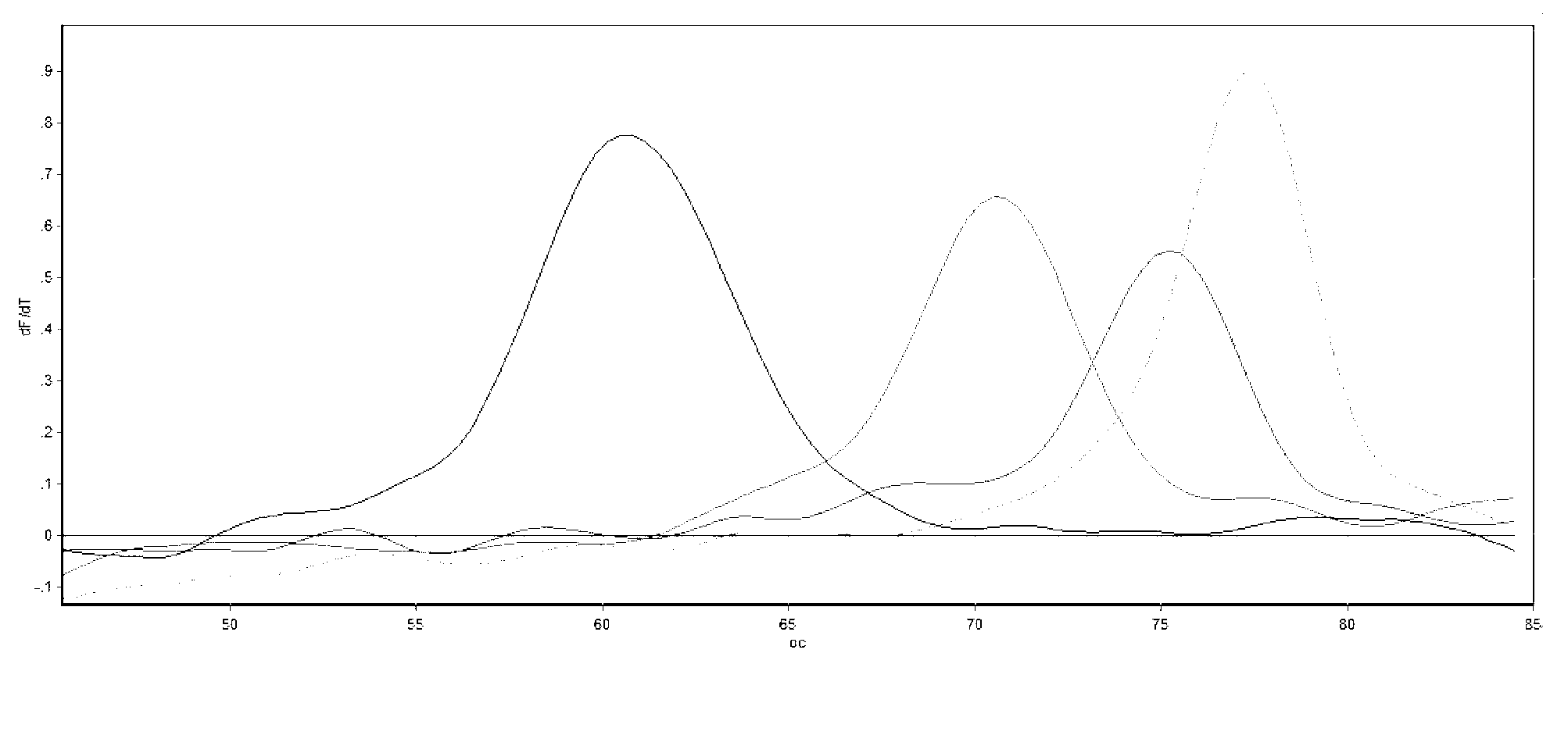
Method for detecting serotyping of streptococcus pneumoniae
ActiveCN103290115AMicrobiological testing/measurementMicroorganism based processesMultiplex ligation-dependent probe amplificationFluorescence
The invention provides a method for carrying out serotyping on streptococcus pneumoniae by using MLPA (multiplex ligation dependent probe amplification). The method comprises the following steps of: firstly, extracting a sample genome DNA of streptococcus pneumoniae to be detected; carrying out pre-amplification on the extracted genome DNA by using a PCR pre-amplification primer; carrying out hybridization on pre-amplification products of the genome DNA by using an MLPA probe; adding a corresponding fluorescence detecting probe in a reaction system; connecting hybridized MLPA probes by using ligase, and carrying out PCR amplification on the hybridized and connected MLPA probes by using an MLPA universal amplification primer; and analyzing PCR products by using a multicolor fluorescence dissolution curve, so that the serotyping on streptococcus pneumoniae can be realized. The detecting method disclosed by the invention is good in specificity and high in sensitivity; according to the method, 10 kinds of serotypes can be subjected to genotyping simultaneously just in 2-4 hours, so that multiple uncapping operations in traditional MLPA detection are avoided, and the pollution possibility is reduced, therefore, the method can meet high-throughput sample detection, and is especially applicable to inspection departments.
Owner:江西南兴医疗科技有限公司 +1
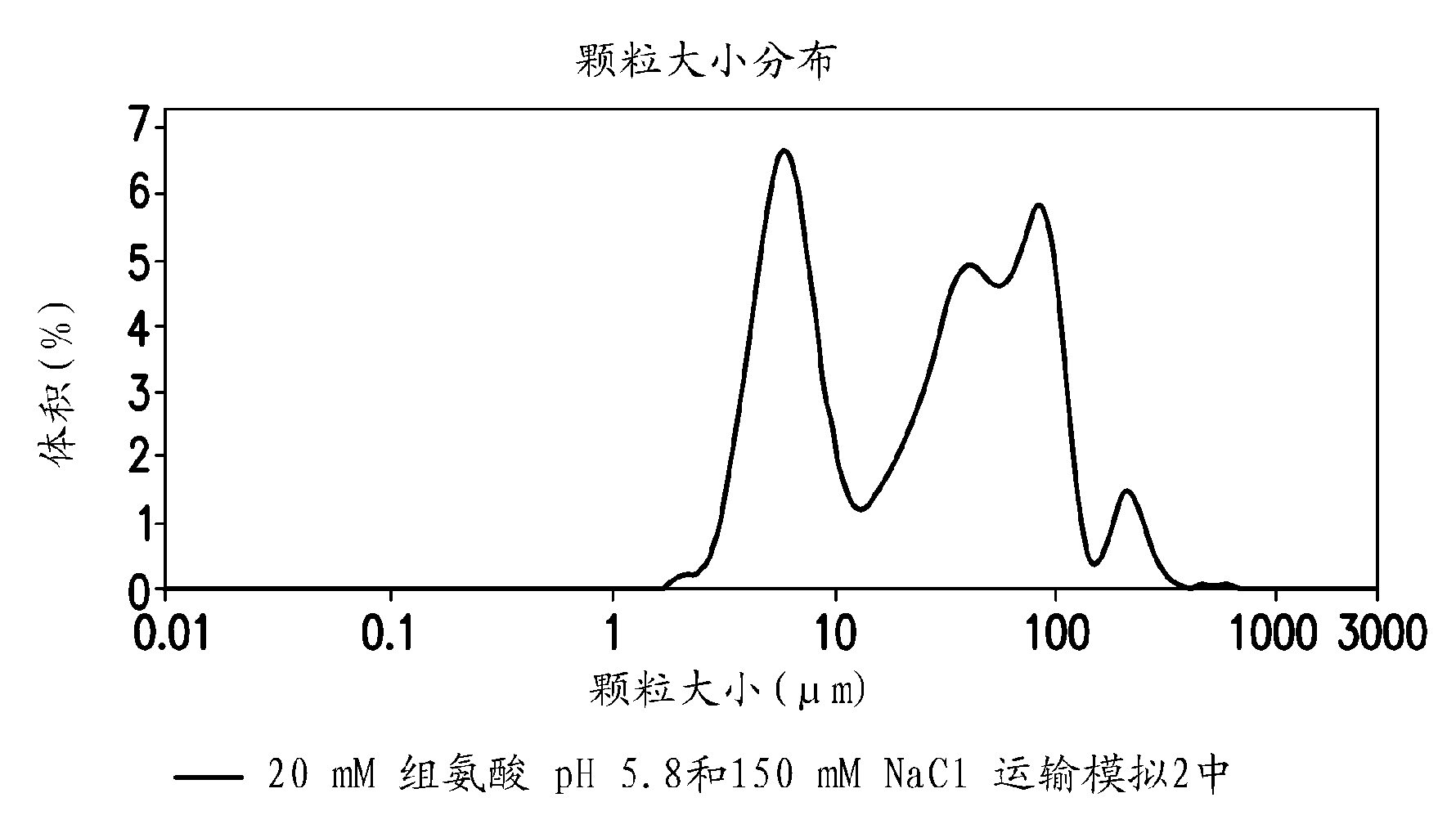
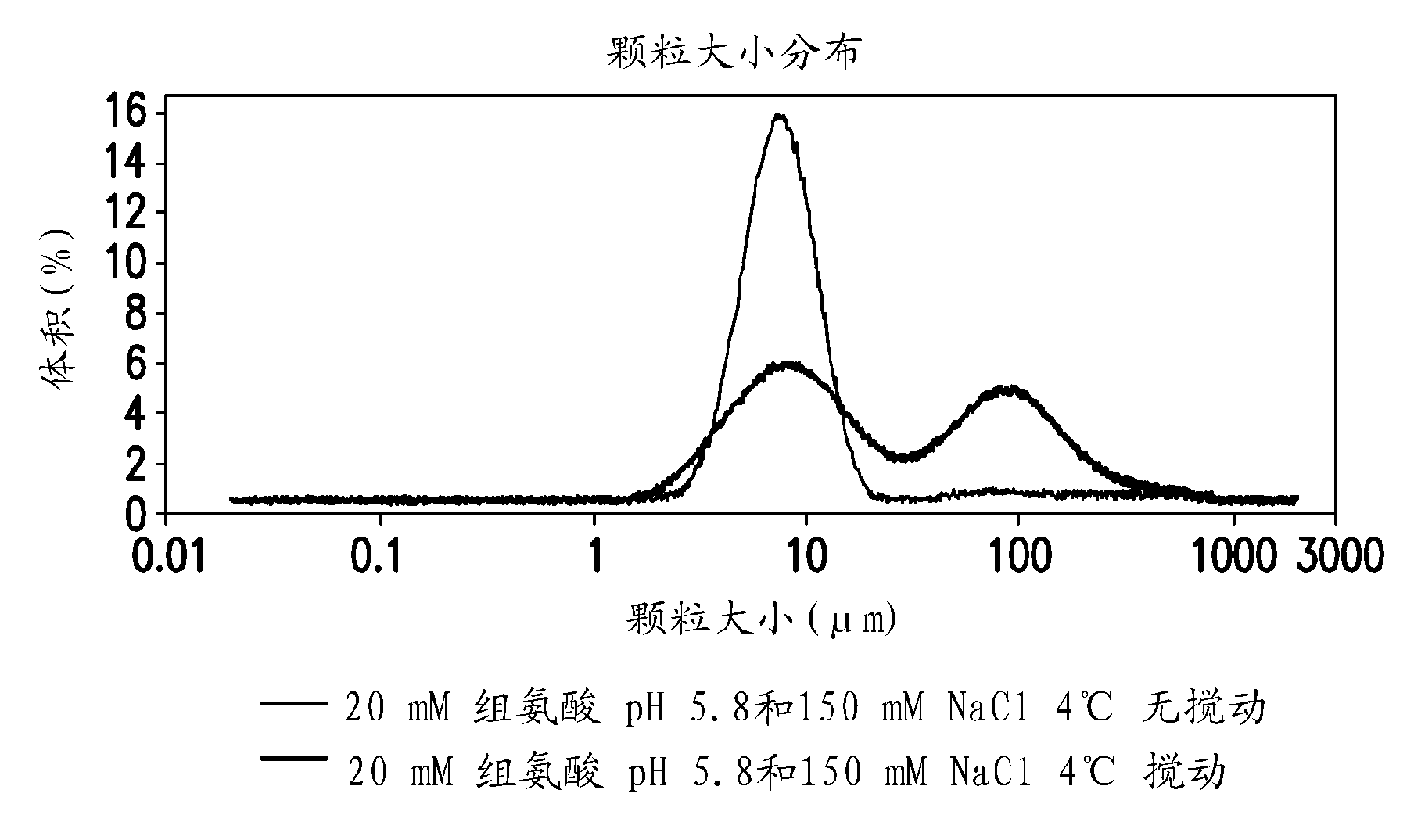
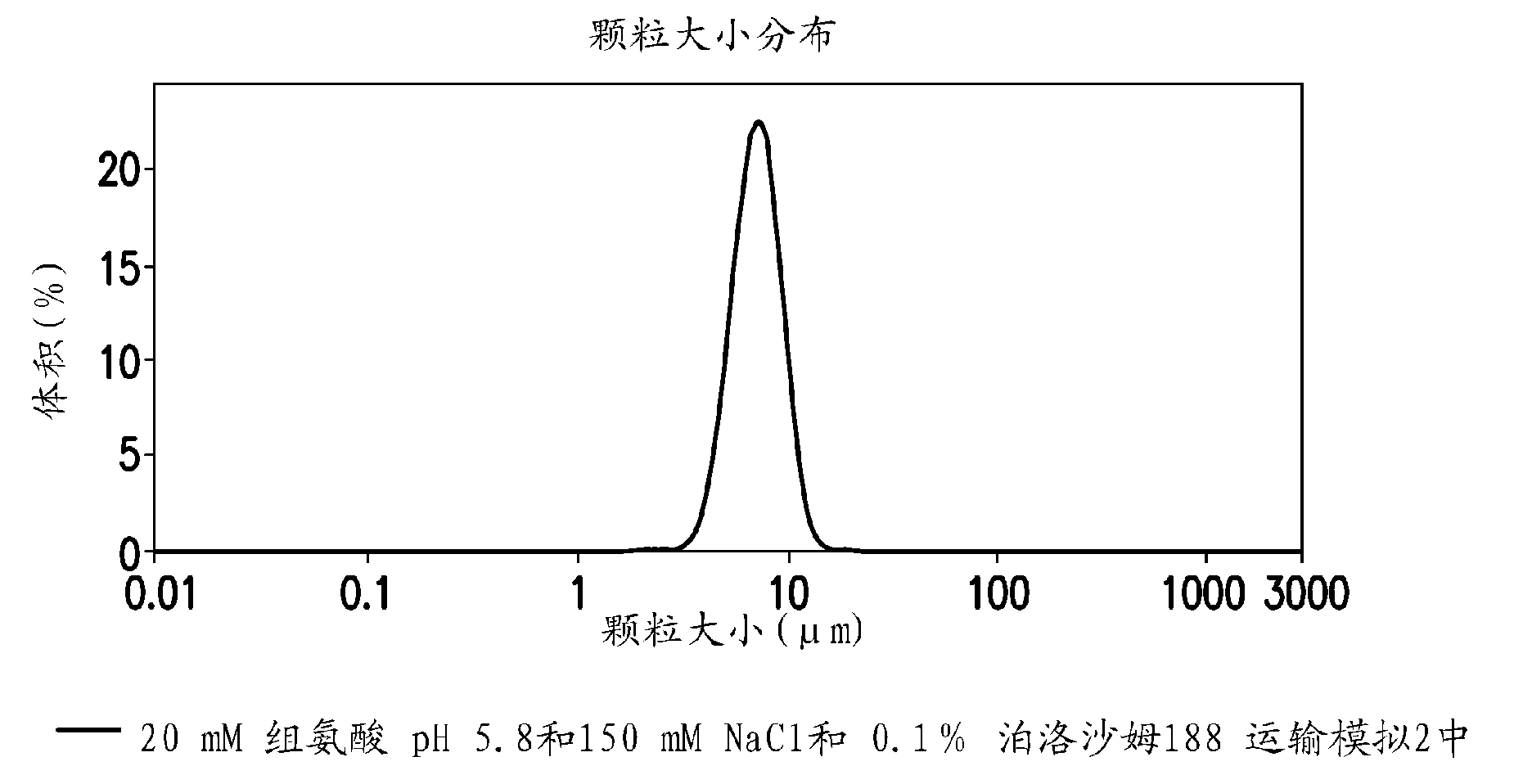
Novel formulations which mitigate agitation-induced aggregation of immunogenic compositions
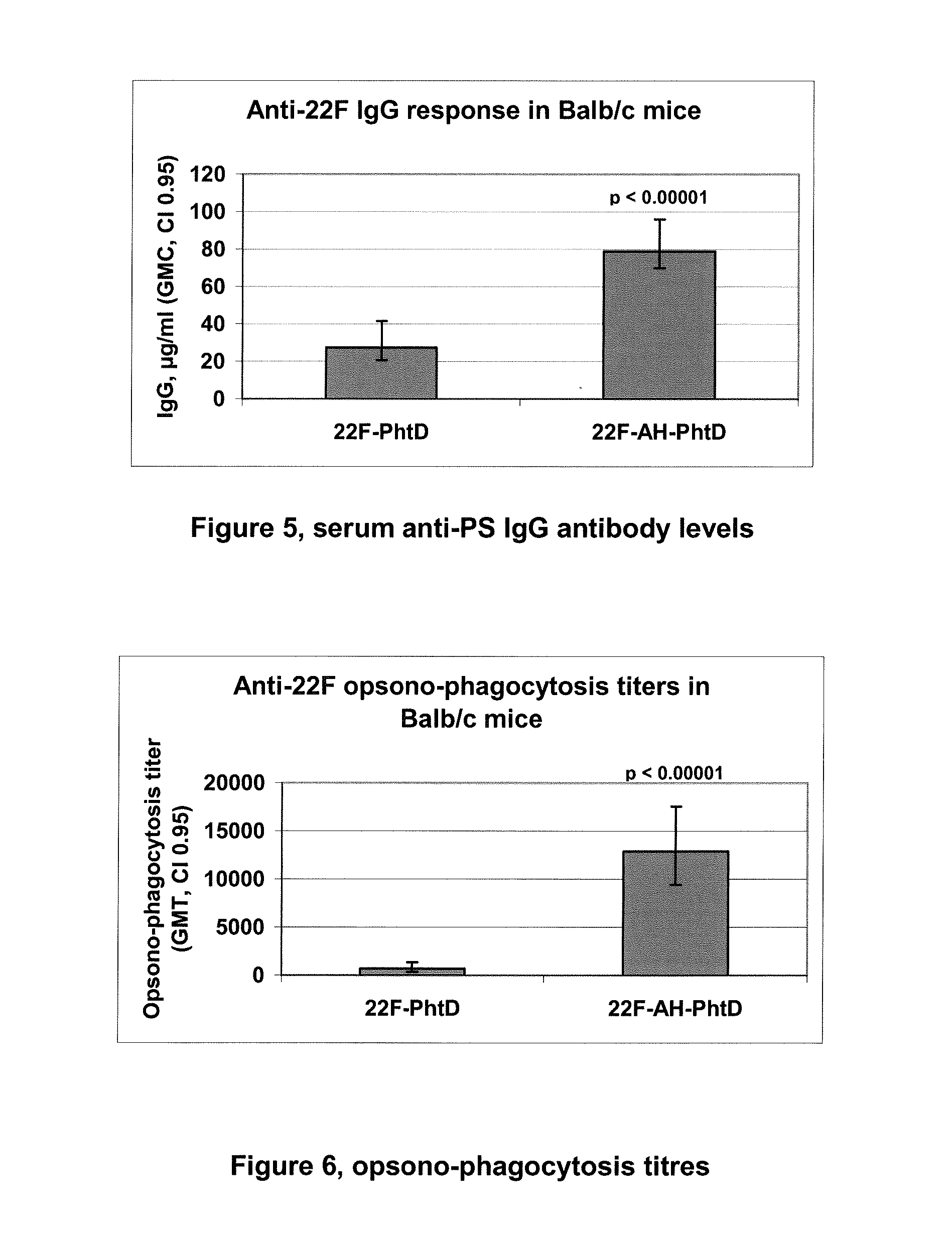
The present invention provides novel formulations which mitigate agitation-induced aggregation of immunogenic compositions particularly those having polysaccharide-protein conjugates. Specifically, the novel formulations comprise a poloxamer within a molecular weight range of 1100 to 17,400 which provides significant advantages over previously used surfactants including polysorbate 80. In one embodiment, the present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates and a poloxamer. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197.
Owner:MERCK & CO INC
Application of streptococcus pneumoniae protein to resisting infection of S. pneumoniae
ActiveCN109456393AIncrease infectionReduced Colonization Protection ExperimentAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaStreptococcus mitis
The invention provides application of S. pneumoniae protein to resisting infection of S. pneumoniae. The endopeptidase O (PepO) of S. pneumoniae is a subcutaneous immunologic adjuvant, and the prepared S. pneumoniae protein vaccines have the good protection effects on resisting infection of S. pneumoniae through mixing and fusing expression of the subcutaneous immunologic adjuvant and 673rd to 863rd amino acid peptide fragment of zinc metal protease B (ZmpB).
Owner:CHONGQING MEDICAL UNIVERSITY
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
The invention is related to multivalent immunogenic compositions comprising more than one S. pneumoniae polysaccharide protein conjugates, wherein each of the conjugates comprises a polysaccharide from an S. pneumoniae serotype conjugated to a carrier protein, wherein the serotypes of S. pneumoniae are as defined herein. In some embodiments, at least one of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. In further embodiments, each of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. Also provided are methods for inducing a protective immune response in a human patient comprising administering the multivalent immunogenic compositions of the invention to the patient. The multivalent immunogenic compositions are useful for providing protection against S. pneumoniae infection and / or pneumococcal diseases caused by S. pneumoniae. The compositions of the invention are also useful as part of treatment regimes that provide complementary protection for patients that have been vaccinated with a multivalent vaccine indicated for the prevention of pneumococcal disease.
Owner:MERCK SHARP & DOHME LLC
Antigenic protein fragments of streptococcus pneumoniae
InactiveUS20110076301A1Antibacterial agentsBacterial antigen ingredientsEpitopeStreptococcus pneumoniae
Antigenic protein fragments of Streptococcus pneumoniae to be used for the preparation of a medicament for the prevention and the treatment of bacterial infections and a method for the detection thereof, and related compositions using said epitopes, are disclosed.
Owner:UNIVERSIT A DEGLI STUDI DI SIENA
Immunogenic streptococcus pneumoniae peptides and peptide-multimers
InactiveUS20120100172A1Increases their immunogenic potential against the bacteriaEffective presentationBacterial antigen ingredientsBacteriaStreptococcus pneumoniaeProtective immunity
The present invention relates to immunogenic peptides, including variants and analogs derived from Streptococcus pneumoniae (S. pneumoniae) proteins, to peptide-multimers, conjugates and fusion proteins that include such peptides, and to vaccines that include such immunogenic entities. In particular, the present invention relates to the use of such vaccines for eliciting protective immunity to S. pneumoniae.
Owner:TAL MICHAEL +3
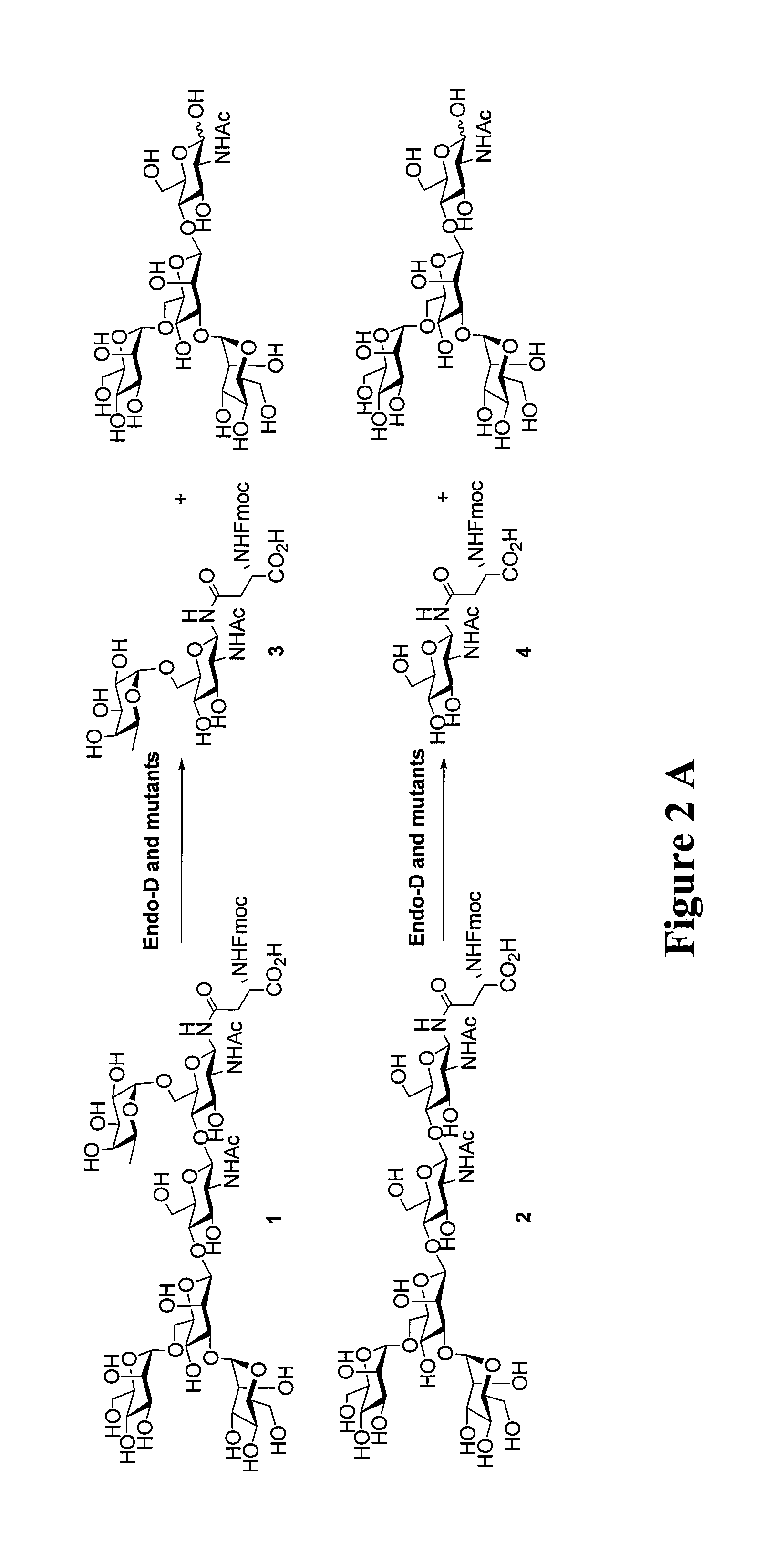
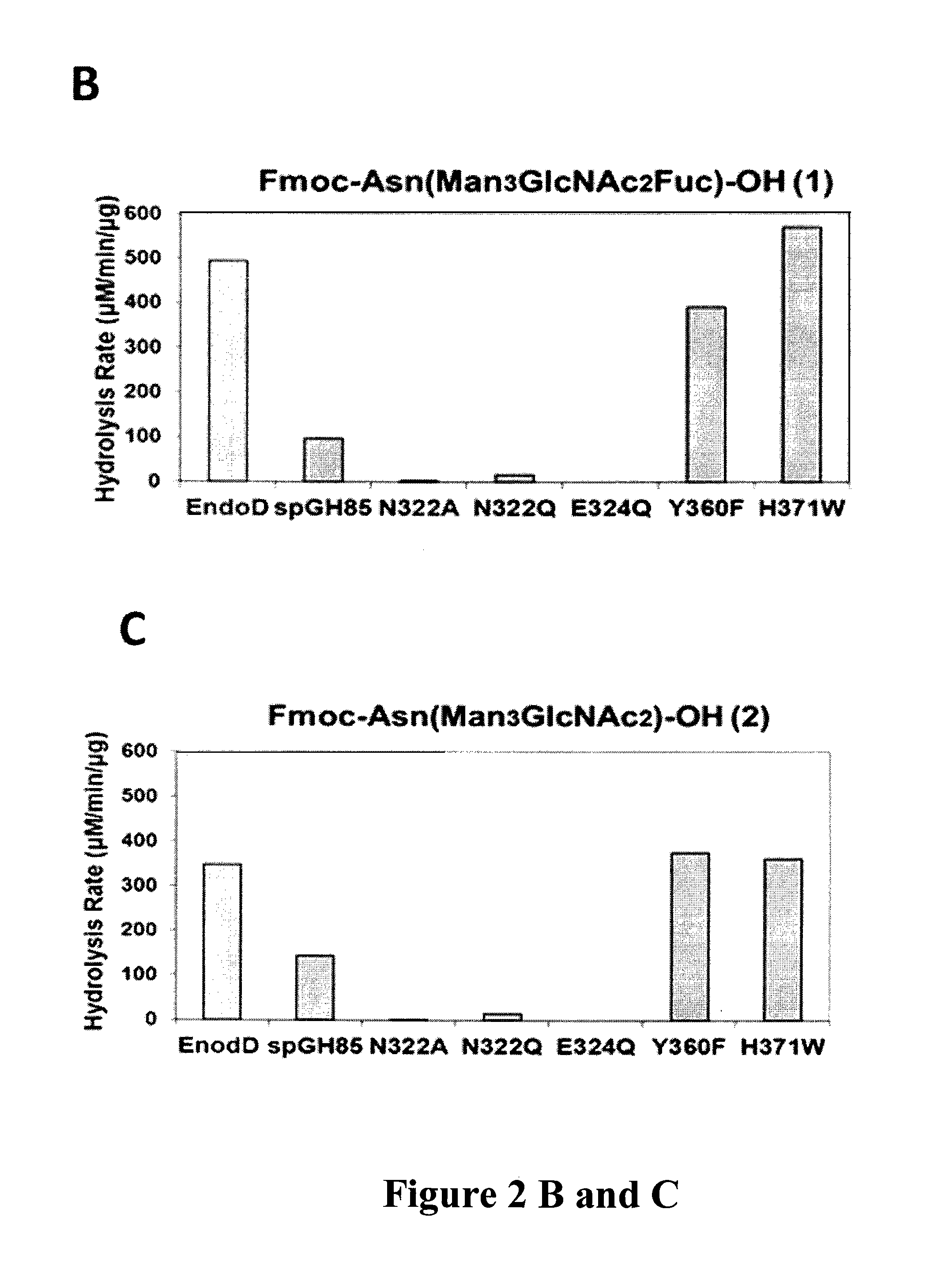
Transglycosylation activity of glycosynthase mutants of an endo-beta-N-acetylglucosaminidase (endo-D) from streptococcus pneumoniae
ActiveUS9175326B2Reduced activityHigh activityImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsStreptococcus pneumoniaeFucosylation
The present invention provides for recombinant Endo-D and selected mutants that exhibit reduced hydrolysis activity and increased transglycosylation activity for the synthesis of glycoproteins wherein a desired sugar chain is added to a core fucosylated or nonfucosylated GlcNAc-protein acceptor by transglycosylation. Such recombinant Endo-D and selected mutants are useful for efficient glycosylation remodeling of IgG1-Fc domain.
Owner:UNIV OF MARYLAND
Immunogenic composition
ActiveUS9561268B2Effective protectionBacterial antigen ingredientsMultivalent vaccineStreptococcus pneumoniae conjugatedImmunogenicity
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Quinoxaline-N1,N4-dioxide derivative capable of inhibiting activity of DNA topoisomerase, preparation method and application of quinoxaline-N1,N4-dioxide derivative
PendingCN110551072AHas inhibitory activityStrong inhibitory activityAntibacterial agentsOrganic chemistryQuinoxalineStaphylococcus aureus
The invention belongs to the technical field of biochemistry, and particularly relates to a quinoxaline-N1,N4-dioxide derivative capable of inhibiting the activity of DNA topoisomerase, a preparationmethod and application of the quinoxaline-N1,N4-dioxide derivative. 4,5,-difluoro-2-nitroaniline is used as a raw material for synthesis of the quinoxaline-N1,N4-dioxide derivative, the quinoxaline-N1,N4-dioxide derivative reacts with sodium hypochlorite under catalysis of a basic catalyst, namely sodium hydroxide, and 5,6-difluoro-N-benzofuroxan is obtained; and then the quinoxaline-N1,N4-dioxidederivative reacts with different substrates in Beirut reaction and substitution reaction, and a series of the quinoxaline-N1,N4-dioxide derivative is obtained. According to the quinoxaline-N1,N4-dioxide derivative, the preparation method and application of the quinoxaline-N1,N4-dioxide derivative, quinoxoline-N1,N4-dioxide has good bacteriostatic activity to previously reported gram-negative bacteria, and also had good bacteriostatic activity to actinobacilluspleuropneumoniae and gram-positive bacteria such as staphylococcus aureus and streptococcus pneumoniae.
Owner:HUAZHONG AGRI UNIV
Immunogenic compositions comprising conjugated capsular saccharide antigens, kits comprising the same and uses thereof
PendingCN108367063AAntibacterial agentsBacterial antigen ingredientsVaccinationStreptococcus pneumoniae conjugated
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates), kits comprising said immunogenic compositions and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR , SYNFLORIX and / or PREVNAR 13 . The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
Synthetic peptide and application thereof
ActiveCN108164586ANo effect on growthGood antibacterial effectAntibacterial agentsPeptide/protein ingredientsStreptococcus pneumoniaeStructure analysis
The invention discloses a synthetic peptide. The synthetic peptide comprises: (a) the amino acid sequence of the synthetic peptide is shown as in SEQ ID No.1; (b) one or more amino acids of a peptideis / are deleted, inserted or replaced in the synthetic peptide defined by the (a), and the peptide derived from (a) has the same biological function with the peptide. The synthetic peptide is designedin combination with the structure analysis of Swiss-Model online software according to the known choline combination sequence. The synthetic peptide has the characteristic of combination with the choline molecule on the surface of streptococcus pneumoniae, and has the capabilities of inhibiting growth and promoting autolysis of the streptococcus pneumoniae. The synthetic peptide is non-toxic and has a certain application potential.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Vaccine containing capsular polysaccharide of type 5 streptococcus pneumoniae and preparation method thereof
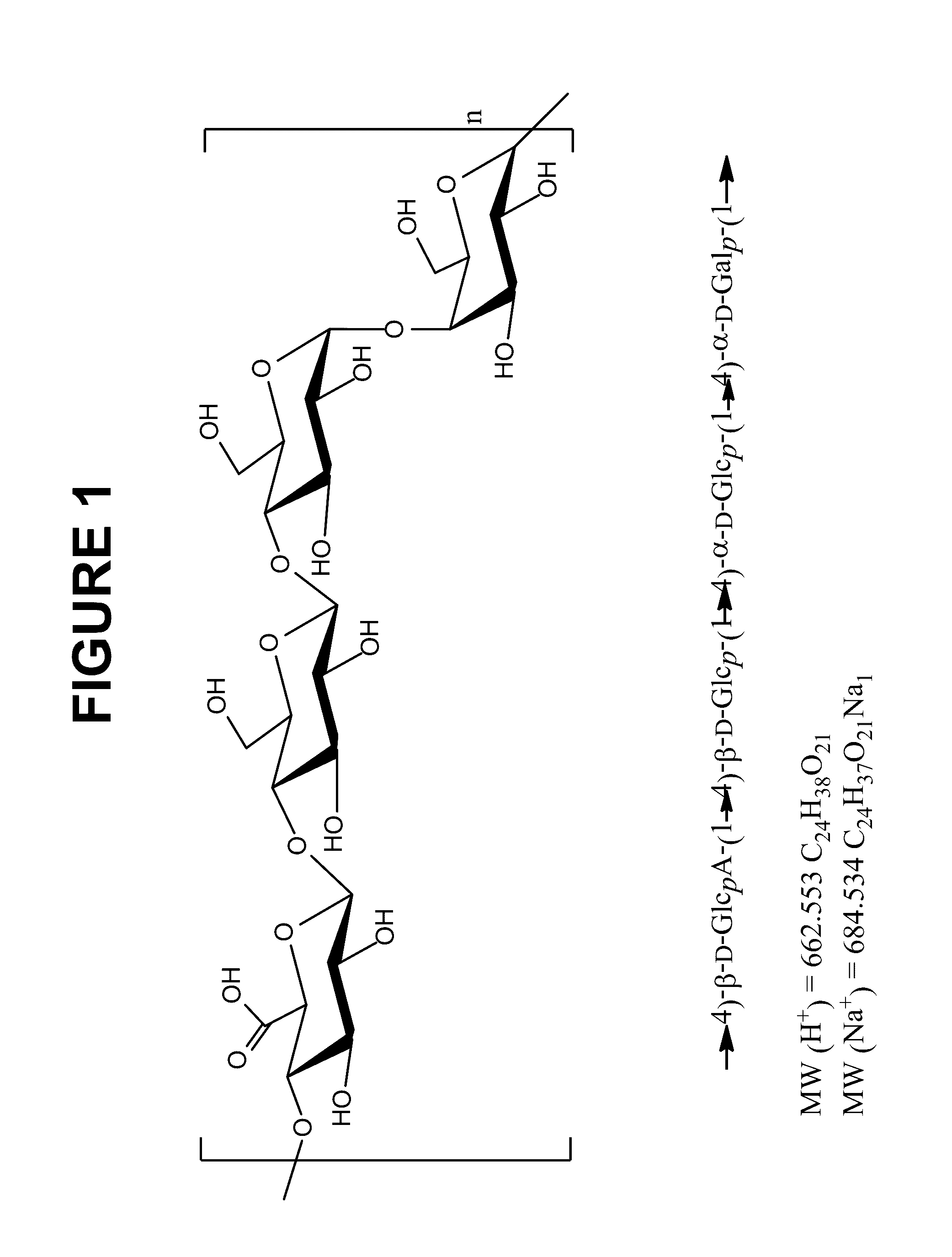
PendingCN112741901AAvoid damageReduce lossAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniae capsular polysaccharideCarrier protein
The invention provides a vaccine containing capsular polysaccharide of type 5 streptococcus pneumoniae and a preparation method of the vaccine, and belongs to the technical field of biological product preparation. The preparation method provided by the invention comprises the following steps: respectively degrading each serotype capsular polysaccharide at least containing type 5 streptococcus pneumoniae; performing activation; carrying out freeze-drying or precipitation; redissolving or dissolving the activated capsular polysaccharide of streptococcus pneumoniae, and conjugating the activated capsular polysaccharide with carrier protein; and purifying the conjugated vaccine stock solution. The antigen-specific epitope in the vaccine stock solution prepared by the method is not lost, the integrity of the antigen is maintained, the immunogenicity in the preparation process of the polysaccharide-protein conjugate is ensured not to be reduced to the greatest extent, and meanwhile, the generation of protein autopolymer is avoided. The streptococcus pneumoniae capsular polysaccharide-protein conjugate vaccine stock solution prepared by the method can be used for preparing streptococcus pneumoniae capsular polysaccharide monovalent and multivalent conjugate vaccines, and can also be used for preparing combined vaccines containing streptococcus pneumoniae capsular polysaccharide.
Owner:SINOVAC RES & DEV
Antigenic protein fragments of Streptococcus pneumoniae
Antigenic protein fragments of Streptococcus pneumoniae to be used for the preparation of a medicament for the prevention and the treatment of bacterial infections and a method for the detection thereof, and related compositions using said epitopes, are disclosed.
Owner:UNIVERSIT A DEGLI STUDI DI SIENA
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
InactiveUS20210177957A1Improve stabilityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsCarrier proteinImmunogenicity
The invention is related to multivalent immunogenic compositions comprising more than one S. pneumoniae polysaccharide protein conjugates, wherein each of the conjugates comprises a polysaccharide from an S. pneumoniae serotype conjugated to a carrier protein, wherein the serotypes of S. pneumoniae are as defined herein. In some embodiments, at least one of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. In further embodiments, each of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. Also provided are methods for inducing a protective immune response in a human patient comprising administering the multivalent immunogenic compositions of the invention to the patient. The multivalent immunogenic compositions are useful for providing protection against S. pneumoniae infection and diseases caused by S. pneumoniae. The compositions of the invention are also useful as part of treatment regimens that provide complementary protection for patients that have been vaccinated with a multivalent vaccine indicated for the prevention of pneumococcal disease.
Owner:MERCK SHARP & DOHME CORP
Antibodies targeting a galactan-based o-antigen of k. pneumoniae
ActiveUS20180066041A1Improve relevanceRapid and reliable mannerAntibacterial agentsImmunoglobulins against bacteriaEpitopeEthylene Homopolymers
The invention provides for an isolated antibody that specifically recognizes a galactan-III epitope of the lipopolysaccharide (LPS) O-antigen structure of Klebsiella pneumoniae , which epitope is incorporated in galactan-III repeating units, wherein the galactan-III repeating unit is a branched galactose homopolymer of Formula (I). The invention further provides for a pharmaceutical or diagnostic preparation comprising said antibody, and a method of producing said antibody.
Owner:X4 PHARMA (AUSTRIA) GMBH
Protein-based Streptococcus pneumoniae vaccine
InactiveUS8691243B2Bacterial antigen ingredientsPeptide/protein ingredientsStreptococcus pneumoniae conjugatedCell membrane
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Processes for the formulation of pneumococcal polysaccharides for conjugation to a carrier protein
ActiveUS20200276316A1Desire propertyPromote dissolutionAntibacterial agentsSugar derivativesSucroseStreptococcus pneumoniae conjugated
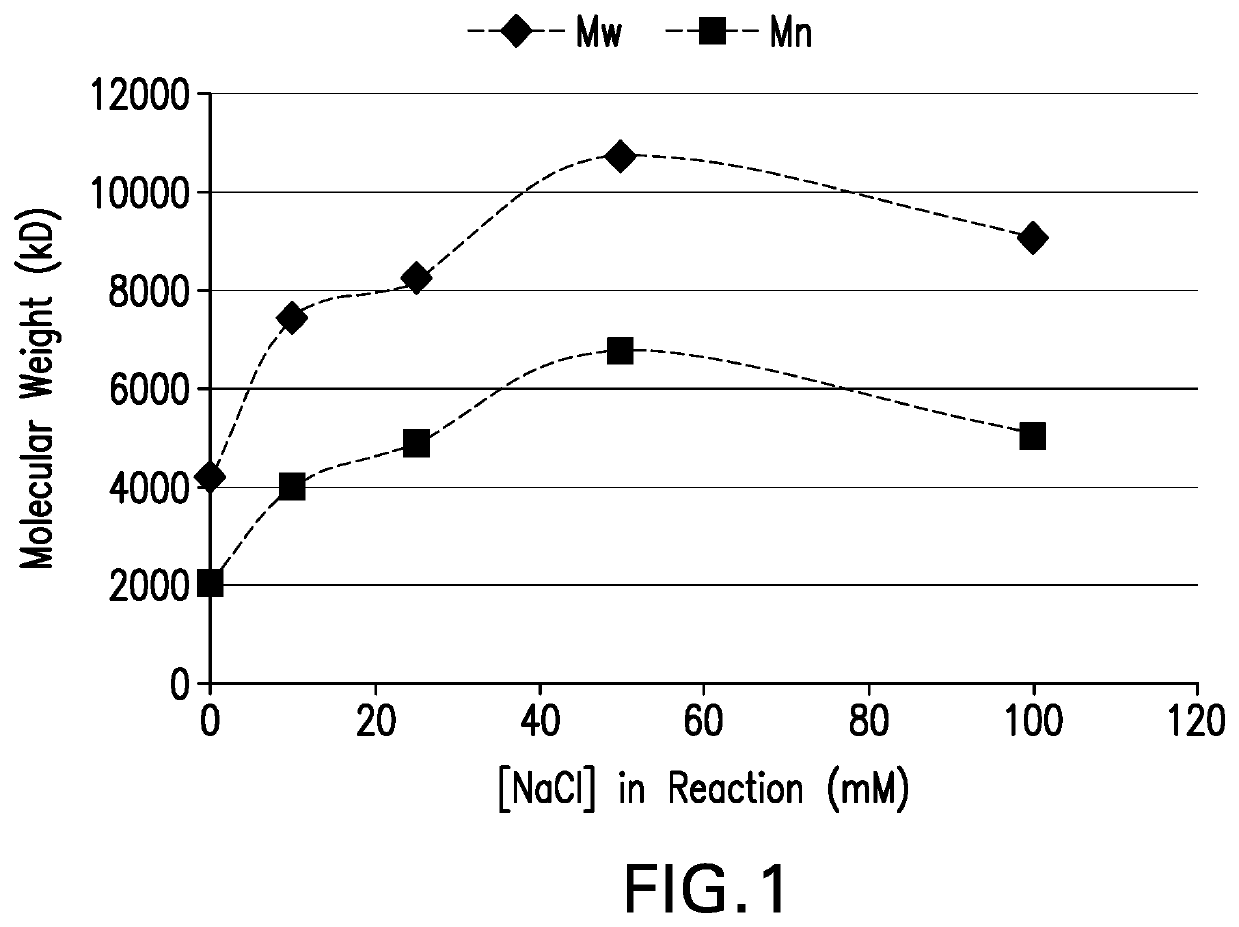
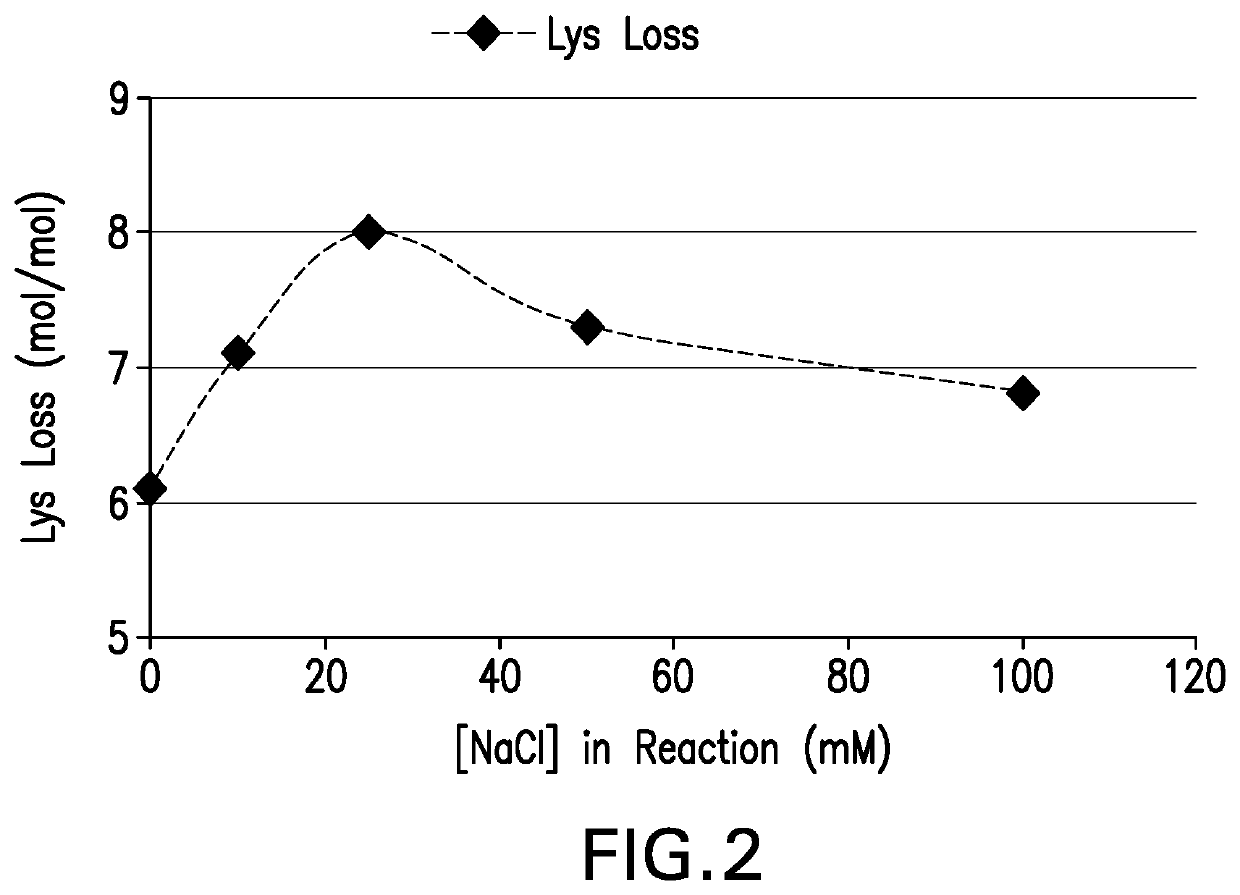
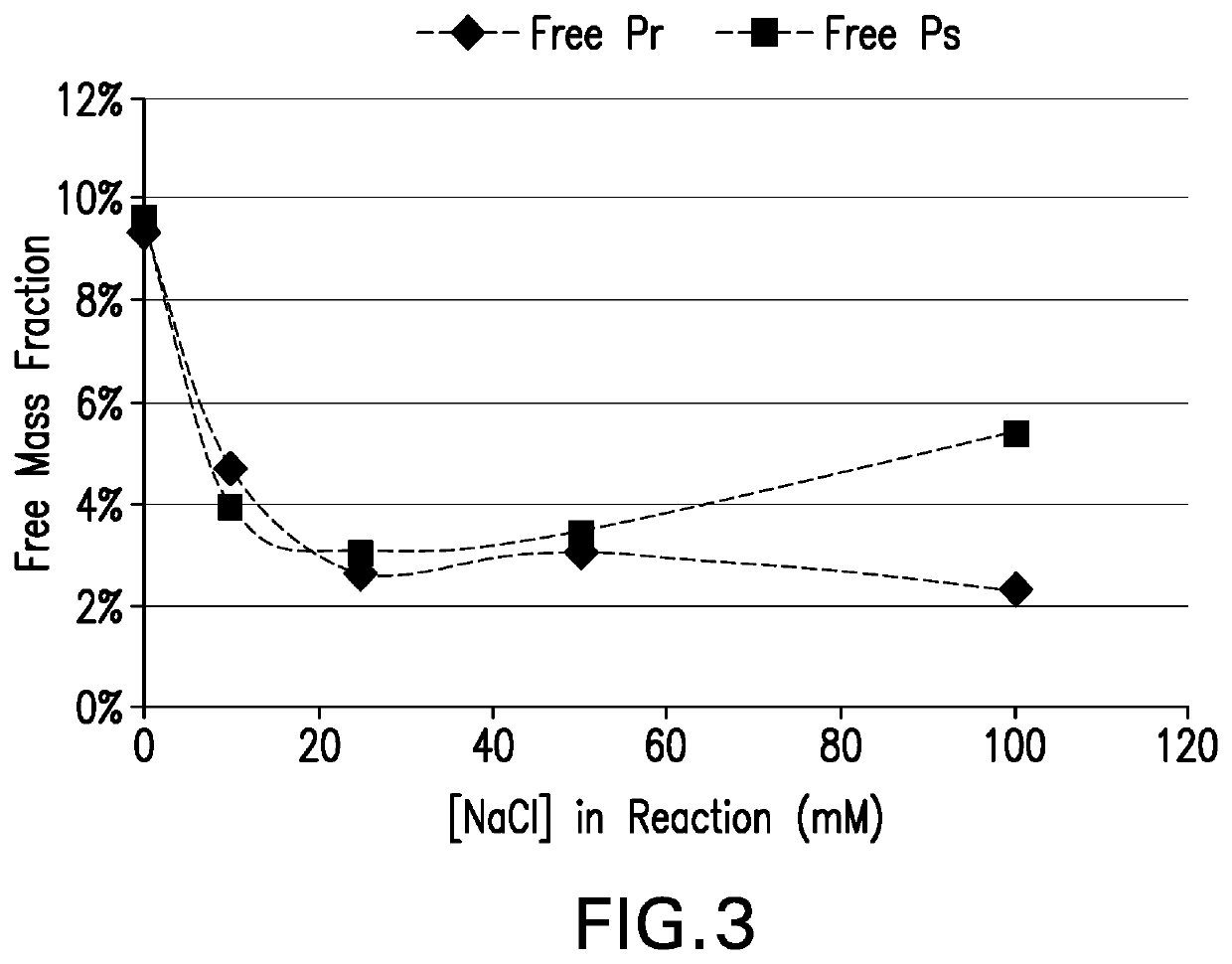
The present invention provides a number of process improvements related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae to a carrier protein. These process are serotype specific and include acid hydrolysis, addition of sodium chloride to the reductive amination reaction, and addition of sucrose to dissolve polysaccharides. Polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccines.
Owner:MERCK SHARP & DOHME LLC
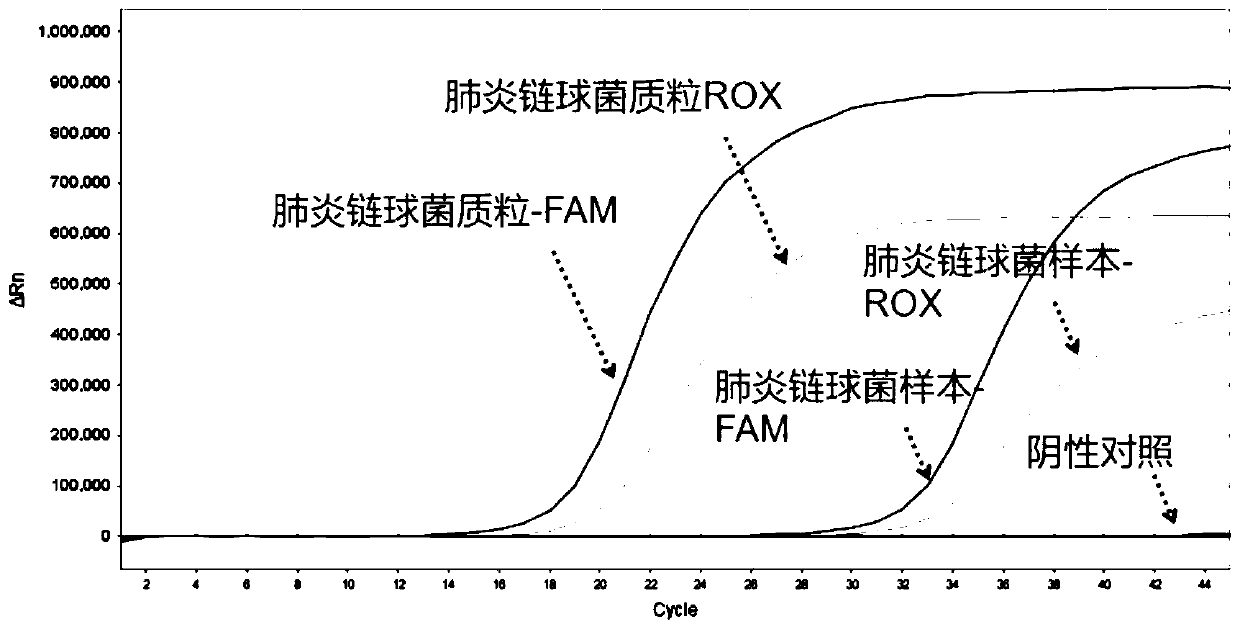
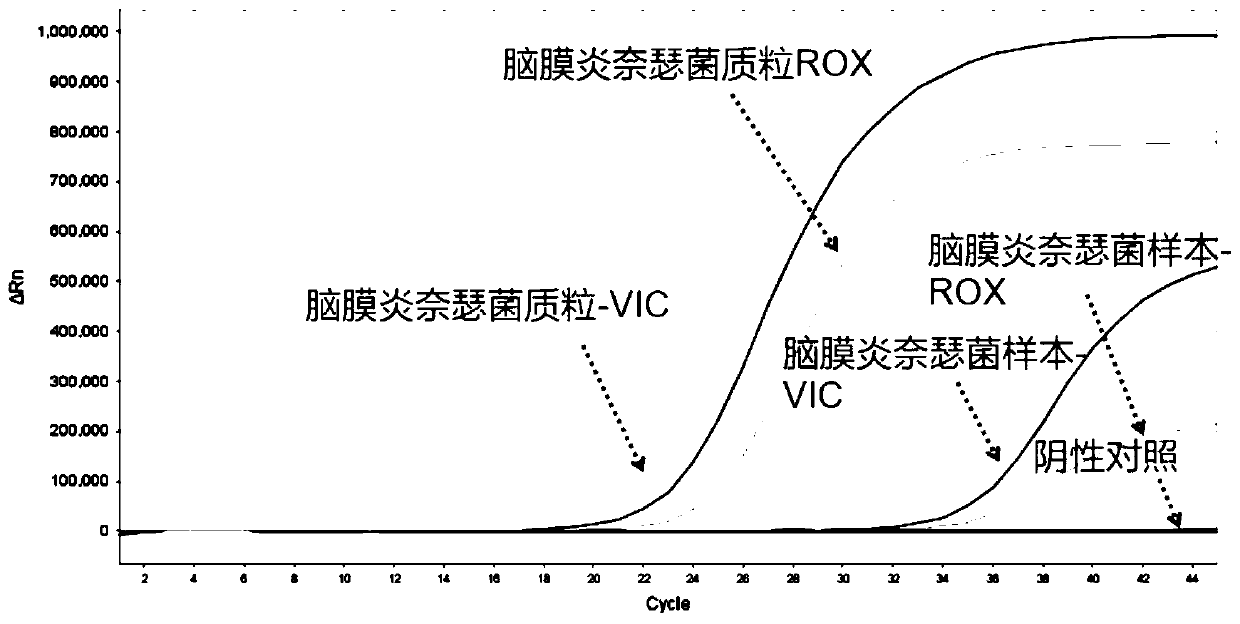
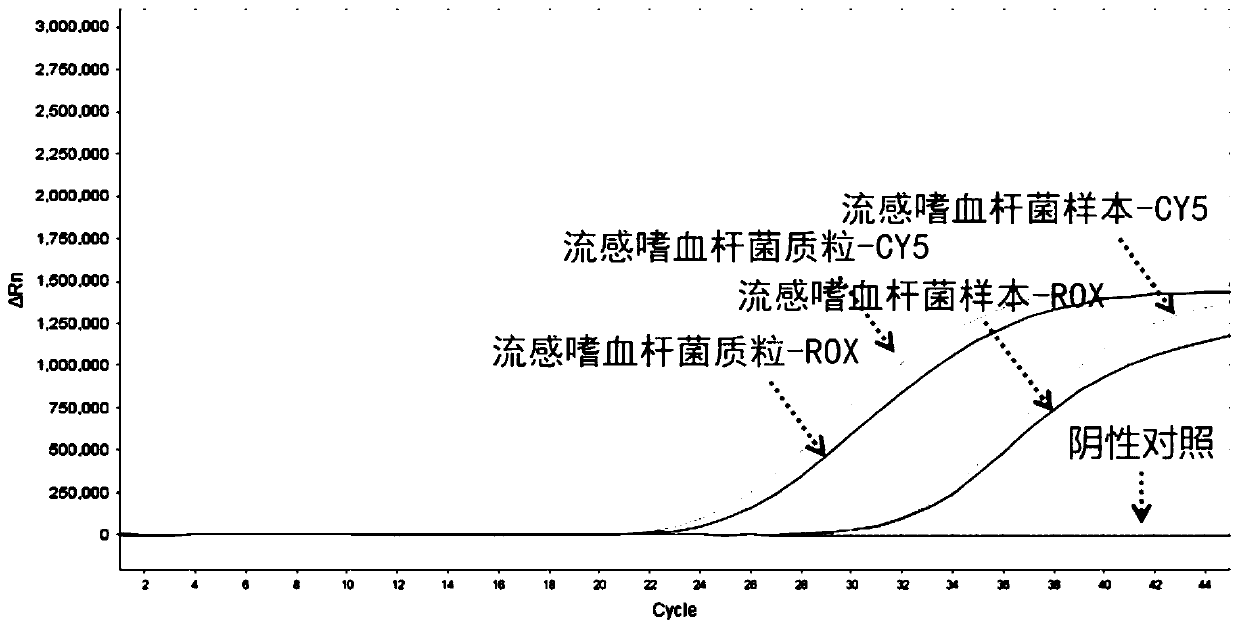
Nucleic acid typing detection kit for encephalitis and meningitis and detection method
PendingCN110904253ARapid diagnosisReduce workloadMicrobiological testing/measurementEncephalitis meningitisNeisseria meningitidis
The invention discloses a nucleic acid typing detection kit for encephalitis and meningitis. The kit comprises a nucleic acid rapid extraction reagent, a PCR amplification reagent, an encephalitis andmeningitis nucleic acid detection reagent, a positive reference substance and a negative reference substance. The encephalitis and meningitis nucleic acid detection reagent comprises an amplificationprimer and a probe of a streptococcus pneumoniae gene, an amplification primer and a probe of a neisseria meningitidis gene, an amplification primer and a probe of a haemophilus influenzae gene, andan amplification primer and a probe of an internal reference RNP gene. The invention further provides a nucleic acid typing detection method for encephalitis and meningitis. According to the invention, multi-channel primers and probes with encephalitis and meningitis specificity are introduced; the workload can be reduced; the problem that other detection methods are low in specificity and prone to missed diagnosis and misdiagnosis is solved; multiple simultaneous detection of three pathogens in the same system can be achieved, and primers and probes in all channels do not interfere with one another; the sensitivity is good; the specificity is high; accuracy and reliability are achieved; rapidness and convenience are achieved; and whether the three pathogens are infected or not is rapidlydiagnosed.
Owner:SHANGHAI BIOGERM MEDICAL TECH CO LTD
(6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid
ActiveCN102863461AQuick effectSmall doseAntibacterial agentsOrganic active ingredientsThio-Carboxylic acid
The invention belongs to the field of chemical pharmaceuticals, and relates to (6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid, a preparation method and application thereof. With the adoption of a two-phase process, 7-amino-3-hydroxymethyl-8-oxo-5-thia-1-azabicycle[4.2.0]-oct-2-ene-2-formic acid and bromoacetyl bromide are reacted to obtain an intermediate A; and the intermediate A and N',N-diisopropylthiourea are reacted to obtain the compound provided by the invention. The compound provided by the invention has the antibacterial effects on staphylococcus aureus, streptococcus pneumoniae, enterococcus hirae and enterococcus faecalis etc., especially, the antimicrobial activity on staphylococcus aureus is very strong and is 10 folds stronger than cefathiamidine, the activity on enterococcus faecalis is 15 folds stronger than cefathiamidine, and the activity on streptococcus pneumoniae is strong as well, so that the compound is a potential antibacterial agent.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


















![(6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid (6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d82a2194-820c-4815-8986-808592e43425/HDA0000074496280000011.png)
![(6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid (6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d82a2194-820c-4815-8986-808592e43425/FDA0000074496260000011.png)
![(6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid (6R, 7R)-3-hydroxymethyl-7-[alpha-(N, N'-diisopropylamidino thio)-acetamido]-8-oxo-5-thia-1-azabicycle [4, 2, 0]-oct-2-ene-2-carboxylic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/d82a2194-820c-4815-8986-808592e43425/FDA0000074496260000012.png)