Application of streptococcus pneumoniae protein to resisting infection of S. pneumoniae
A Streptococcus pneumoniae, protein technology, applied in the direction of immunoglobulin, antibacterial immunoglobulin, application, etc., can solve the problems of insufficient immunogenicity, adverse reactions, etc., to enhance resistance to Streptococcus pneumoniae infection, improve the body's IgG Effect of Antibody Response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Recombinant expression plasmid pET28a(+)-PepO, pET28a(+)-ZmpB 673-863 , pET28a(+)-ZmpB 673-863 -PepO and pET28a(+)-PepO-ZmpB 673-863 Construction of expression vectors.
[0089] (1) Materials:
[0090] Plasmid pET28a(+) was purchased from Novagen, Prime Star high-fidelity enzyme, dNTPs, Buffer, MgCl used in PCR 2 Purchased from Bao Biological Engineering (Dalian) Co., Ltd., PTC-200 PCR instrument was a Perkin Elmer product, and fluorescent quantitative PCR instrument RG-3000 was purchased from Corbett Research.
[0091] (2) Design and synthesis of primers:
[0092] Using Streptococcus pneumoniae D39 genomic DNA as a template, refer to its complete sequence (GeneBank number CP000410.2), use Premier5.0 to design primers, and synthesize them by Sangon Bioengineering (Shanghai) Co., Ltd.
[0093] ZmB 673-863 : Upstream primer: 5'-GCCATGGTTGAAGAAGTTGTTGTT-3', containing NcoI site;
[0094] Downstream primer: 5'-CCCTCGAGATCTCCAAGACTGTTAAT-3', containing XhoI site;
[...
Embodiment 2
[0162] Prokaryotic expression plasmids pET28a(+)-PepO, pET28a(+)-ZmpB 673-863 , pET28a(+)-ZmpB 673-863 -PepO and pET28a(+)-PepO-ZmpB 673-863 Expression, identification and purification in Escherichia coli.
[0163] (1) Recombinant plasmids pET28a(+)-PepO, pET28a(+)-ZmpB 673-863 , pET28a(+)-ZmpB 673-863 -PepO and pET28a(+)-PepO-ZmpB 673-863 Transformed into the host strain BL21(DE3).
[0164] (2) IPTG induces PepO and ZmpB 673-863 , ZmpB 673-863 -PepO (fusion protein) and PepO-ZmpB 673-863 (Fusion protein) expression in large quantities.
[0165] (3) Purification of the recombinant protein: After the bacteria were broken by ultrasonic, the supernatant of the broken bacteria was taken for purification; 4°C, 10000rpm×10min, the supernatant was filtered with a 0.45μm membrane filter, and the filtrate was collected for later use.
[0166] Affinity chromatography purification: pipette 2ml of 50% Ni 2+ -NTA resin suspension in the chromatography column, equilibrate with 20m...
Embodiment 3
[0180] Detection of ZmpB by Western blot 673-863 Recognition reaction of protein antiserum to Streptococcus pneumoniae bacterial protein.
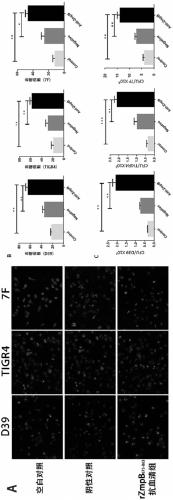
[0181] (1) Streptococcus pneumoniae CMCC 31109 (type 1), D39 (type 2), CMCC 31436 (type 3), TIGR4 (type 4), CMCC 31207 (type 6B), CMCC31507 (type 7F), CMCC31216 (type 9V) , CMCC 31614 (Type 14), CMCC31687 (Type 18C), CMCC31693 (Type 19F) and CMCC31759 (Type 23F). A total of 11 different serotypes of bacteria were inoculated in 30ml C+Y medium, 37°C, 5% CO 2 Cultivate until the bacteria are in the logarithmic growth phase OD600=0.4-0.6, collect the bacteria, centrifuge at 12000g for 5min, wash twice with sterile PBS, resuspend the bacterial cell pellet with 100μl PBS, add 5×loadingbuffer in proportion, boil in boiling water for 30min, Centrifuge at 12000g for 5min, and take the supernatant for Western Blot analysis.
[0182](2) PAGE electrophoresis: the loading amount of protein per hole is 2 μg, 8% separating gel+5% stacking gel, 80V, 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com