Quinoxaline-N1,N4-dioxide derivative capable of inhibiting activity of DNA topoisomerase, preparation method and application of quinoxaline-N1,N4-dioxide derivative
A topoisomerase, dioxide technology, applied in the field of biochemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
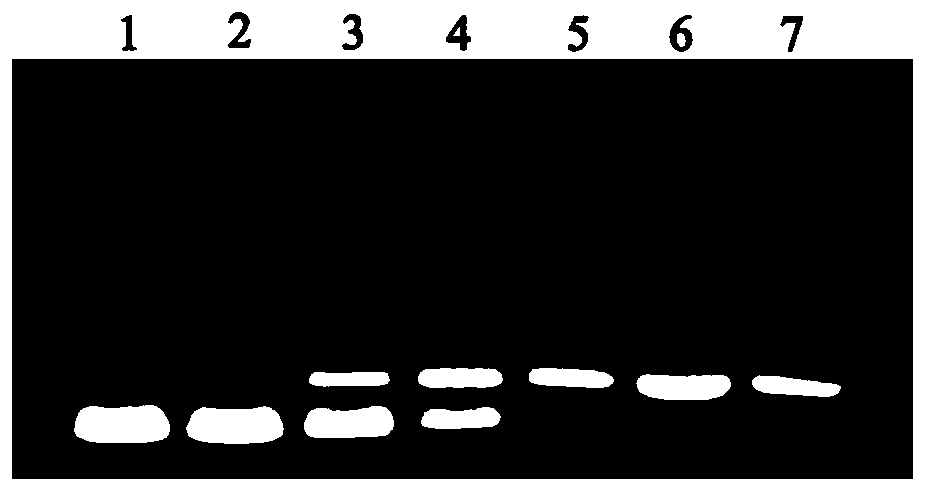
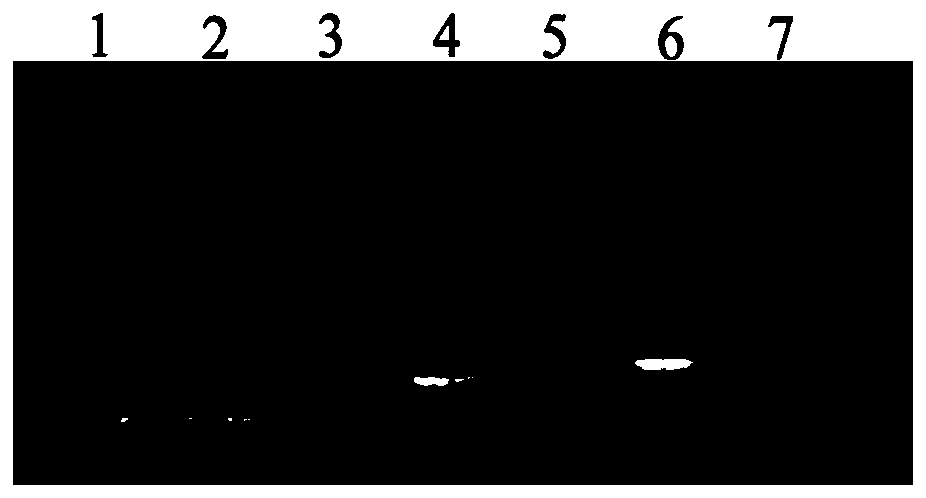
Image
Examples
Embodiment 1
[0034] With quinoxaline ring C 3 methyl substitution, C 7 Take fluorine atom substitution as an example to illustrate
[0035] In this embodiment, quinoxaline-N 1 ,N 4 -The synthetic reaction formula of the dioxide derivative is as follows:
[0036]
[0037] Concrete preparation steps:
[0038] 1. Add 25mL tetrahydrofuran to a 50mL round-bottom reaction flask, then add 3g 4,5-difluoro-2-nitroaniline, 80mL sodium hypochlorite, and 0.2g sodium hydroxide in sequence, and react at 0°C for 2 hours. Pour into a separatory funnel, extract twice with dichloromethane (25mL×2), combine the dichloromethane layers, evaporate the solvent under reduced pressure to obtain a light yellow solid, which is 5,6-difluoro-N-oxybenzene Furazan;
[0039] 2. Quinoxaline ring C 2 Position is ethyl formate, C 3 When the position is substituted with a methyl group:
[0040] Take 3.5g of 5,6-difluoro-N-oxybenzofurazan and place it in a 50mL round-bottomed reaction flask, add 25mL of acetone to...
Embodiment 2
[0051] 7-F-6-piperazine-3-CH 3 -Ethyl 2-quinoxalinecarboxylate-N 1 ,N 4 -The synthetic reaction formula of dioxide oxide is as follows:
[0052]
[0053] The preparation steps are as follows:
[0054] (1) Add 8.7g (0.05mol) 4,5-difluoro-2-nitroaniline to a 500mL three-necked flask, add 75mL tetrahydrofuran to fully dissolve it, then add 0.6g (0.015mol) sodium hydroxide as a catalyst, Add 240mL of sodium hypochlorite dropwise in an ice bath (0°C), and stir for 2 hours to react. After the reaction is complete, the reaction solution is extracted twice with dichloromethane (400mL × 2). The organic phase is collected and the solvent is distilled off under reduced pressure to obtain a light yellow solid. 7.0 g is 5,6-difluoro-N-oxybenzofurazan, and the yield is 81.4%.
[0055] (2) Add 3.44g (0.02mol) of 5,6-difluoro-N-oxybenzofurazan to a 50mL single-necked flask, add 25mL of acetone to dissolve, then add 3.9g (0.03mol) of ethyl acetoacetate, and 2.5 g potassium carbonate, s...
Embodiment 3
[0058] 7-F-6-piperazine-3-CH 3 -2-quinoxalinecarboxylic acid-N 1 ,N 4 -The synthetic reaction formula of dioxide oxide is as follows:
[0059]
[0060] The preparation steps are as follows:
[0061] Add the 7-F-6-piperazine-3-CH obtained in Example 2 to the 50mL one-necked flask 3 -Ethyl 2-quinoxalinecarboxylate-N 1 ,N 4 - Dioxide 3.5g (0.01mol), dissolved in tetrahydrofuran / water (v:v=2:1), add 1mol / L sodium hydroxide 24.5mL, carry out hydrolysis reaction at 50°C, after the reaction, add Dilute hydrochloric acid (1mol / L) to adjust the pH value of the reaction solution to 5-6, freeze and precipitate a solid at -20°C, filter and wash with ice ethanol to obtain 1.4 g of a khaki solid powder, which is 7-F-6-piperazine-3 -CH 3 -2-quinoxalinecarboxylic acid-N 1 ,N 4- Dioxide, 43.8% yield. Melting point 218.2-220.5℃; MS: [M+H] + 323.1077; 1 H NMR (600MHz,D 2 O)δ8.08(d,J=12.4Hz,1H),7.82(d,J=7.9Hz,1H), 3.61–3.50(m,4H),3.48–3.39(m,4H),2.53(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com