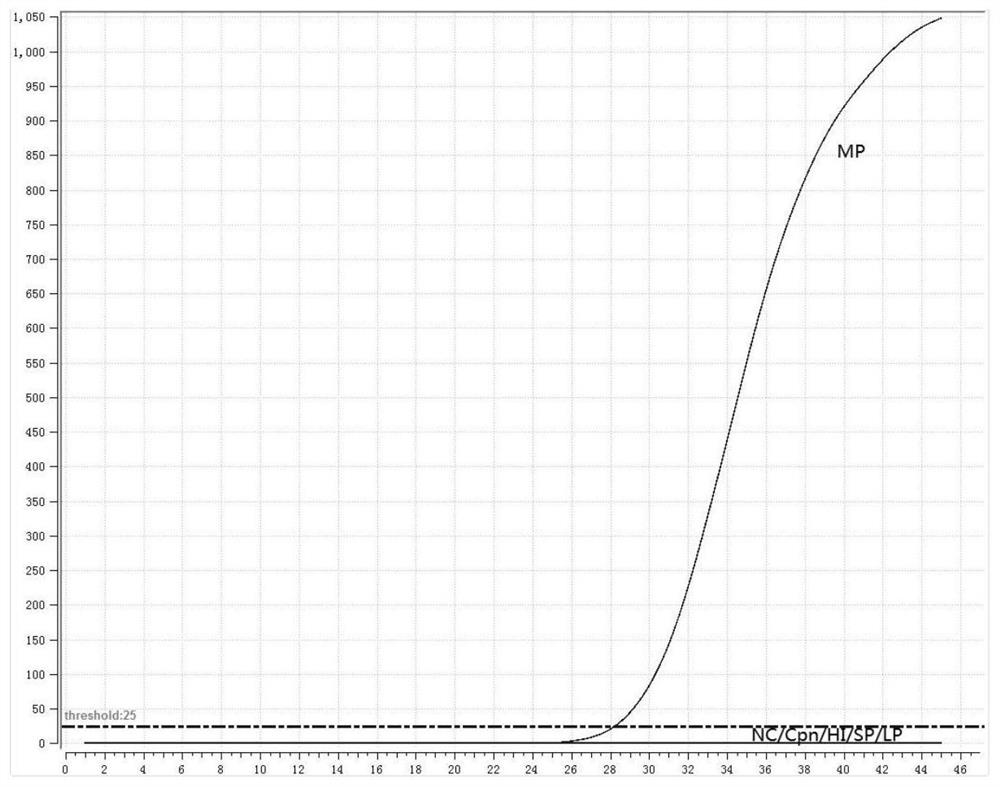
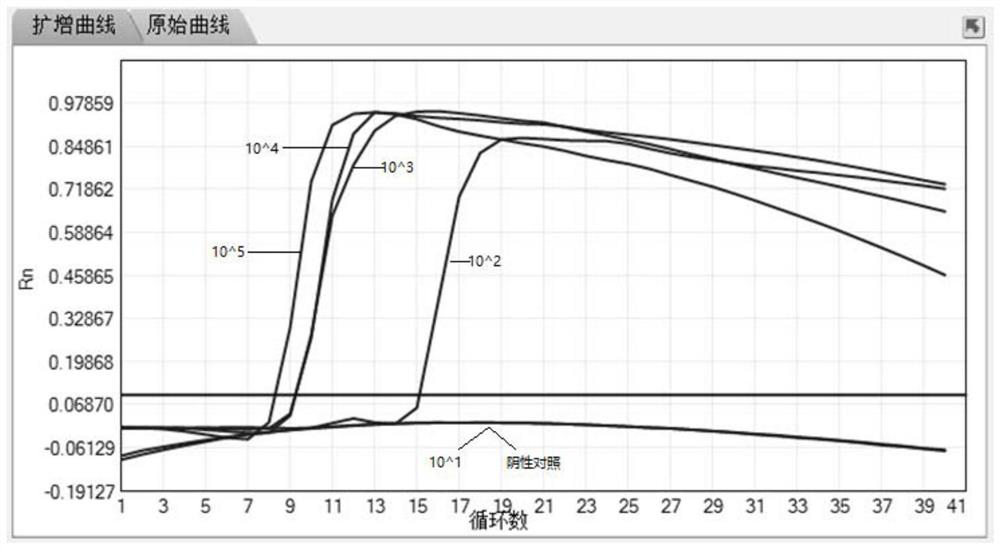
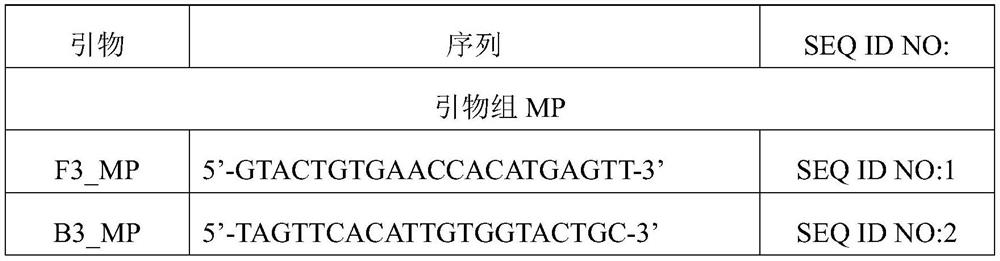
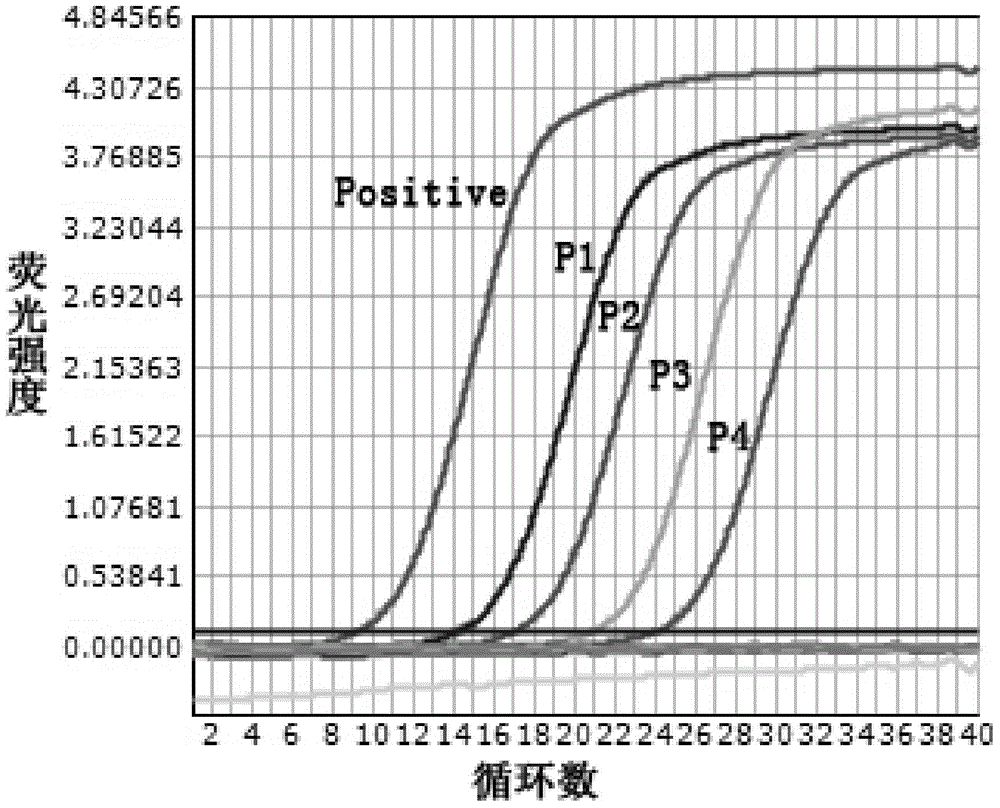
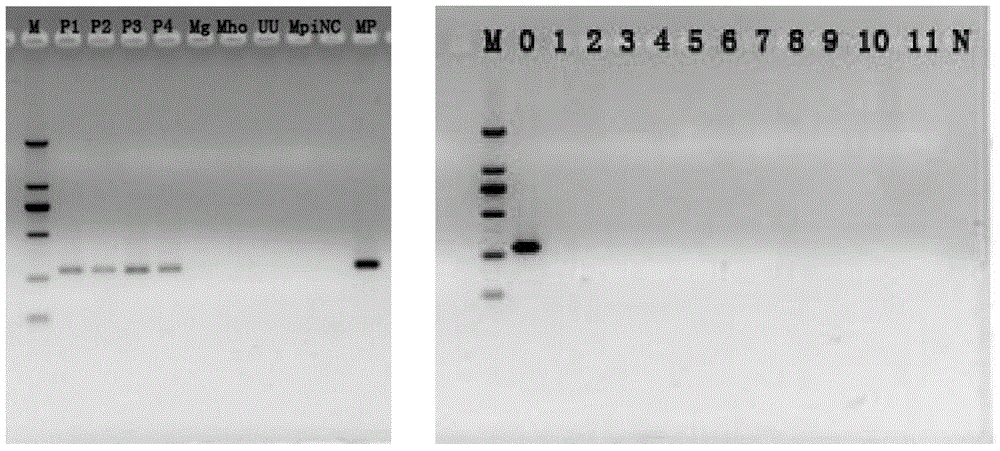
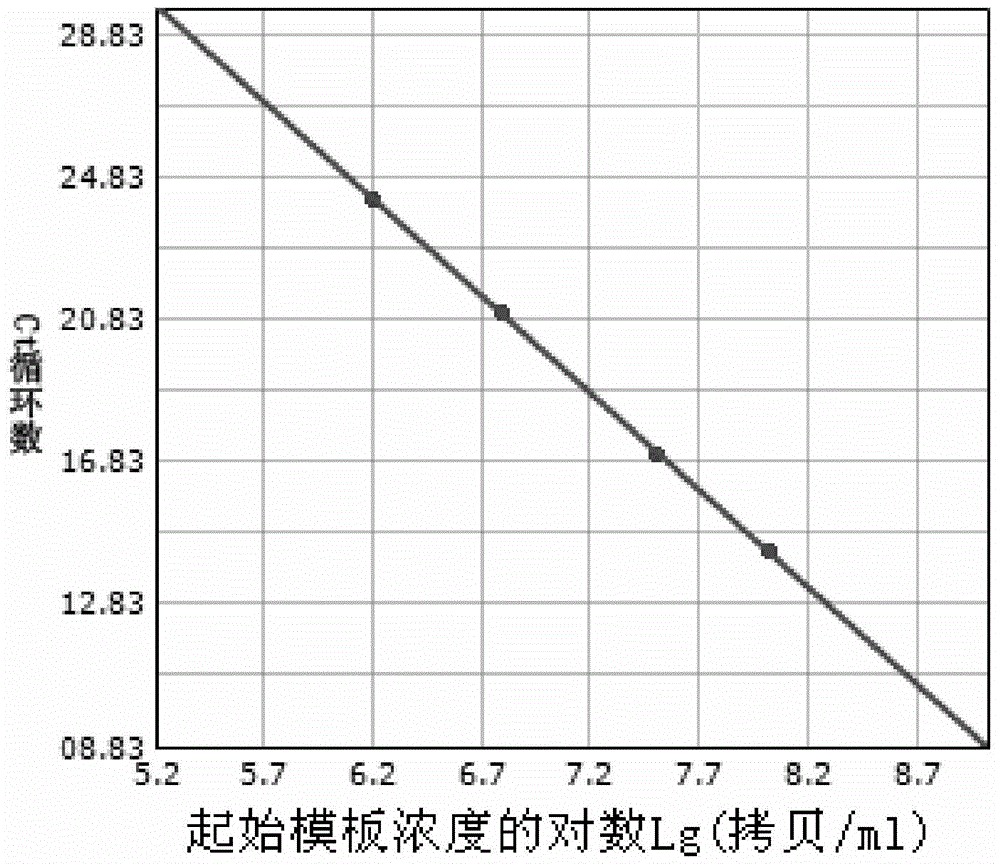
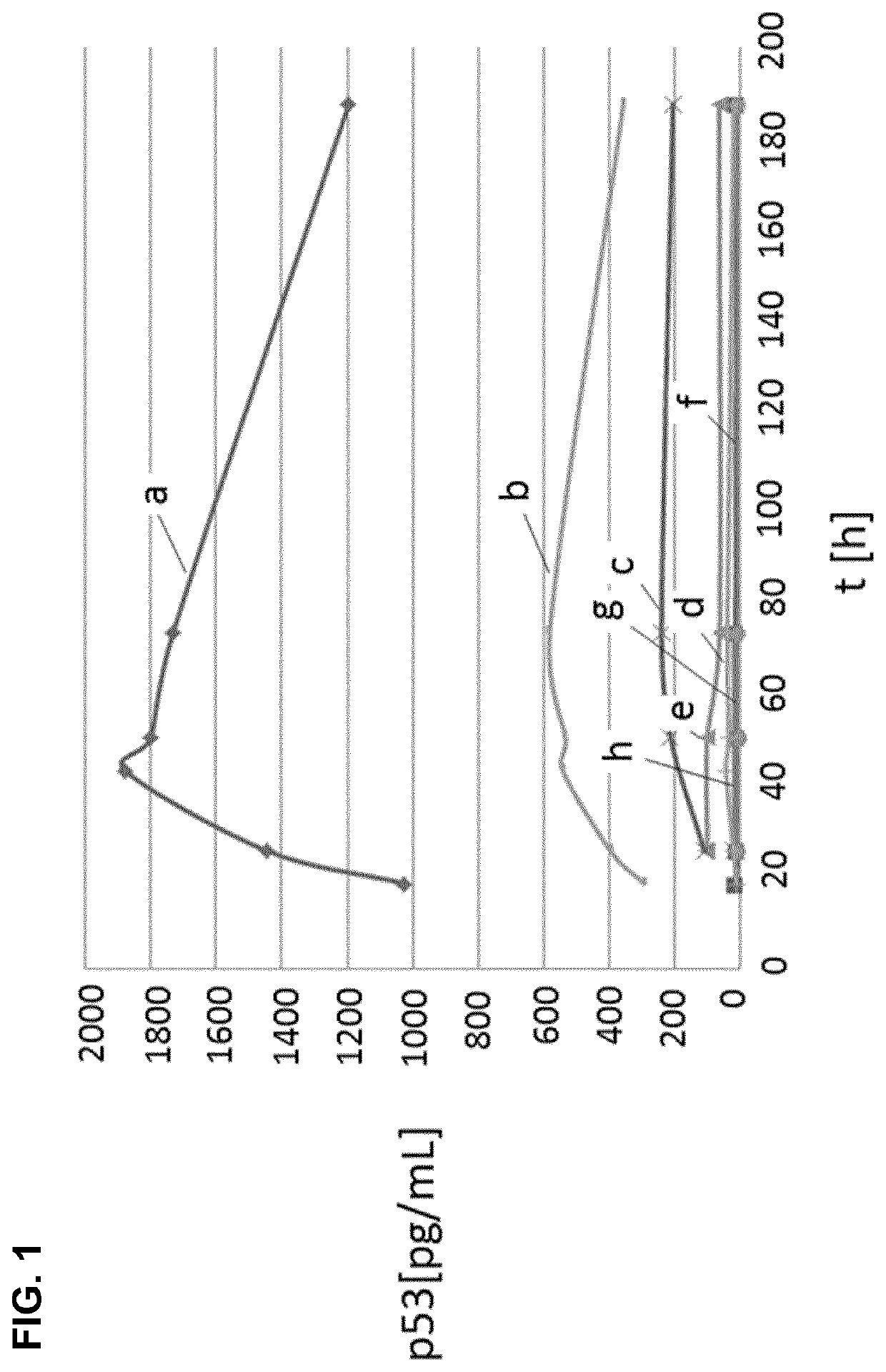
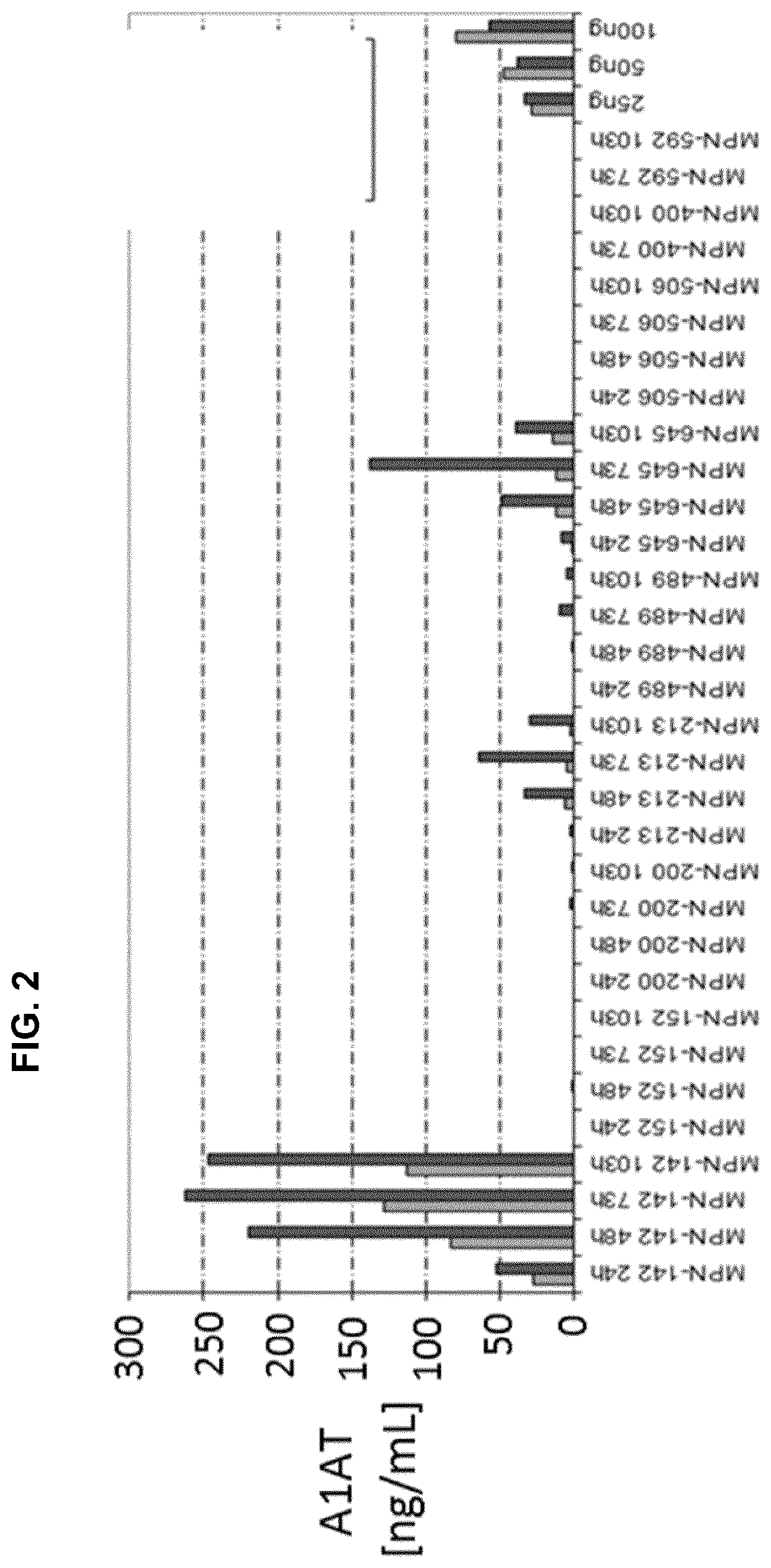
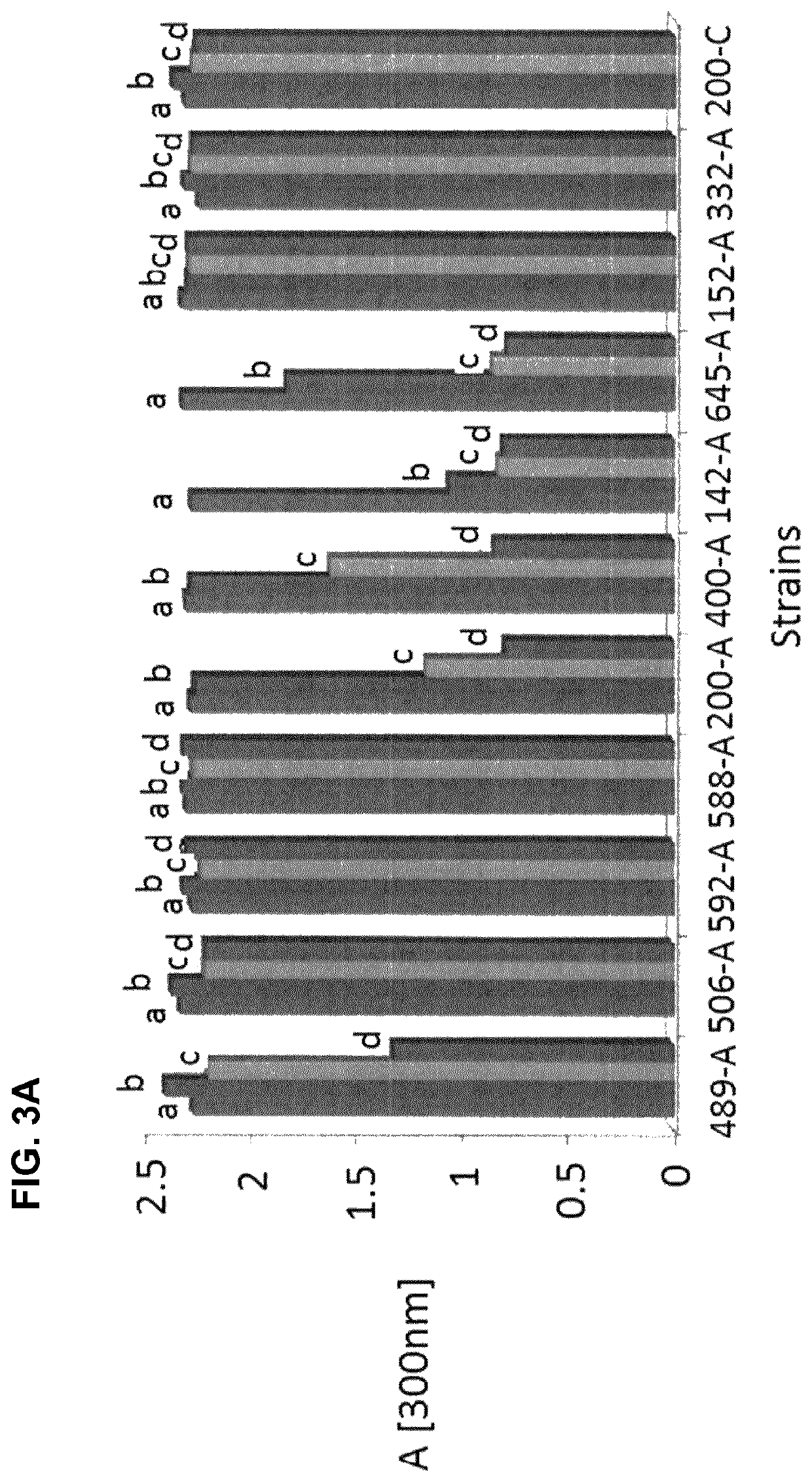
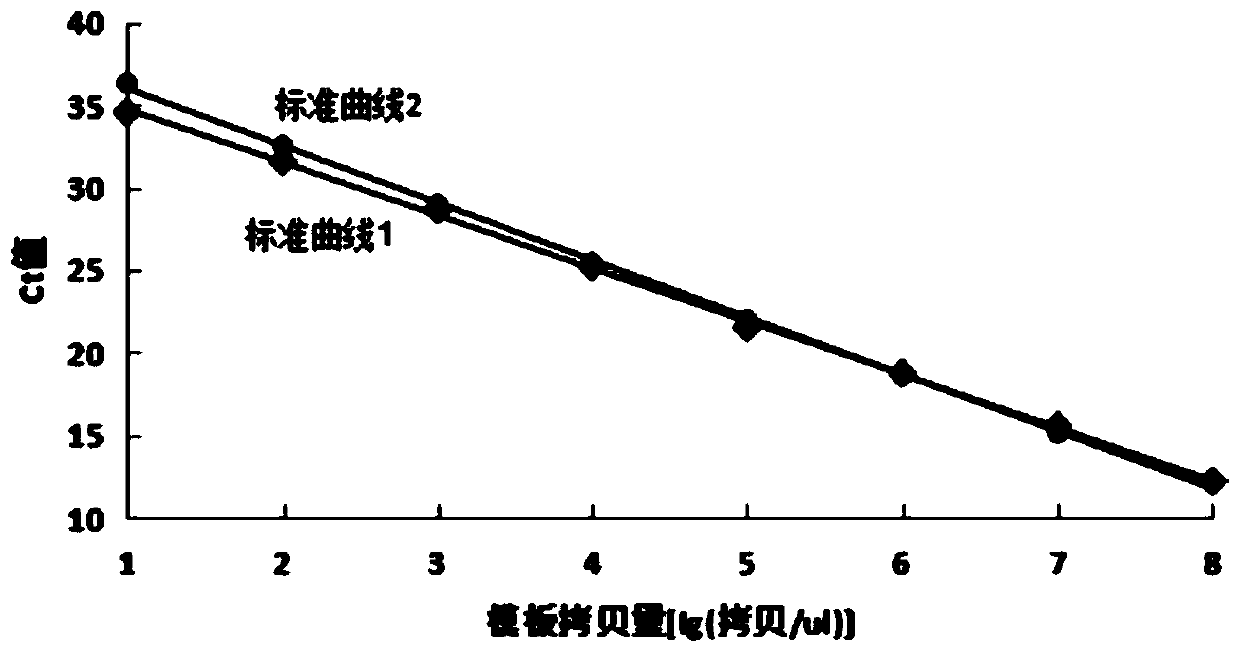
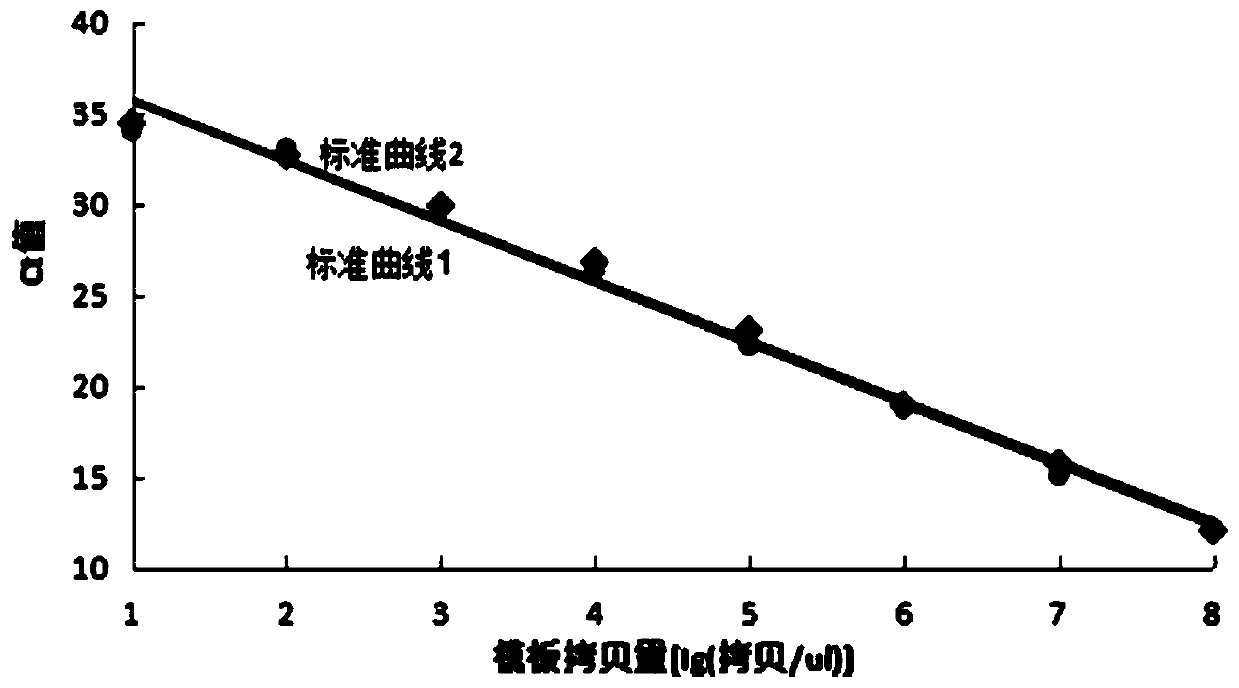
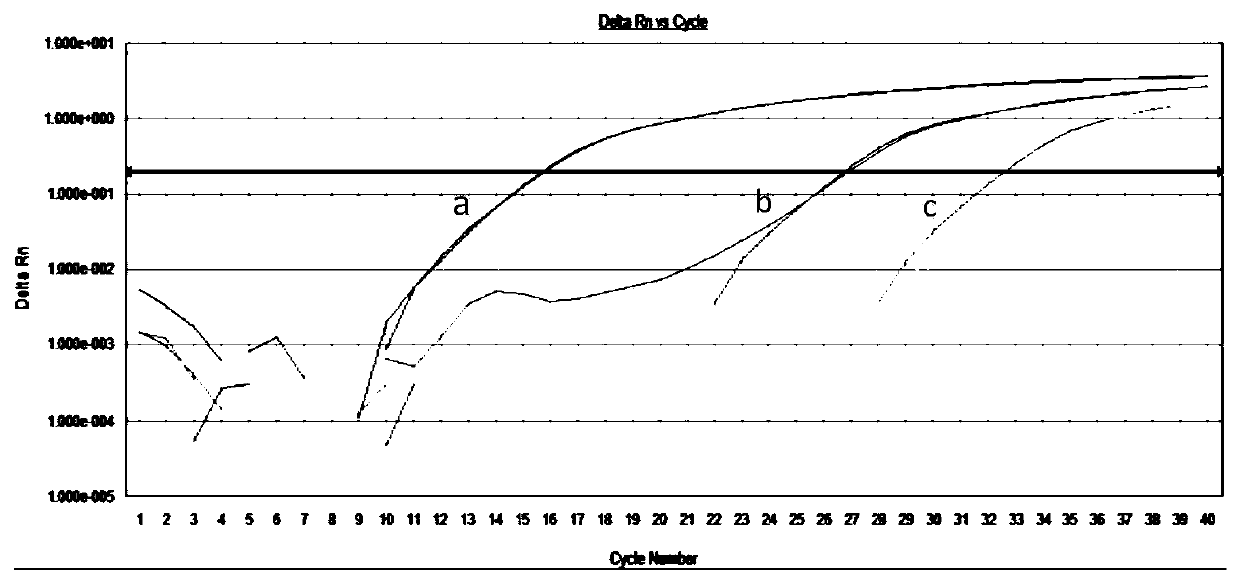
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "M. pneumoniae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
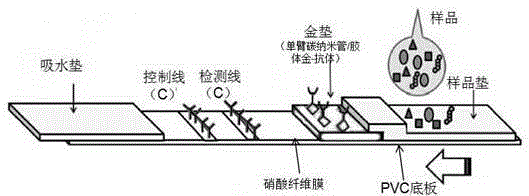
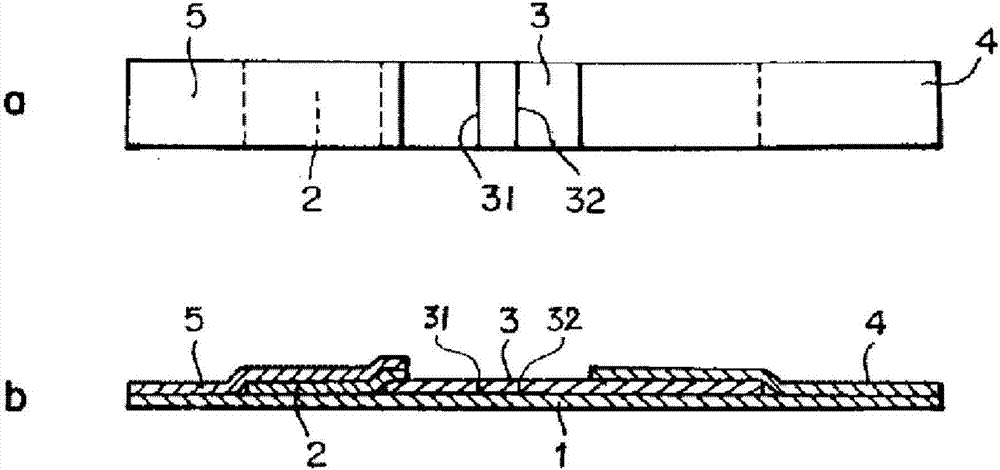
Collaurum immunochromatography test strip detecting mycoplasma pneumoniae and preparing method thereof
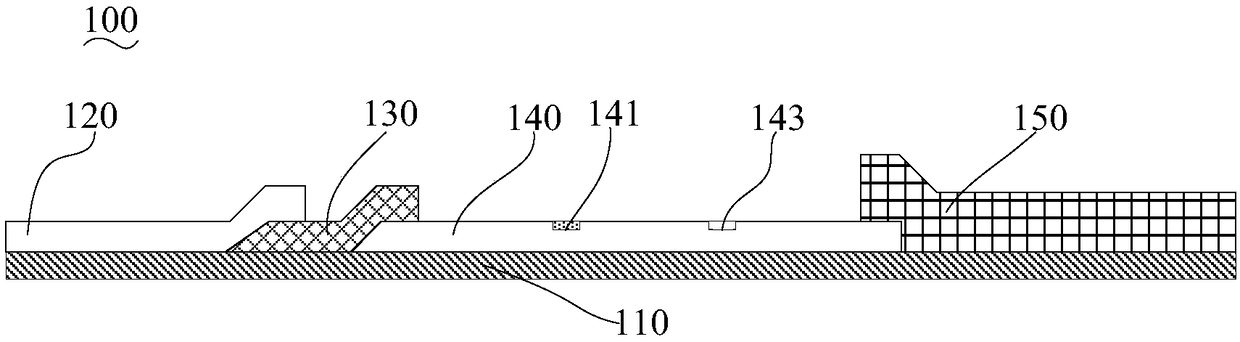
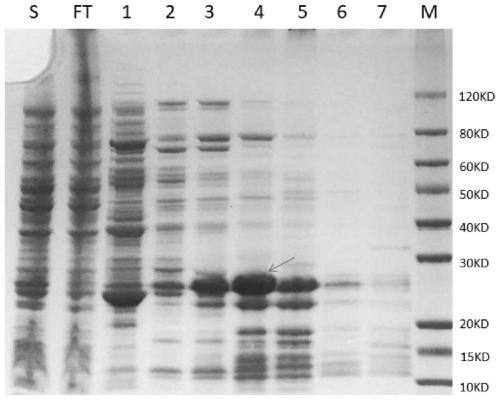
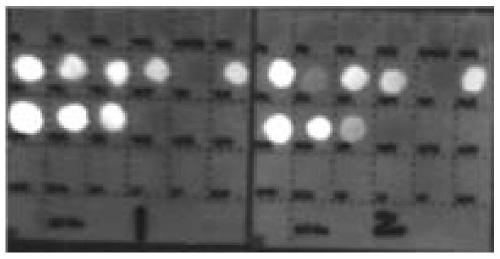
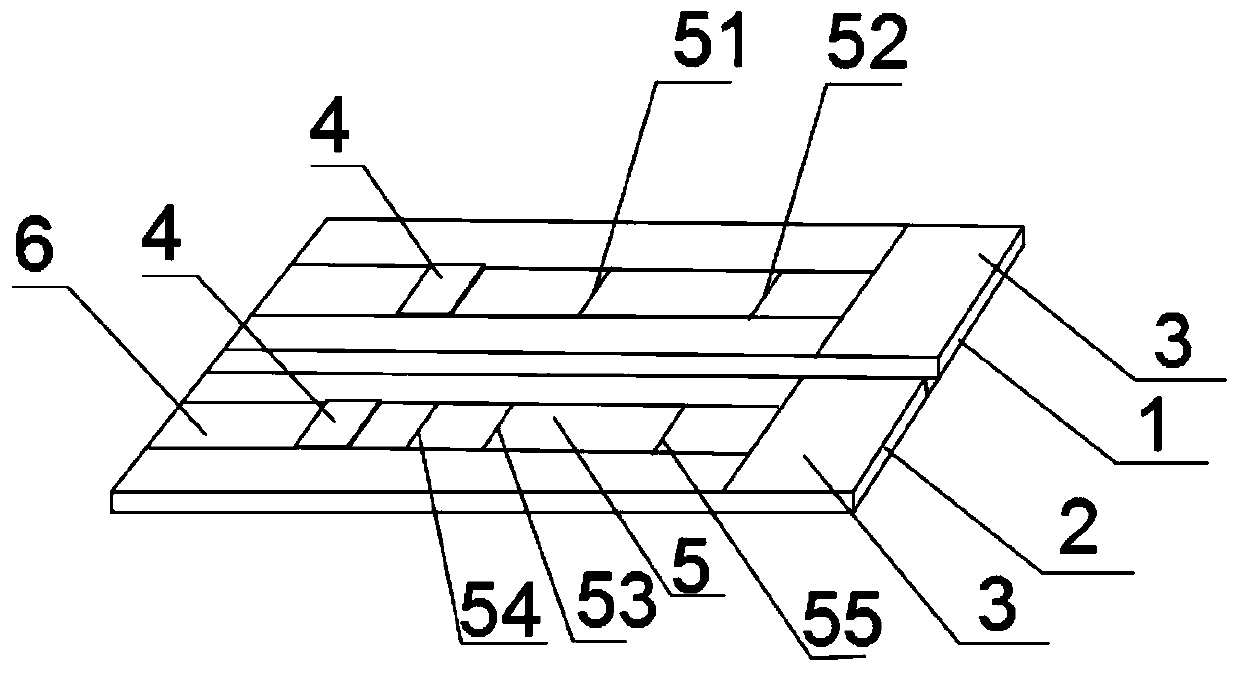
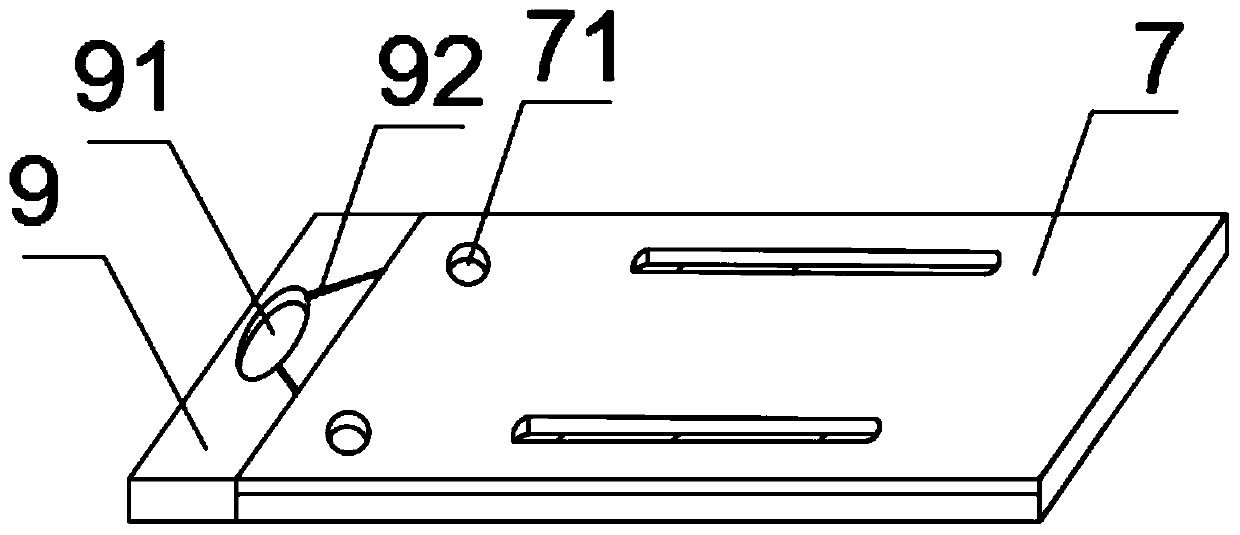
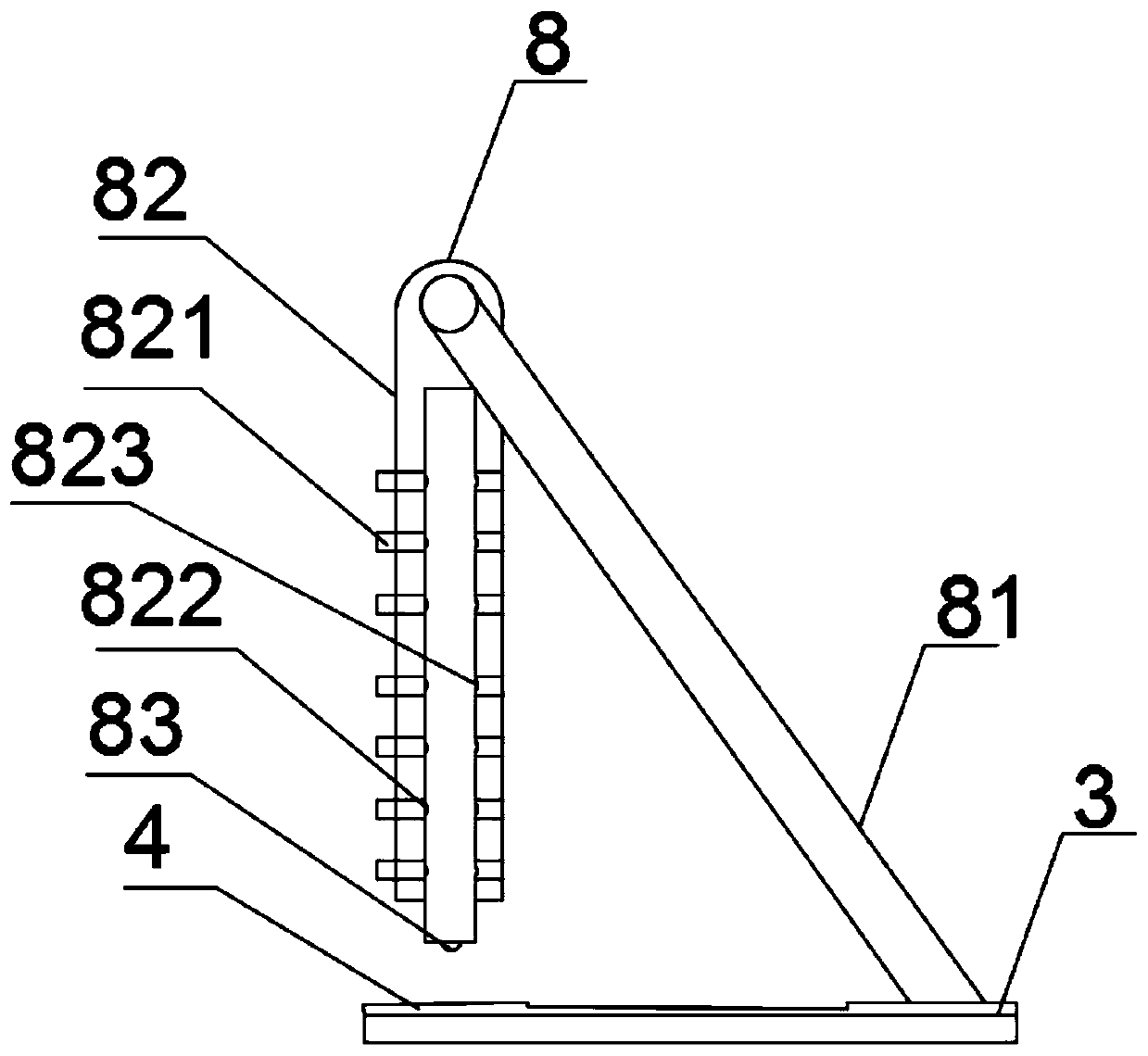
The invention belongs to a collaurum immunochromatography test strip detecting mycoplasma pneumoniae, comprising a sample pad, a combining pad, a nitrocellulose coating film, a water absorption pad and a PVC bottom plate; the sample pad, the combining pad, the nitrocellulose coating film and the water absorption pad are overlapped on the PVC bottom plate in sequence; the combining pad is coated with a mycoplasma pneumoniae antibody MPh2-collaurum-carbon nanotube marker; the nitrocellulose coating film is provided with a detection line and a quality control line; the detection line is coated with a mycoplasma pneumoniae antibody MPh1 and the quality control line is coated with a goat anti mouse IgG. The invention further comprises a preparing method of the collaurum immunochromatography test strip, comprising the steps of preparing of collaurum, preparing of a gold marker antibody, purifying and assembling of the test strip. The collaurum immunochromatography test strip has the characteristics of high specificity, high sensitivity, simple and convenient operation, fast detection, accuracy and suitability for field use.
Owner:姜竹泉 +1
Bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma hyopneumoniae and preparation method of bigeminy inactivated vaccine
ActiveCN103127497ALong-lasting immunityImprove efficiencyAntibacterial agentsViral antigen ingredientsMycoplasma hyopneumoniaeKilled Vaccine
The invention provides a bigeminy inactivated vaccine of porcine circovirus type 2 and swine mycoplasma pneumoniae and a preparation method of the bigeminy inactivated vaccine. The bigeminy inactivated vaccine comprises inactivated porcine circovirus type 2, inactivated swine mycoplasma pneumoniae, a vaccine adjuvant, and an excipient, wherein the content of the porcine circovirus type 2 is at least105.5 tissue culture inoculated dose (TCID) 50 / head, and the content of the swine mycoplasma pneumoniae is at least 2*109 MHDCE / head. The bigeminy inactivated vaccine has the advantages that the immune effect is equal to or better than the effect of sum of commodity single vaccines in the market, two antigens do not interfere each other, immune persistent period is long, potency is lasting, and due to the fact that one time immunization only needs, cost is lowered, and stress reaction of animals is also reduced. The bigeminy inactivated vaccine can be used for preventing porcine circovirus disease and at the same time preventing the swine mycoplasma pneumoniae.
Owner:PU LIKE BIO ENG +1
Use of Hydrolytic and Oxidative Enzymes to Dissolve Biofilm in Airway Passages
ActiveUS20160144004A1Prevent and inhibit inflammationAntibacterial agentsOrganic active ingredientsPeroxidaseProtein molecules
A composition for removal of biofilm in the airway passage is useful for the treatment of infections such as pneumonia cause by Mycoplasma pneumoniae. In general, the composition comprises: (1) a quantity of at least one enzyme that catalyzes the hydrolysis of a bond that connects two monosaccharides in a polysaccharide or that connects a monosaccharide with a protein molecule in a glycoprotein sufficient to break down biofilm in the airway; and (2) a pharmaceutically acceptable carrier suitable for administration into the airway. The composition can further include ingredients such as a steroid, lysozyme, lactoferrin, or a peroxidase; if a peroxidase is included, the composition can further include an oxidase to generate peroxide as well as a substrate for the oxidase. The composition can be used in methods for treatment of an infection based on the ability of the composition to dissolve biofilm in the airway.
Owner:LACLEDE
Primer probe combination and detection kit for detecting mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus
PendingCN112410472ASolve efficiency problemsSolve the characteristicsMicrobiological testing/measurementMicroorganism based processesMicrobiologyVirology
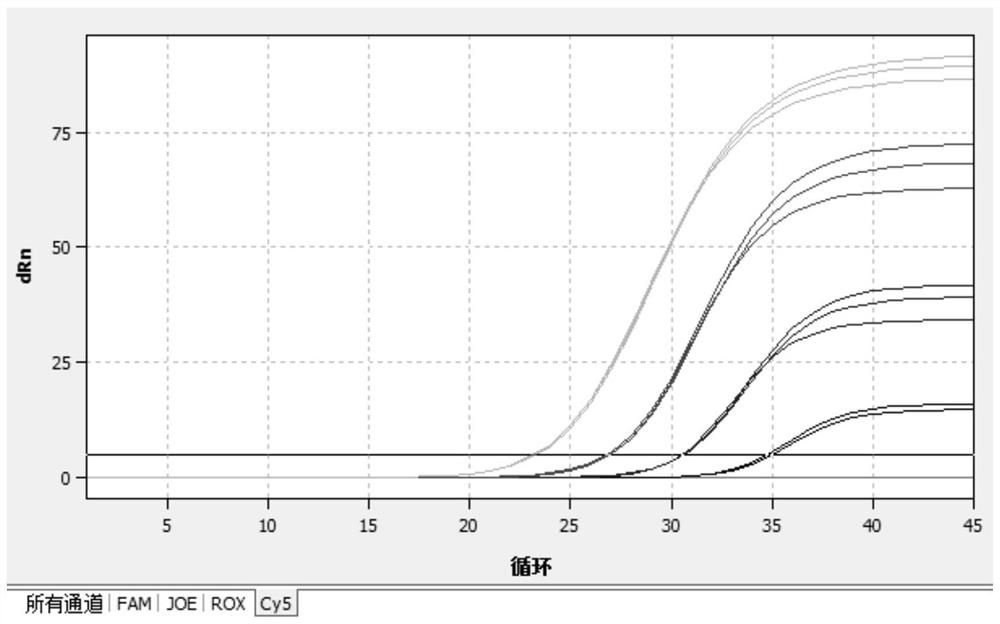
The invention relates to the technical field of biology, in particular to a primer probe combination and a detection kit for detecting mycoplasma pneumoniae, chlamydia pneumoniae and adenovirus. The primer probe combination comprises a specific primer pair of mycoplasma pneumoniae shown in SEQ ID NO: 1-2, a specific probe of mycoplasma pneumoniae shown in SEQ ID NO:3, a specific primer pair of chlamydia pneumoniae shown in SEQ ID NO: 4-5, a specific probe of chlamydia pneumoniae shown in SEQ ID NO:6, a specific primer pair of adenovirus shown in SEQ ID NO: 7-8 and a specific probe of adenovirus shown in SEQ ID NO: 9. The primers and the probes have good specificity, and rapid, accurate and sensitive identification of MP, CP and ADV can be achieved in combination with a real time PCR detection method.
Owner:AUTOBIO DIAGNOSTICS CO LTD
SERS-immunochromatography detection method for rapidly and highly sensitively detecting Mycoplasma pneumoniae infection
InactiveCN110133255AHigh detection sensitivityImprove the detection rateBiological testingSurface-enhanced Raman spectroscopyDTNB
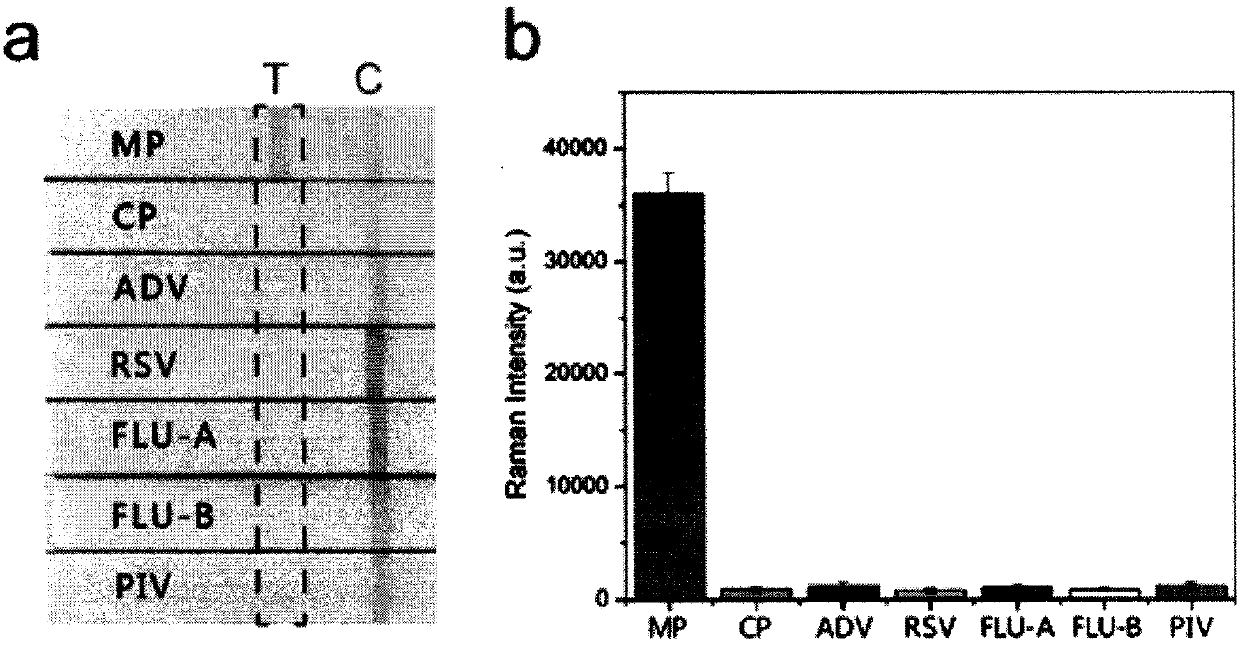
The invention relates to a SERS-immunochromatography detection method for rapidly and highly sensitively detecting Mycoplasma pneumoniae infection. The detection principle is that Nitrocellulose membranes (NC) are used as a carrier, a double-layer dye 5,5'-dithiobis (2-nitrobenzoic acid){5,5'-dithiobis- (2-nitrozoic acid), DTNB} labeled Au@Ag nano material coupling detection antibody is used as anSERS probe, a novel surface enhanced Raman spectroscopy (SERS-ICA)-based immunochromatography technology is established by combining the traditional Immunochromatography assay (ICA), and the technology is used for detecting human IgM and Mycoplasma pneumoniae (MP) specific IgM positive serum samples. The detection comprises the processes of sample dilution, SERS probe release, antigen-antibody reaction, result analysis and the like. The SERS-immunochromatography detection method for rapidly and highly sensitively detecting Mycoplasma pneumoniae infection can improve the detection sensitivityof human IgM and the detection rate of the lung branch positive serum specimen, and has important significance for clinical treatment.
Owner:ACADEMY OF MILITARY MEDICAL SCI
CRISPR detection primer group for mycoplasma pneumoniae and application of CRISPR detection primer group
ActiveCN110804669AGet rid of dependenceShorten detection timeMicrobiological testing/measurementMicroorganism based processesMycoplasmaSequenceome
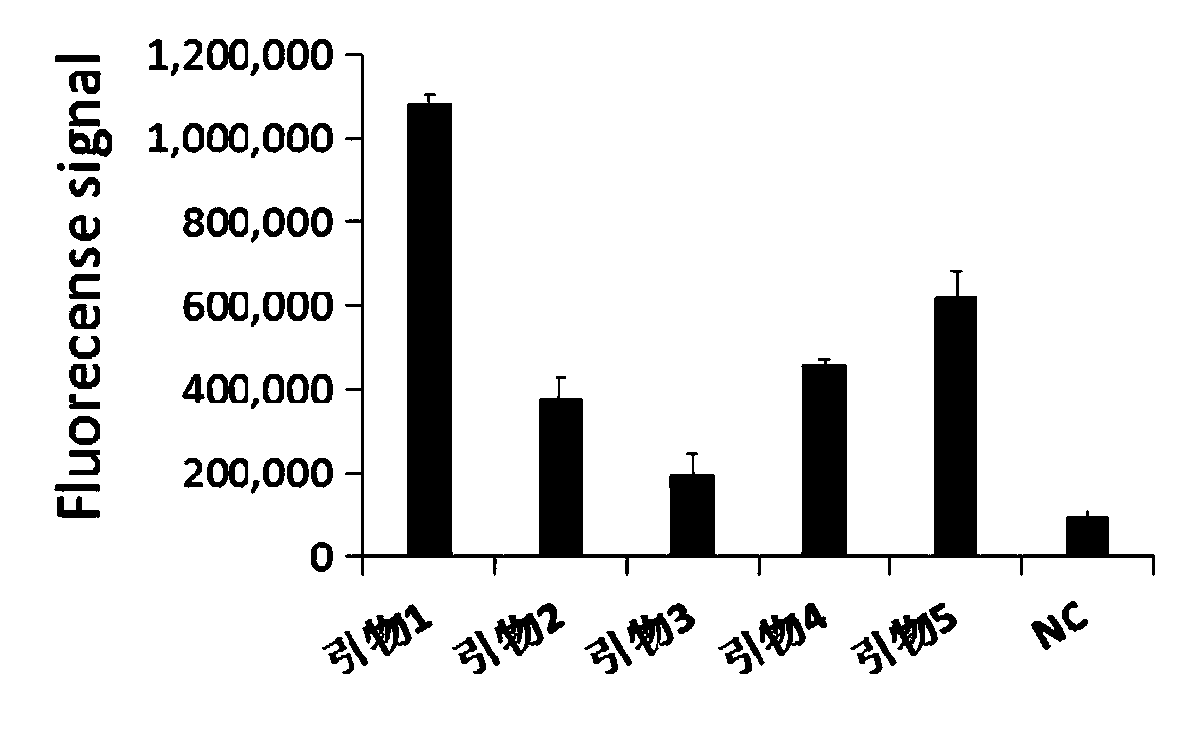
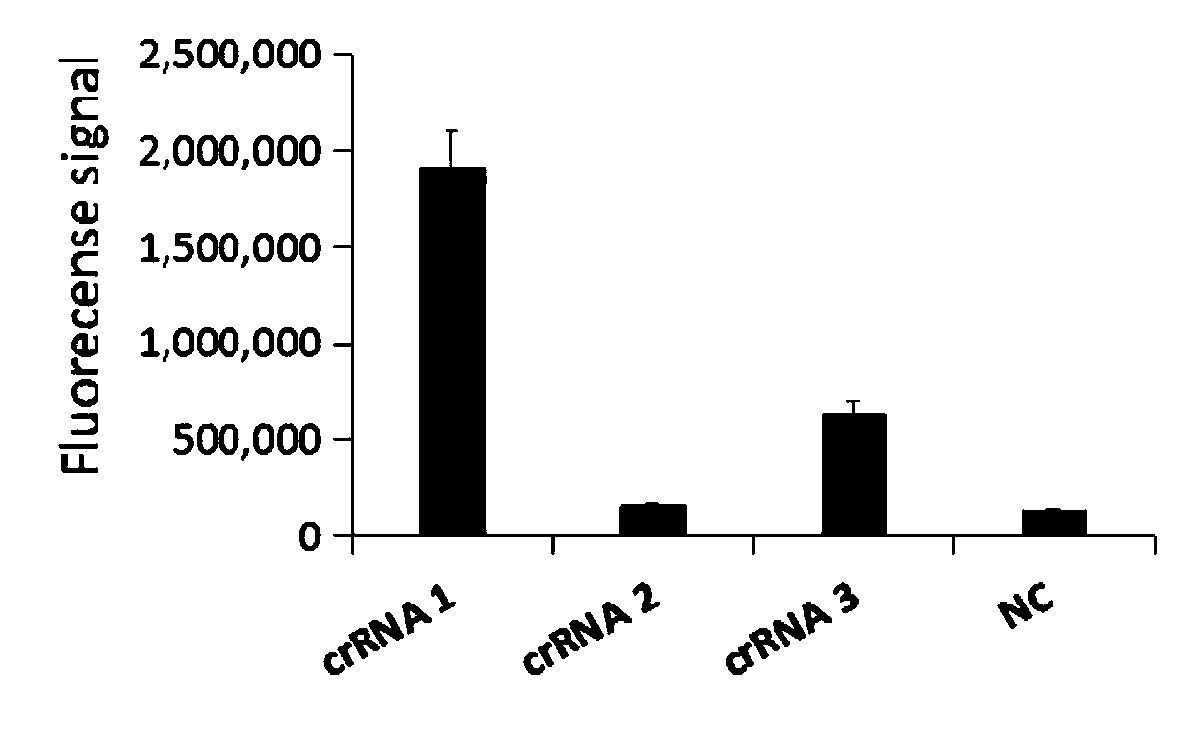
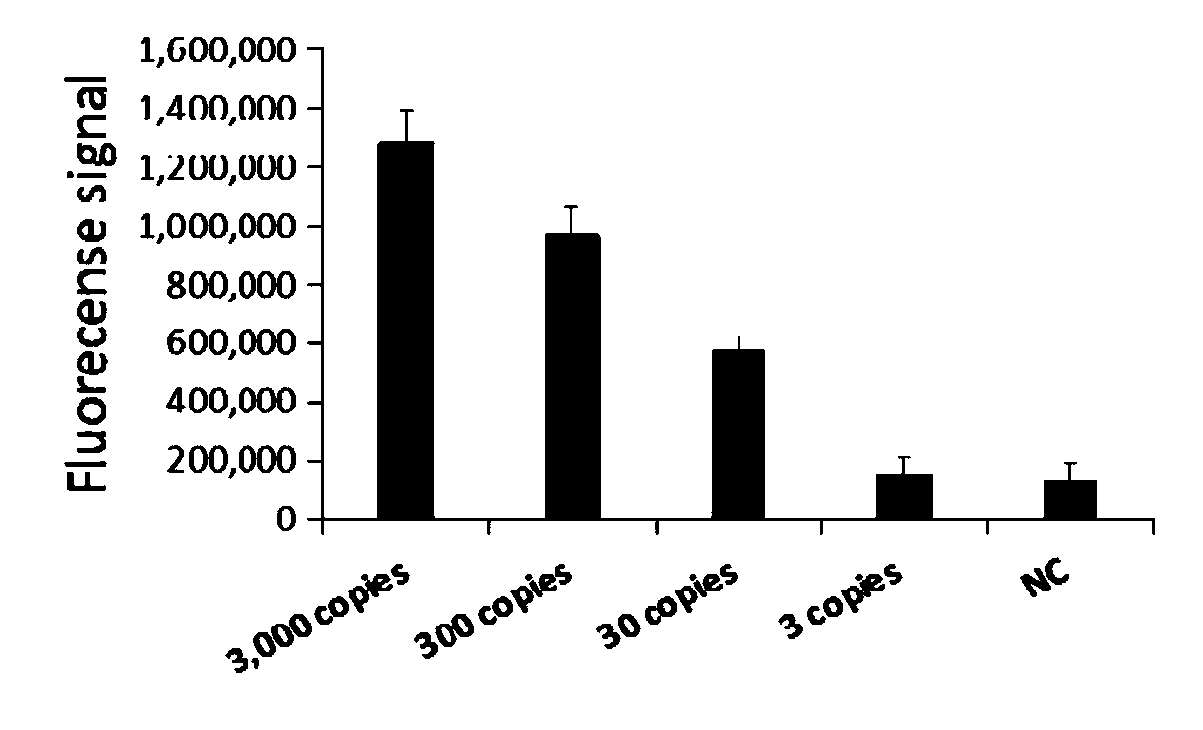
The invention relates to a CRISPR (clustered regularly interspaced short palindromic repeat) detection primer group for mycoplasma pneumoniae and an application of the CRISPR detection primer group, and belongs to the technical field of gene detection of a CRISPR technology. The primer group comprises an amplification primer pair and crRNA (CRISPR-derived ribonucleic acid), wherein the amplification primer pair is used for amplifying a sequence shown as SEQ ID NO. (sequence identifier number) 1 of the mycoplasma pneumoniae; crRNA comprises an anchor sequence and a guide sequence; the anchor sequence specifically identifies a Cas protein; and the guide sequence is matched with a target sequence segment in the sequence of SEQ ID NO. 1. The primer group detects the mycoplasma pneumoniae through the CRISPR technology; the detection time of the mycoplasma pneumoniae is shortened; and the detection can be accomplished within 60min. A specific sequence combination obtained by screening acts as the primer group for detection, the primer group has the advantages of high sensitivity and strong specificity, and a limit of detection of the primer group can reach 30 copies. The primer group isused for the CRISPR detection of the mycoplasma pneumoniae, is independent of a complicated variable temperature amplification instrument such as a qPCR (quantitative polymerase chain reaction) instrument, and a CRISPR-Cas technology has wide application prospects in instant diagnosis of the mycoplasma pneumoniae.
Owner:广州微远医疗器械有限公司 +3
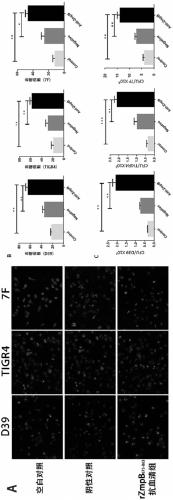
Application of streptococcus pneumoniae protein to resisting infection of S. pneumoniae
ActiveCN109456393AIncrease infectionReduced Colonization Protection ExperimentAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaStreptococcus mitis
The invention provides application of S. pneumoniae protein to resisting infection of S. pneumoniae. The endopeptidase O (PepO) of S. pneumoniae is a subcutaneous immunologic adjuvant, and the prepared S. pneumoniae protein vaccines have the good protection effects on resisting infection of S. pneumoniae through mixing and fusing expression of the subcutaneous immunologic adjuvant and 673rd to 863rd amino acid peptide fragment of zinc metal protease B (ZmpB).
Owner:CHONGQING MEDICAL UNIVERSITY
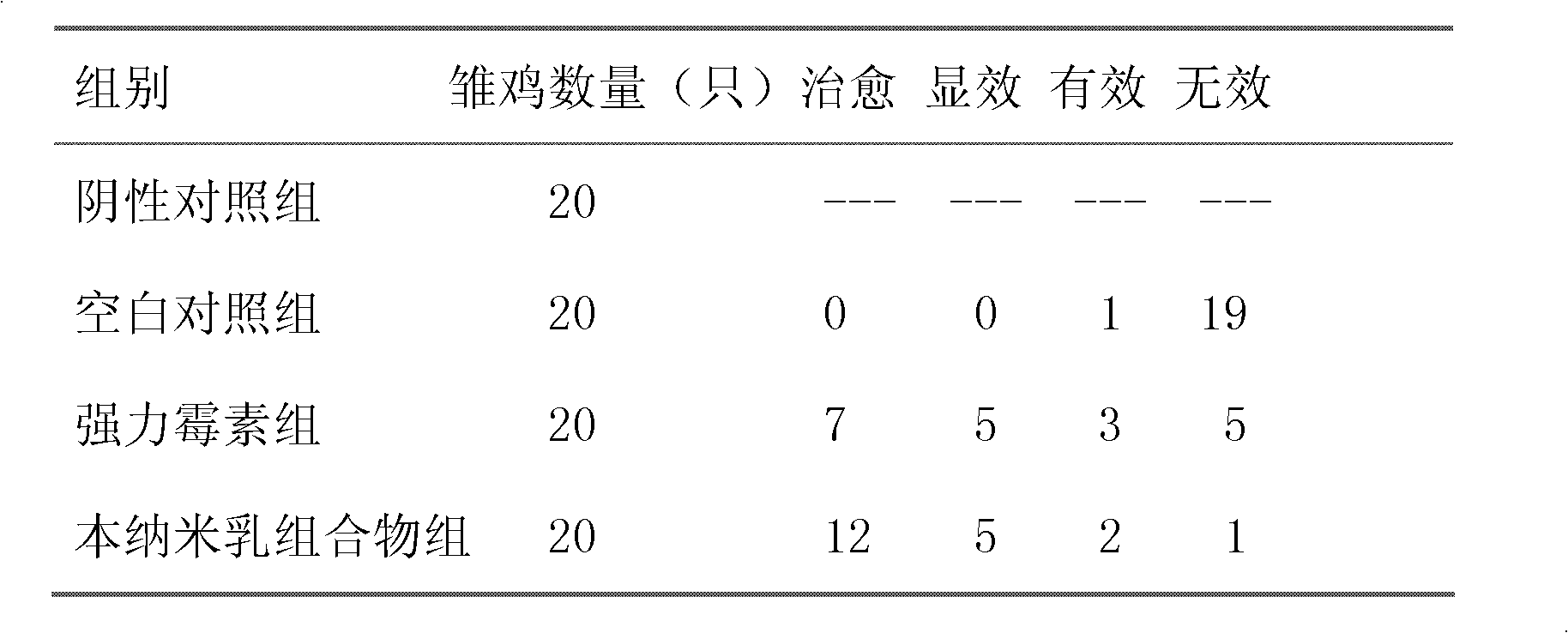
Nanoemulsion composition for treating mycoplasma pneumonia of livestock and preparation method of nanoemulsion composition
InactiveCN102600304AGood water solubilityWill not break milkAntibacterial agentsSulfur/selenium/tellurium active ingredientsBiotechnologyDisease
The invention discloses a nanoemulsion composition for treating mycoplasma pneumonia of livestock, which consists of raw materials including, by mass percent, from 0.1 to 8.0% of amyris oil, from 0.1 to 5.0% of vitex oil, from 0.1 to 3.0% of perilla oil, from 0.1 to 2.0% of bupleurum oil, from 0.01 to 0.1% of allicin, from 0.1 to 4.0% of tiamulin fumarate, from 19.0% to 31.0% of surfactant and the balance distilled water. The sum of the mass ratios of the ingredients is 100%. The nanoemulsion composition has effects of dilating the trachea of the livestock, suppressing cough reflex, increasing immunity of the organism, resisting inflammation and killing mycoplasma pneumoniae or other mixed infected causative agent, can be used for preventing and curing respiratory diseases including chronic respiratory diseases of the livestock, mycoplasma pneumoniae of swine, porcine contagious pleuropneumonia and the like, is fine in stability, is high in intestinal absorption utilization rate after being orally taken, can avoid liver first pass effect, and is remarkable in curative effect.
Owner:NORTHWEST A & F UNIV
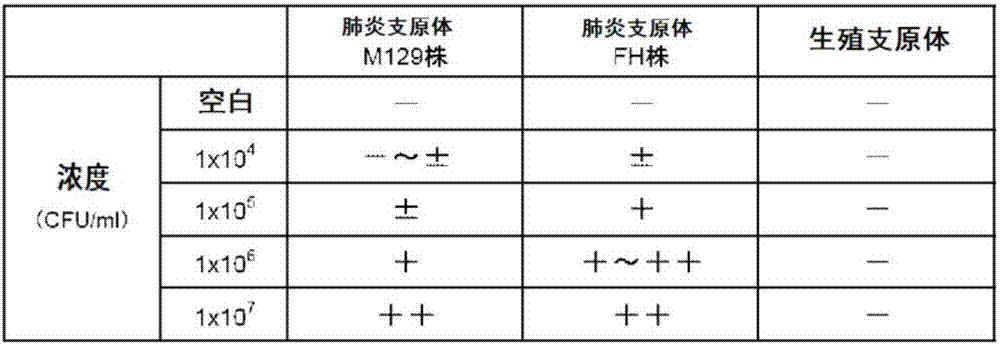
Mycoplasma pneumoniae recombinant antigen and application thereof
ActiveCN108314710AReduced pair screening effortImprove the detection rateBacteriaDepsipeptidesEpitopeBinding peptide
The invention relates to a mycoplasma pneumoniae recombinant antigen and application thereof. The mycoplasma pneumoniae recombinant antigen comprises a PIA fragment, a first binding peptide, a PIB fragment, a second binding peptide and a P30 fragment. Experiment results show that the recombinant antigen can obtain different types of antigen dominant epitopes after being purified in different ways,and the various types of antigen dominant epitopes are stable in expression, namely the different types of antigen dominant epitopes can be stably expressed as required. The matching and screening works of the antigen can be greatly reduced during detection; the detection rate is high, and the specificity is high during the detection of mycoplasma pneumoniae.
Owner:GUANGDONG WESAIL BIOTECH CO LTD
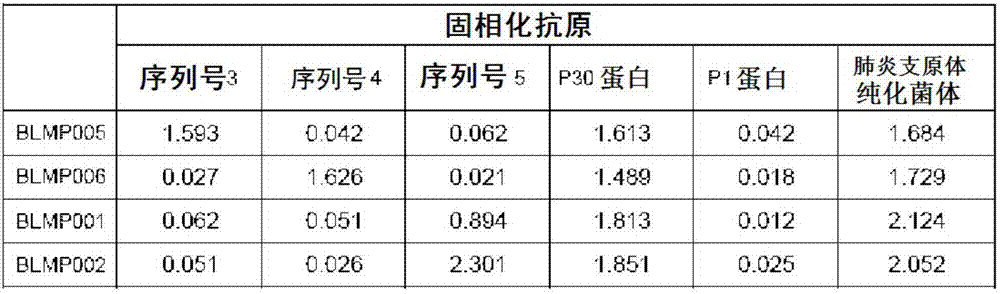
Mycoplasma pneumoniae antigen as well as preparation method and application thereof
ActiveCN111253478AStrong specificityHigh sensitivityNucleic acid vectorDepsipeptidesMycoplasma antigenImmuno detection
The invention relates to the field of in-vitro diagnostic immunoassay, and in particular provides a mycoplasma pneumoniae antigen as well as a preparation method and application thereof. The mycoplasma pneumoniae antigen provided by the invention is protein composed of an antigenic protein sequence P1M:residues 1340-1518, specifically an amino acid sequence shown in SEQ ID NO.1, or protein which is obtained by performing substitution and / or deletion and / or addition on the amino acid sequence shown in the SEQ ID NO.1 by one or more amino acid residues and has same function. The antigen is verified by an immunoserological detection technology to have strong specificity and high sensitivity, and the antigen is easy to culture and purify, is more conducive to industrial production and saves costs; and in addition, the antigen is suitable for preparation of MP antibody detection products, can be used for any forms of products in the field of in-vitro immunodiagnosis, and has broad market prospects.
Owner:ZHUHAI LIVZON DIAGNOSTICS
Novel application of minor radix buplenri granules combined chloroquine phosphate tablets
ActiveCN111329982AImprove the level ofImprove the ability to scavenge free radicalsAntibacterial agentsOrganic active ingredientsAzithromycinSuperoxide dismutases
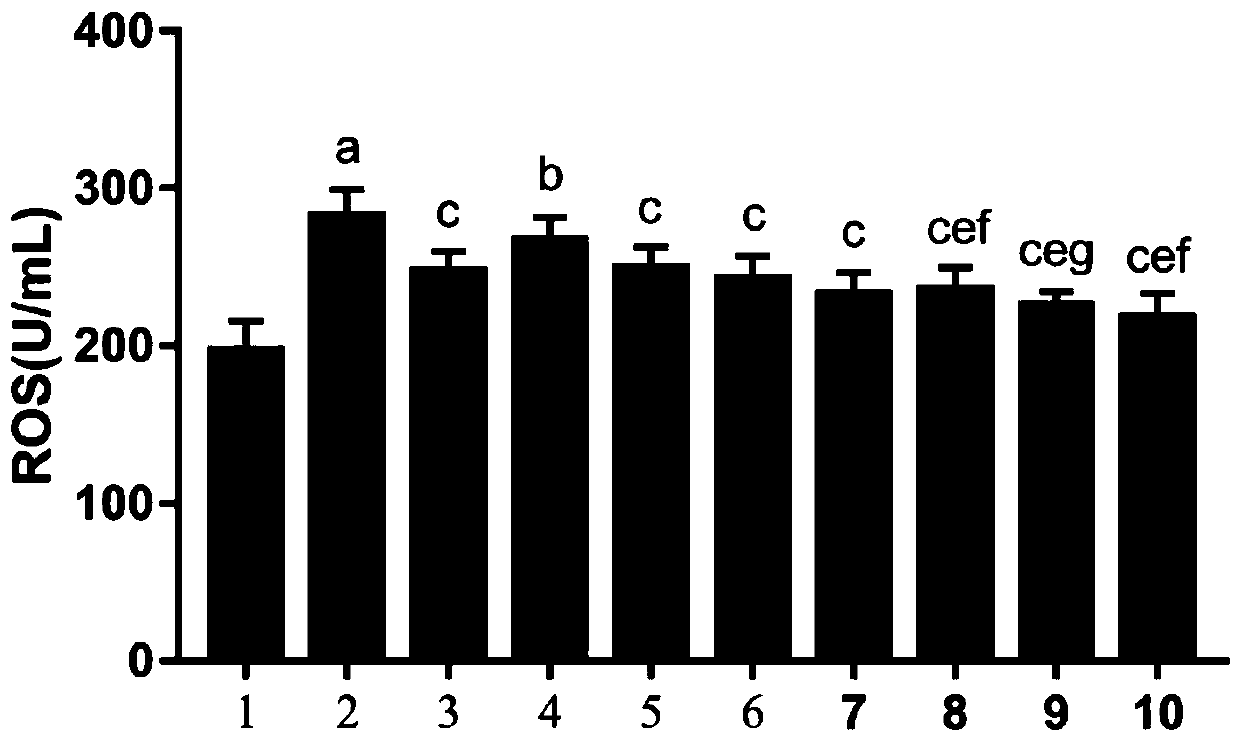
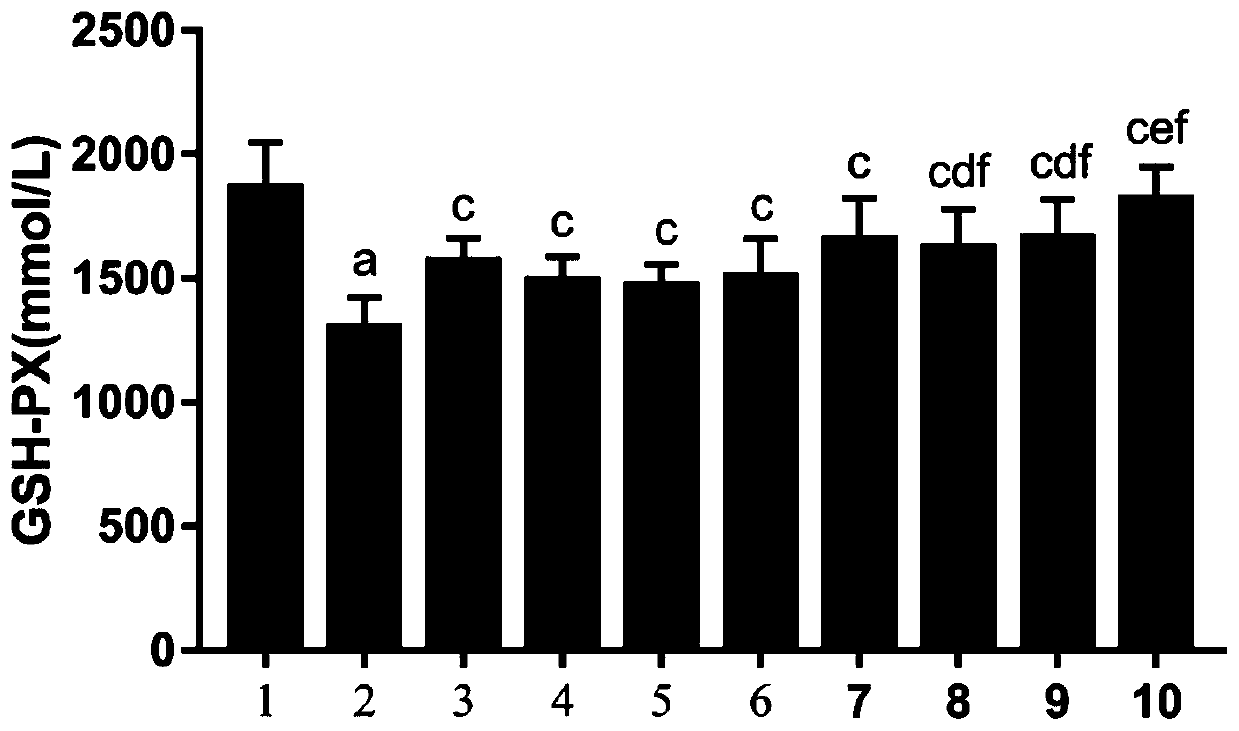
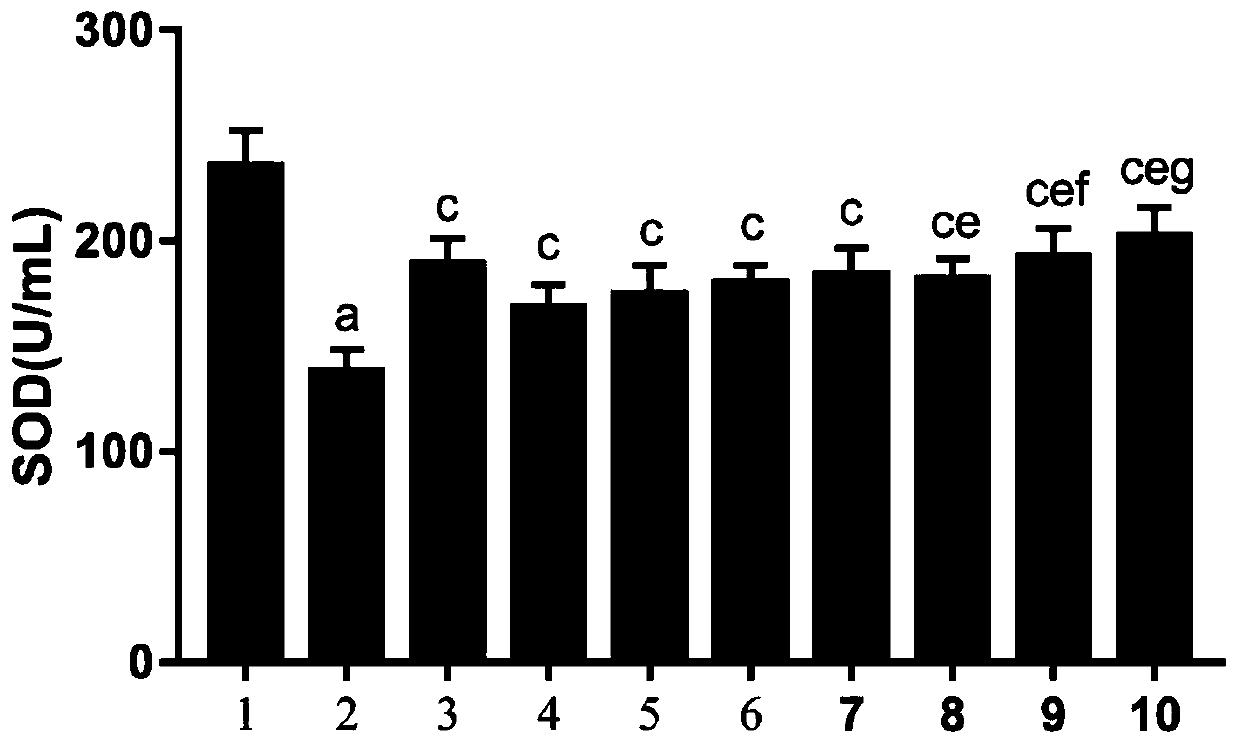
According to the invention, an SD rat model infected by mycoplasma pneumoniae is adopted for experiments, and it is found that minor radix buplenri granules can remove excessive free radicals caused by mycoplasma pneumoniae infection. In particular, through combination of the minor radix buplenri granules and chloroquine phosphate, the levels of glutathione peroxidase, superoxide dismutase and catalase in serum can be obviously increased, and the content of malondialdehyde can be reduced, so that combined administration can improve the free radical removing capability of an organism and reducecytotoxicity generated by free radical accumulation. The minor radix buplenri granules combined chloroquine phosphate tablets have a purpose of removing excessive free radicals caused by mycoplasma pneumoniae infection, solve the problem of excessive free radicals in mycoplasma pneumoniae infection, have a better sensitization effect on azithromycin, and thus can be used for preventing and treating mycoplasma pneumoniae.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Mycoplasma pneumoniae fusion antigen as well as preparation method and application thereof
ActiveCN111548423AStrong specificityHigh sensitivityBacteriaAntibody mimetics/scaffoldsSerologyBiomedical engineering
The invention provides a mycoplasma pneumoniae fusion antigen as well as a preparation method and application thereof, and relates to the technical field of biology. The mycoplasma pneumoniae fusion antigen is a fusion protein containing a P1M antigen fragment and a P30A antigen fragment. The mycoplasma pneumoniae fusion antigen provided by the invention is verified by an immunoserological detection technology, and compared with an existing mycoplasma pneumoniae antigen, the mycoplasma pneumoniae fusion antigen is stronger in specificity, higher in sensitivity, easy to culture and purify, morebeneficial to industrial production and lower in cost. The mycoplasma pneumoniae fusion antigen is suitable for preparation of MP antibody detection products, can be processed into products in any form in the field of in-vitro immunodiagnosis, and has a wide market prospect.
Owner:ZHUHAI LIVZON DIAGNOSTICS
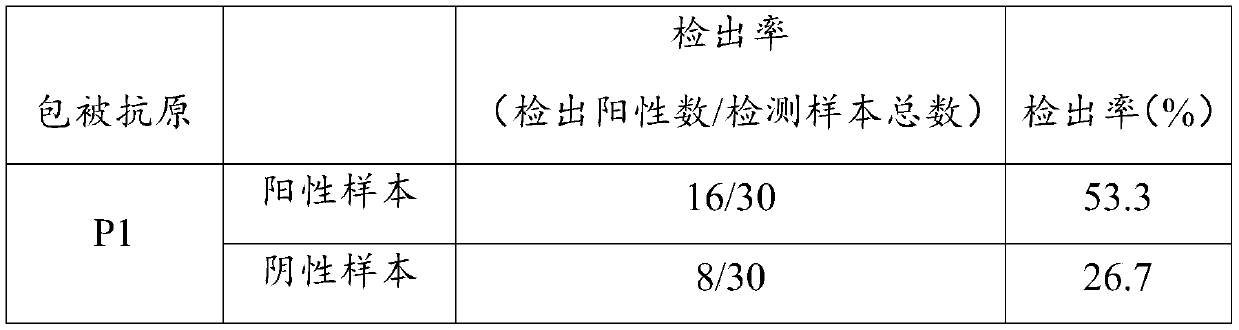
Mycoplasma pneumoniae antigen and application thereof in simultaneous quantitative detection of Mycoplasma pneumoniae IgG and IgM content in peripheral blood
ActiveCN110964089AImprove filtering effectEnhance immune responseDepsipeptidesGenetic engineeringImmune profilingAssay
The invention discloses a Mycoplasma pneumoniae antigen and its application in simultaneous quantitative detection of Mycoplasma pneumoniae IgG and IgM content in peripheral blood. The amino acid sequence of the mutant Mycoplasma pneumoniae antigen P80 of the invention is as shown in SEQ ID NO.1. The immunoreactivity of the Mycoplasma pneumoniae antigen p80 and the antibody is about 34.6% higher than that of the natural antigen. By combining the Mycoplasma pneumoniae antigen P80 with the quantum dot immunochromatography technology, the shortcoming that existing detection methods of Mycoplasmapneumoniae antibody, such as immunochromatography assay (ICA) and enzyme-linked immunosorbent assay (ELISA), can only detect qualitatively or semi-quantitatively, and the MP-IgM and MP-IgG detection must be carried out twice is solved; and the detection of MP-IgM and MP-IgG can be realized at the same time, so as to distinguish the patients with recent infection or previous infection and reduce the misdiagnosis rate.
Owner:NANJING VAZYME MEDICAL TECH CO LTD
Composition, kit and method for detecting pathogens causing respiratory tract infection and identifying pathogen species and application
ActiveCN113943836AReduce wasteReduce psychological burdenMicrobiological testing/measurementMicroorganism based processesRespiratory syncytial virus (RSV)Influenza Viruses Type A
The invention belongs to the field of molecular biological detection. Specifically, the invention belongs to the field of detection of pathogens causing respiratory tract infection; and more specifically, the present invention can simultaneously detect influenza A virus, influenza B virus, rhinovirus, respiratory adenovirus, respiratory syncytial virus, and mycoplasma pneumoniae. By using a composition provided by the invention, the six pathogens causing respiratory tract infection can be detected at the same time, and specific infection of one or more pathogens can be identified. Meanwhile, the detection rate is higher than that of the existing composition, and the detection is more accurate.
Owner:SANSURE BIOTECH INC
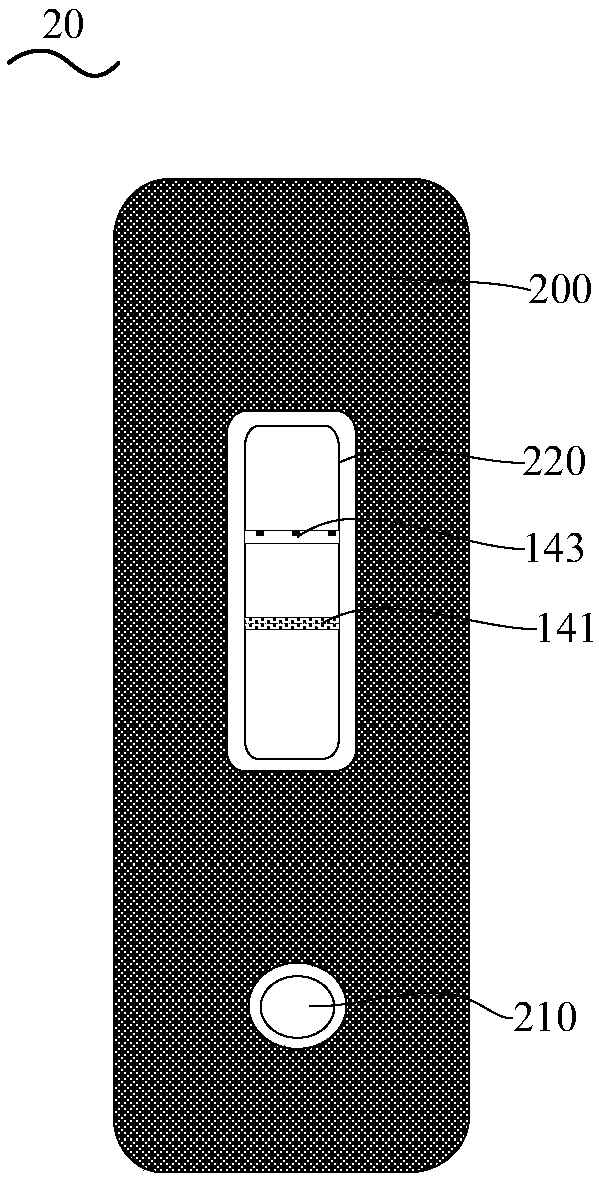
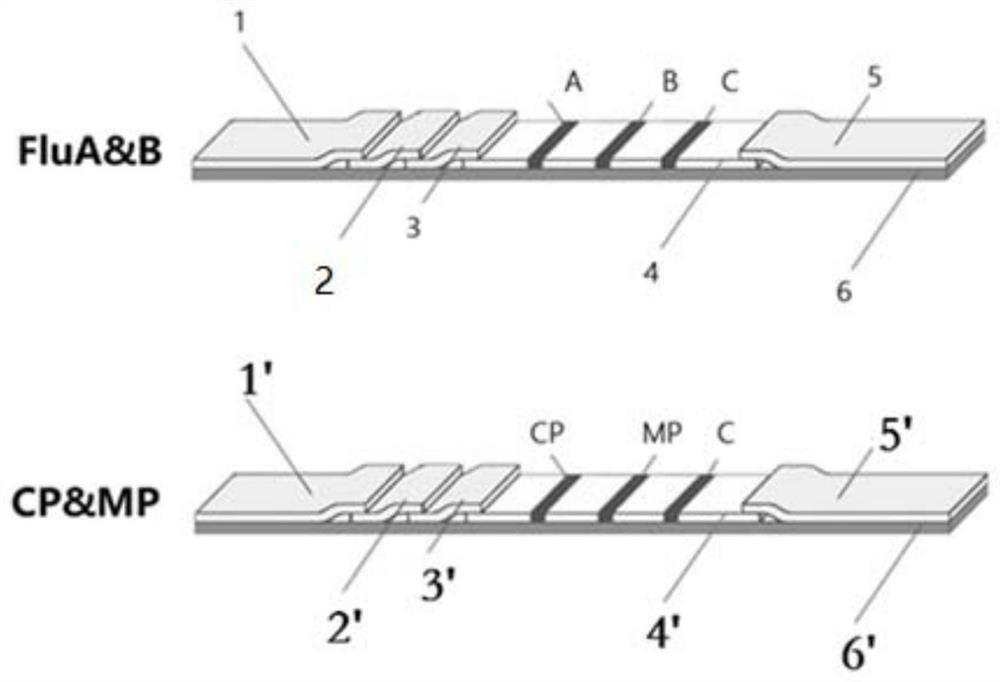
Combined device and detection method for synchronously detecting influenza A virus, influenza B virus, chlamydia pneumoniae IgM antibody and mycoplasma IgM antibody
PendingCN111610329ASynchronize independent test resultsIndependent Testing ProcessMaterial analysisReceptorIgm antibody
The invention belongs to the field of medical detection equipment, and provides a combined device for synchronously detecting IgM antibodies of influenza A and B viruses and chlamydia pneumoniae and mycoplasma pneumoniae. The device comprises a double-channel clamping shell, a test strip FluA & B and a test strip CP & MP which are arranged in the double-channel clamping shell in parallel; influenza virus A detection lines arranged on a nitrocellulose membrane of the test strip FluA & B at intervals are coated with high-specificity influenza virus A antigens, influenza virus B detection linesare coated with high-specificity influenza virus B antigens, and first quality control lines are coated with quality control line coating receptors; chlamydia pneumoniae detection lines (CP) arranged on a nitrocellulose membrane of the test strip CP & MP at intervals are coated with high-specificity chlamydia pneumoniae antigens, mycoplasma pneumoniae detection lines (MP) are coated with high-specificity mycoplasma pneumoniae antigens, and second quality control lines are coated with quality control line coating receptors.
Owner:北京柏兆嘉业科技有限公司
Quantum dot nucleic acid detection kit and method for simultaneously detecting 24 respiratory tract pathogens
ActiveCN111394514AReduce detection stepsShorten detection timeBacteriaMicrobiological testing/measurementReverse transcriptaseGenomic DNA
The invention discloses a quantum dot nucleic acid detection kit and method for simultaneously detecting 24 respiratory tract pathogens. The kit comprises reaction solutions I-V, a positive control, an internal label and a negative control. The reaction solutions I-III comprise a respiratory tract pathogen detection primer, dN(U)TP, UDG, a PCR buffer system and an RNA enzyme inhibitor; the reaction solution IV comprises DNA polymerase; the reaction solution V comprises reverse transcriptase and DNA polymerase; the positive control comprises influenza A viruses, coronavirus OC43 pseudoviruses and mycoplasma pneumoniae recombinant plasmids; the internal label comprises exogenous fragment pseudoviruses free of target genes; the negative control comprises human genomic DNA; a fluorescence detection solution comprises a streptavidin-coupled quantum dot; a denaturant comprises an alkali solution; a neutral agent comprises an acid solution; and a membrane strip comprises a nylon membrane comprising a coupling-specific detection probe. The 24 common respiratory tract infection pathogens can be quickly, sensitively and specifically detected, and the occurrence of false negatives can be effectively monitored.
Owner:杭州千基生物科技有限公司 +1
Diagnostic kit for combined diagnosis of mycoplasma pneumonia and application of diagnostic kit
ActiveCN111505313AHigh sensitivityStrong specificityBiological testingImmunoassaysMurine antibodyMycoplasma antigen
The invention relates to a diagnostic kit, in particular to the diagnostic kit for the combined diagnosis of mycoplasma pneumonia. The kit comprises a single-side detection test strip and a double-side detection test strip, a mycoplasma pneumoniae antibody marked by time-resolved fluorescent microspheres is fixed on a combination pad of the single-side detection test strip, a mycoplasma pneumoniaeantibody is coated on a first detection line, and a goat-anti-mouse IgG antibody is coated on a first quality control line. A time-resolved fluorescent microsphere labeled mouse anti-human IgM monoclonal antibody and a mouse anti-human IgG antibody are fixed on a combination pad of the double-detection test strip, a second detection line and a third detection line are both coated with natural MP-Ag, and the second quality control line is coated with a goat anti-mouse IgG antibody. According to the invention, the time-resolved fluorescent microsphere is used as a chromogenic marker, the detection test strip for detecting the mycoplasma pneumoniae IgM and IgG three times is combined into a whole, and the human mycoplasma pneumoniae antigen and the mycoplasma pneumoniae IgM and IgG are simultaneously detected, so that the kit has higher sensitivity and stronger specificity compared with a colloidal gold method.
Owner:厦门万渤生物技术有限公司
Swine mycoplasma pneumonia inactivated vaccine and preparation method thereof
ActiveCN104399070AGood spiritsNormal feedingAntibacterial agentsBacterial antigen ingredientsAdjuvantAntibody level
The invention discloses a swine mycoplasma pneumonia inactivated vaccine and a preparation method thereof and belongs to the field of swine mycoplasma pneumonia inactivated vaccines. The swine mycoplasma pneumonia inactivated vaccine comprises a water phase and an oil phase according to a volume ratio of 1: 1-3. The water phase comprises Tween-80 and swine pneumoniae mycoplasma liquid according to a ratio of 2-5: 95-98. The oil phase comprises polyoxyl-40-hydrogenated castor oil, Span-80 and white oil according to 1-2: 5-8: 90-94. The invention further discloses a method for preparing the swine mycoplasma pneumonia inactivated vaccine. An emulsification adjuvant of the swine mycoplasma pneumonia inactivated vaccine is added with polyethyleneglycol-40-hydrogenated castor oil with hydrophilic and hydrophobic groups, substantially improves vaccine emulsification effects, substantially reduces a use amount of white oil and vaccine consistency, reduces injection difficulty and animal stress response, realizes a high antibody level in a long period and has substantial immunization effects on piglets.
Owner:哈药集团生物疫苗有限公司
Luchuan pig mycoplasma pneumoniae P36 gene in-situ hybridization detection method, and probes and kit thereof
InactiveCN104894116AFast and accurate identificationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationMycoplasma pneumoniae DNALung tissue
The invention discloses a Luchuan pig mycoplasma pneumoniae P36 gene in-situ hybridization detection method, and probes and a kit thereof. Three oligonucleotide probes are designed and synthesized according to three specific DNA (deoxyribonucleic acid) sequences in the Luchuan pig mycoplasma pneumoniae P36 whole-genome segment. The invention also discloses a related detection method and kit. The specific probes have high specificity and high sensitivity. The detection method and kit established on such basis can be conveniently and quickly used for digoxin labeled in-situ hybridization detection of Luchuan pig mycoplasma pneumoniae P36 gene. The method can quickly and accurately identify Luchuan pig mycoplasma pneumoniae when being used for detection and monitoring research of Gangxi Luchuan pig mycoplasma pneumoniae, thereby determining the distribution conditions of the mycoplasma pneumoniae DNA in the Luchuan pig lung tissues. The Luchuan pig mycoplasma pneumoniae pathogen nucleic acid distribution conditions are combined with the related pathology tissue changes to achieve the goal of accurate detection and prediction.
Owner:GUANGXI VETERINARY RES INST +1
Primer probe group and kit for combined detection of mycoplasma pneumoniae and chlamydia pneumoniae based on fluorescence RMA method
PendingCN112592992AChange defectsIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesPolyethylene oxideReverse transcriptase
The invention belongs to the technical field of detection of mycoplasma pneumoniae and chlamydia pneumoniae, and particularly relates to a primer probe group, a kit and a detection method for combineddetection of mycoplasma pneumoniae and chlamydia pneumoniae based on a fluorescence RMA method. The kit comprises a detection tube containing an amplification reaction reagent, a buffer solution, magnesium acetate, standard positive plasmids and sterile double distilled water. The amplification reaction reagent is prepared from primers and probes of MP and Cpn, M-MLV reverse transcriptase, escherichia coli RecA protein, UvsY protein, single-stranded binding protein GP32, Bst polymerase, exonuclease III, polyethylene oxide, trehalose, mannitol, ATP, dNTPs, creatine kinase and creatine phosphate. The standard positive plasmids are recombinant plasmids containing MP and Cpn amplified gene sequences, and are used for positive control of MP and Cpn nucleic acid detection.
Owner:济南国益生物科技有限公司
Primer group for detecting mycoplasma pneumoniae, kit and method
ActiveCN112831578ASimple extraction methodAvoid long time-consuming disadvantagesMicrobiological testing/measurementMicroorganism based processesVirologyBioinformatics
The invention discloses a primer group for detecting mycoplasma pneumoniae (MP), a kit and a method, the primer group comprises an MP outer primer pair, an MP inner primer pair and an MP loop primer pair, the sequence of the MP outer primer pair is shown as SEQ ID NO: 1 and SEQ ID NO: 2, the sequence of the MP inner primer pair is shown as SEQ ID NO: 3 and SEQ ID NO: 4, and the sequence of the MP loop primer pair is shown as SEQ ID NO: 5 and SEQ ID NO: 6. The primer group, the kit and the method provided by the invention have the advantages of high sensitivity (capable of reaching 100 copies / reaction), accurate detection, high detection speed and simple detection process.
Owner:SHANGHAI UPPER BIO TECH PHARMA
PCR (Polymerase Chain Reaction) primers for detecting mycoplasma pneumoniae (MP) and application thereof
ActiveCN103740833BStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMycosisMycobates parmeliae
The invention discloses fluorescent quantitative PCR (Polymerase Chain Reaction) primers for detecting mycoplasma pneumoniae (MP) and application thereof. A primer pair provided by the invention can be a primer pair 1 (shown by a sequence 1 and a sequence 2) or a primer pair 2 (shown by a sequence 3 and a sequence 4) or a primer pair 3 (shown by a sequence 5 and a sequence 6). Shown by experiments, compared with other commercialized detection primers, IgM (Immunoglobulin M) antibody detection methods and culture methods, a method in which the primer pair provided by the invention is used for carrying out fluorescent quantitative PCR detection on test samples has the advantage that the MP can be specifically detected without being interfered by four kinds of common pathogenic mycoplasmas which have a relatively close genetic relationship with the MP, as well as common respiratory tract bacteria and fungal pathogenic bacteria. The method can be used for qualitatively detecting the MP and detecting the MP quantitatively very well, is a quantitative PCR detection technology which is quick, is strong in specificity and high in sensitivity and is suitable for clinically detecting the MP of all strains, and has good detection effect and application value in clinical detection.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV
Peptides and uses thereof
ActiveUS10745450B2Easy and more compatibleEfficient secretionPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleic acid sequencingCell biology
Owner:FUNDACIO CENT DE REGULACIO GENOMICA +1
Specific primer set and detection kit for detecting drug resistance mutation gene of Mycoplasma pneumoniae
ActiveCN106566874BStrong specificityGood repeatabilityMicrobiological testing/measurementMicroorganism based processesWild typeGenotype
The invention discloses a specific primer pair for detecting drug resistance mutation gene of mycoplasma pneumonia. The primer pair is designed by using mycoplasma pneumonia 23S rRNA V-zone wild-type sequence and A2063G and A2064G mutant-type sequence as template. The primer pair is composed of wild-type upstream primer sequence as shown in SEQ ID No. 1, A2063G mutant-type upstream primer sequence as shown in SEQ ID No.2, A2064G mutant-type upstream primer sequence as shown in SEQ ID No.3, and universal downstream primer sequence as shown in SEQ ID No. 4. The detection kit has very high sensitivity and specificity, and can quantify mutant DNA and wild template DNA so as to effectively distinguish various genotypes and calculate corresponding ratio of various genotypes. Dynamic monitoring of genotypes and content changes of microflora in the body of a patient with clinical mycoplasma pneumoniae infection can be realized. The detection kit is beneficial to clinical researches on drug resistance mechanism and progress of mycoplasma pneumonia and is convenient for observation of clinic therapy effect.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Kit and detection method for detecting mycoplasma pneumoniae nucleic acid and drug resistance gene variation of mycoplasma pneumoniae nucleic acid
PendingCN113462794AShorter amplification reaction timesAvoid False Positive ResultsMicrobiological testing/measurementMicroorganism based processesResistant genesDrug resistance
The invention provides a kit and a detection method for detecting mycoplasma pneumoniae nucleic acid and drug-resistant gene variation, which are beneficial for detecting the mycoplasma pneumoniae nucleic acid and drug-resistant gene variation of the mycoplasma pneumoniae nucleic acid and the drug-resistant gene variation of the mycoplasma pneumoniae nucleic acid. The kit and the detection method for detecting mycoplasma pneumoniae nucleic acid and drug-resistant gene variation of the mycoplasma pneumoniae nucleic acid are characterized in that the kit comprises KOD DNA polymerase, a primer MPN-F, a primer MPN-R and a specific targeting fluorescent probe. According to the kit and the detection method disclosed by the invention, the aim of rapidly, simply, conveniently and accurately detecting the mutations of the mycoplasma pneumoniae nucleic acid, the 23S rRNA gene of the mycoplasma pneumoniae nucleic acid and the 2063th or 2064th gene locus of the 23SrRNA is fulfilled by designing the primers and the probes with specific sequences.
Owner:TOYOBO CO LTD
Immunological detection method and kit for mycoplasma pneumoniae
ActiveCN107407679ARapid and specific diagnosisEasy and quick diagnosisMicrobiological testing/measurementDepsipeptidesEpitopeMycoplasma pneumonia
The present invention aims at providing a specific antibody that can simply and rapidly detect Mycoplasma pneumoniae which is a causative bacterium of mycoplasma pneumonia, with high sensitivity, and also an immunological detection method and a kit containing the same antibody. The present invention makes it possible to diagnose infection with Mycoplasma pneumoniae more rapidly and specifically than the conventional method, by producing an antibody recognizing a specific epitope of P30 protein of Mycoplasma pneumoniae and performing an immunological detection using the antibody. Also, the present invention enables easy and rapid detection of Mycoplasma pneumoniae and diagnosis of infection with the same at a hospital or the like without need of specialized instruments or skilled techniques.
Owner:TAUNS CO LTD
Primers and kit for detecting mycoplasma pneumoniae
InactiveCN112662793AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationNucleotideMycoplasma pneumoniae Infections
The invention discloses a loop-mediated isothermal amplification primer group for detecting mycoplasma pneumoniae. The loop-mediated isothermal amplification primer group is composed of the following three pairs of primers: MP2-F3 and MP2-B3, MP2-FIP and MP2-BIP, and MP2-LF and MP2-LP, wherein the nucleotide sequence of the MP2-F3 is shown as SEQ ID No. 1, the nucleotide sequence of the MP2-B3 is shown as SEQ ID No. 2, the nucleotide sequence of the MP2-FIP is shown as SEQ ID No. 3, the nucleotide sequence of the MP2-BIP is shown as SEQ ID No. 4, the nucleotide sequence of the MP2-LF is shown as SEQ ID No. 5, and the nucleotide sequence of the MP2-LP is shown as SEQ ID No. 6. The primer group provided by the invention can significantly improve detection efficiency, shortens detection time to be within 30 minutes, a decrease by a half compared with 60 minutes or more needed in existing loop-mediated isothermal amplification methods, can be used for rapid detection of mycoplasma pneumoniae infection, provides powerful help for clinical early diagnosis and treatment, and buys time for timely isolation of infectors and control of infection diffusion.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
A kind of pcr primer for detecting mycoplasma pneumoniae and its application
InactiveCN103740837BStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationMycosisMycobates parmeliae
The invention discloses fluorescent quantitative PCR (Polymerase Chain Reaction) primers for detecting mycoplasma pneumoniae (MP) and application thereof. A primer pair provided by the invention can be a primer pair 1 (shown in a sequence 1 and a sequence 2) or a primer pair 2 (shown in a sequence 3 and a sequence 4) or a primer pair 3 (shown in a sequence 5 and a sequence 6). Shown by experiments, compared with other commercialized detection primers, IgM (Immunoglobulin M) antibody detection methods and culture methods, a method in which the primer pair disclosed by the invention is used for carrying out fluorescent quantitative PCR detection on test samples has the advantage that the MP can be specifically detected without being interfered by four kinds of common pathogenic mycoplasmas which have a relatively close genetic relationship with the MP, as well as common respiratory tract bacteria, and fungal pathogenic bacteria. The method can be used for qualitatively detecting the MP and detecting the MP quantitatively very well, is a quantitative PCR detection technology which is quick, is strong in specificity and high in sensitivity and is suitable for clinically detecting the MP of all strains, and has good detection effect and application value in clinical detection.
Owner:高宇辉
Mycoplasma pneumoniae drug sensitivity rapid detection kit and preparation method thereof
PendingCN113151395APromote growthShort color development timeMicrobiological testing/measurementPenicillinAntibiotic drug
The invention discloses a mycoplasma pneumoniae drug sensitivity rapid detection kit and a preparation method thereof. The kit comprises mycoplasma pneumoniae culture solution, a drug sensitive plate and a drug for treating mycoplasma pneumoniae; the mycoplasma pneumoniae culture solution comprises the following components: 7 to 10 g of agar powder, 5 to 8 g of peptone, 2 to 3 g of short-chain peptide, 700 to 800 mg of morroniside, 3 to 5 g of yeast powder, 2 to 4 g of glucose, 2.5 to 3.5 g of sodium pyruvate, 90 to 120 mL of MEM culture medium, 2 mL of 1% (w / v) phenol red solution, 140 to 180 mL of horse serum, 300 thousand units of penicillin and 750 to 850 mL of distilled water; and the short-chain peptide is composed of 3 to 5 amino acid residues. The kit is stable in performance and high in specificity, and can accurately detect which antibiotic in various clinically common antibiotics is sensitive to a patient when the patient is infected with mycoplasma pneumoniae, so that medication is accurately guided, blind medication and excessive medication are avoided, and the recovery rate is increased.
Owner:河南省医疗器械检验所
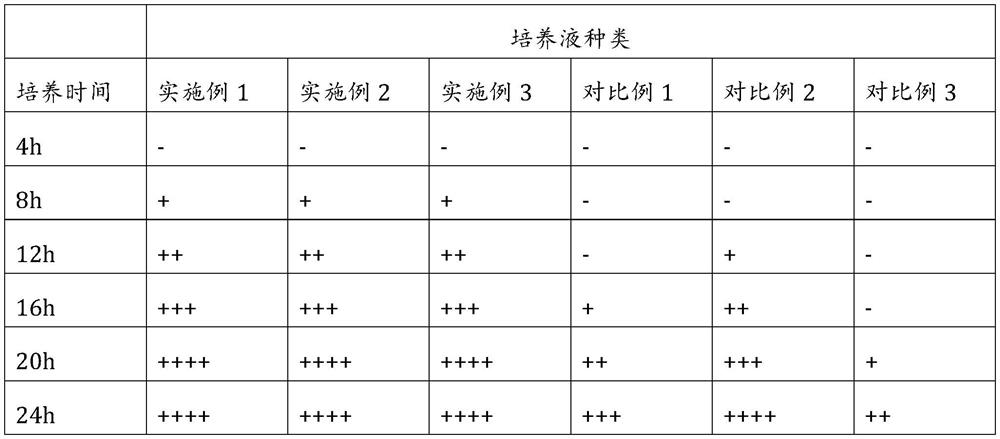
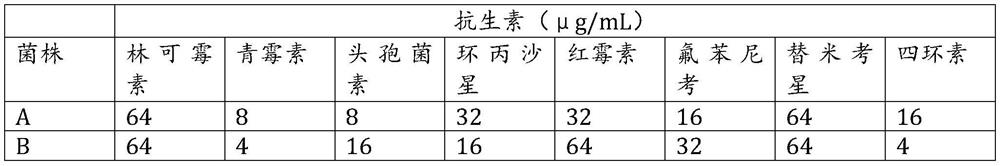
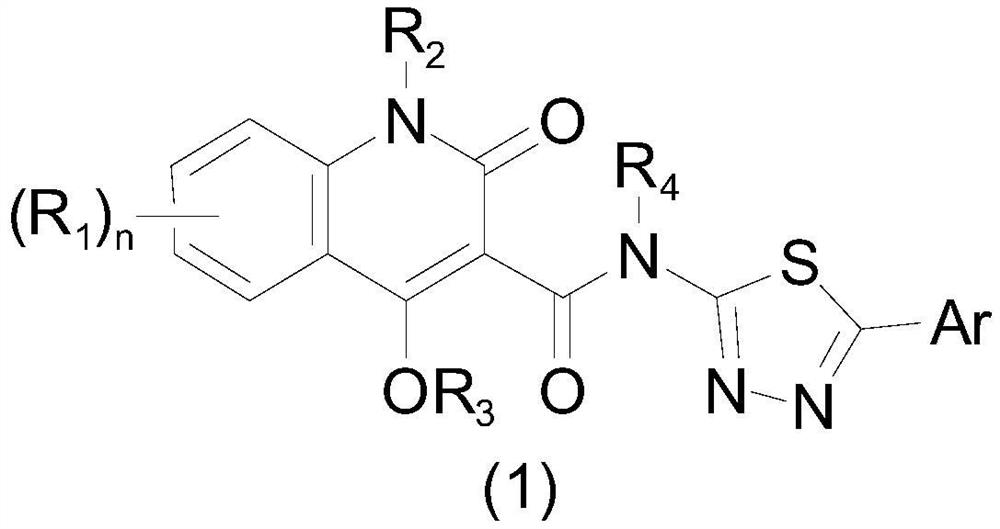
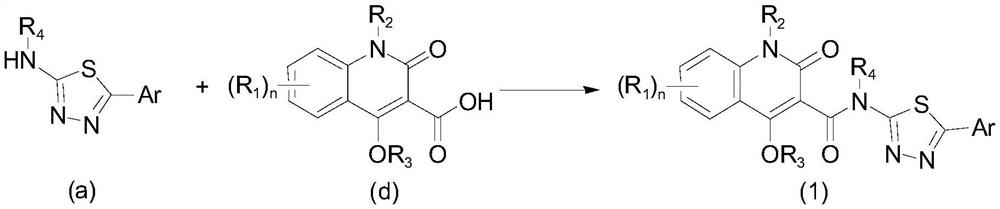
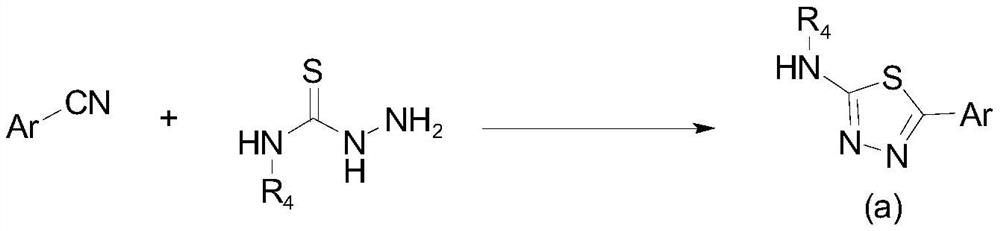
Aryl or heteroaryl substituted thiadiazole compound and antibacterial application thereof
ActiveCN112279845ABroad spectrum activityEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsIntraperitoneal routeAntimicrobial drug
The invention belongs to the technical field of medicines, and particularly relates to an aryl or heteroaryl substituted thiadiazole compound, a preparation method and application thereof as an antibacterial drug. The compound is represented by formula (1), wherein Ar represents a substituted or unsubstituted aryl group or heteroaryl group. The aryl or heteroaryl substituted thiadiazole compound provided by the invention is a compound with a new structure, and the series of compounds have a relatively strong inhibition effect on gram-positive bacteria and mycoplasma like staphylococcus aureus,staphylococcus epidermidis, enterococcus and clostridium difficile, especially drug-resistant gram-positive bacteria (MRSA, MRSE and VRE); certain inhibition is achieved on macrolide drug-resistant mycoplasma pneumoniae, intragastric administration has a good protection effect on systemic infection mice caused by MRSA intraperitoneal injection, and the compound is worthy of further research and development.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com