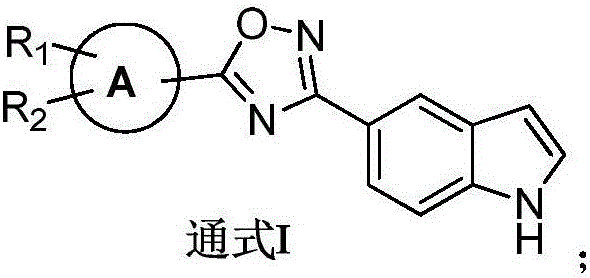
Indole derivative with 1, 2, 4-oxadiazole structure, preparation method thereof and application thereof in preparation of antibacterial medicines
A technology of indole derivatives and oxadiazoles, applied in the field of drug synthesis, can solve the problems of death of patients and uncontrollable antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
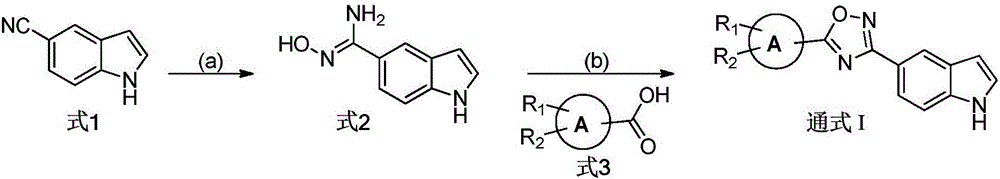
[0027] The preparation of formula 2 compound
[0028]
[0029] Dissolve 1.42 g of powdered p-cyanindole (Formula 1) in 50 ml of absolute ethanol, cool in ice to 0° C., add 4.04 g of triethylamine and 1.67 g of hydroxylamine hydrochloride, stir for 0.5 h, then heat to reflux (80 °C) overnight. TLC detected that the reaction was complete, and the reaction solution was concentrated. Extracted with ethyl acetate (50ml*3), the combined organic layers were washed 3 times with saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel chromatography to obtain 1.44 g of yellow viscous N'-hydroxy-1H-indole-5-carboxamidine (Formula 2), with a yield of 82.3%. 1 H NMR (DMSO-d 6 ,300MHz)δ11.15(s,1H),9.36(s,1H),7.85(m,1H),7.45(dd,J=1.59Hz,J=8.45Hz,1H),7.36-7.33(m,2H ),6.44(m,1H),5.69(s,2H).ESI-MS m / z:176.1[M+H] +
Embodiment 2
[0031] The preparation of general formula I compound
[0032]
[0033] Dissolve 2mmol of the compound of formula 3 in 10ml of dichloromethane, add 2.2mmol of N,N-diisopropylethylamine and 2.2mmol of 2-(7-azobenzotriazole)-tetramethyluronium hexafluorophosphate (HATU) , stirred at room temperature for 3 h, and TLC detected that the reaction was complete. 2 mmol of N'-hydroxy-1H-indole-5-carboxamidine (Formula 2) dissolved in dichloromethane was added to the above reaction liquid, stirred at room temperature for three hours, TLC detected that the reaction was complete, and the reaction liquid was concentrated. Extracted with ethyl acetate (25ml*3), the combined organic layer was washed 3 times with saturated sodium chloride solution, dried over anhydrous sodium sulfate and concentrated under reduced pressure to obtain a residue. The above residue was dissolved in 10 ml of ethanol, 4 mmol of anhydrous sodium acetate was added, and heated to reflux (80° C.) overnight, TLC dete...
Embodiment 3
[0108] Bacteriostatic activity test
[0109] In this study, the half-inhibitory concentration (MIC) of the compound against methicillin-resistant Staphylococcus aureus (MRSA) was determined by the microbroth dilution method. 50 ): First, the compounds were diluted to different concentrations with dimethyl sulfoxide (DMSO), and 5 μL of each concentration of the drug solution was added to 195 μL of bacterial suspension (10 5 CFU / mL), the final concentration of the compound in the medium was 125 μg / mL, 62.5 μg / mL, 31.25 μg / mL, 15.625 μg / mL, 7.8125 μg / mL, 3.9063 μg / mL, 1.95315 μg / mL, 0.976575 μg / mL, 0.4882875 μg / mL, 0.24414375 μg / mL, 0.122071875 μg / mL, 0.061035938 μg / mL. Incubate at 37°C for 24 hours to observe the results, and calculate the half inhibitory concentration MIC of the compounds 50 value.
[0110] The antibacterial activities of some compounds such as compounds 4, 20, 23, 25, 28, 29, 31 and 34 in the examples are shown in the table below:
[0111] compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com