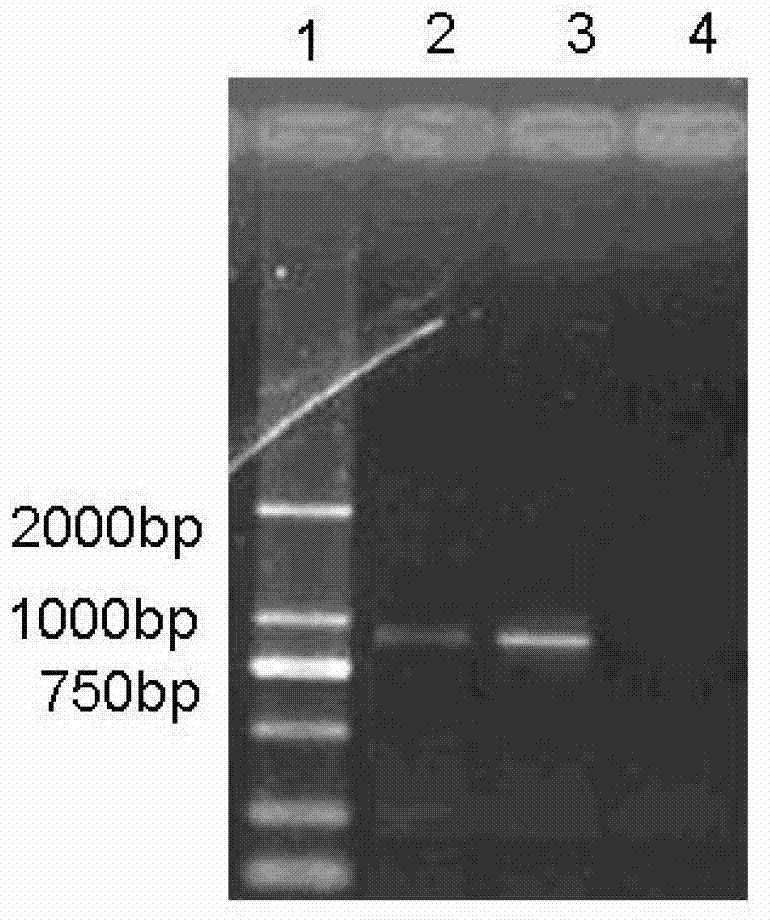
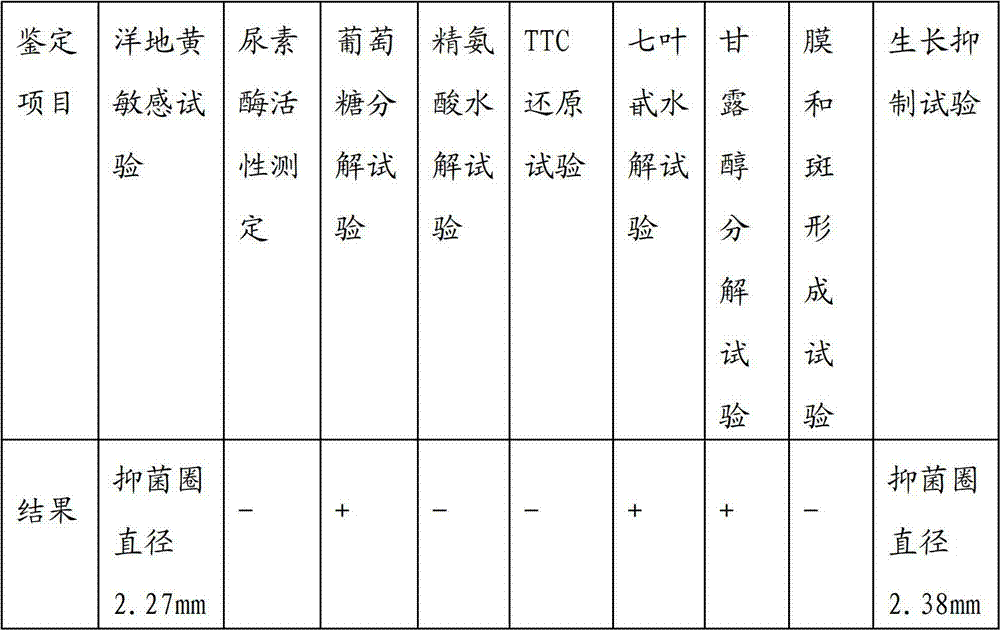
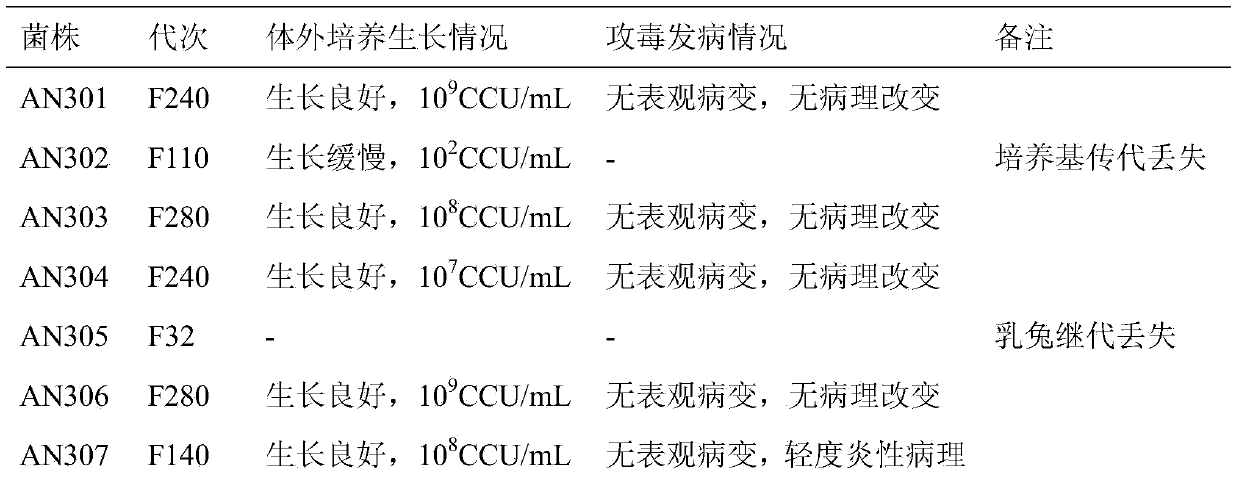
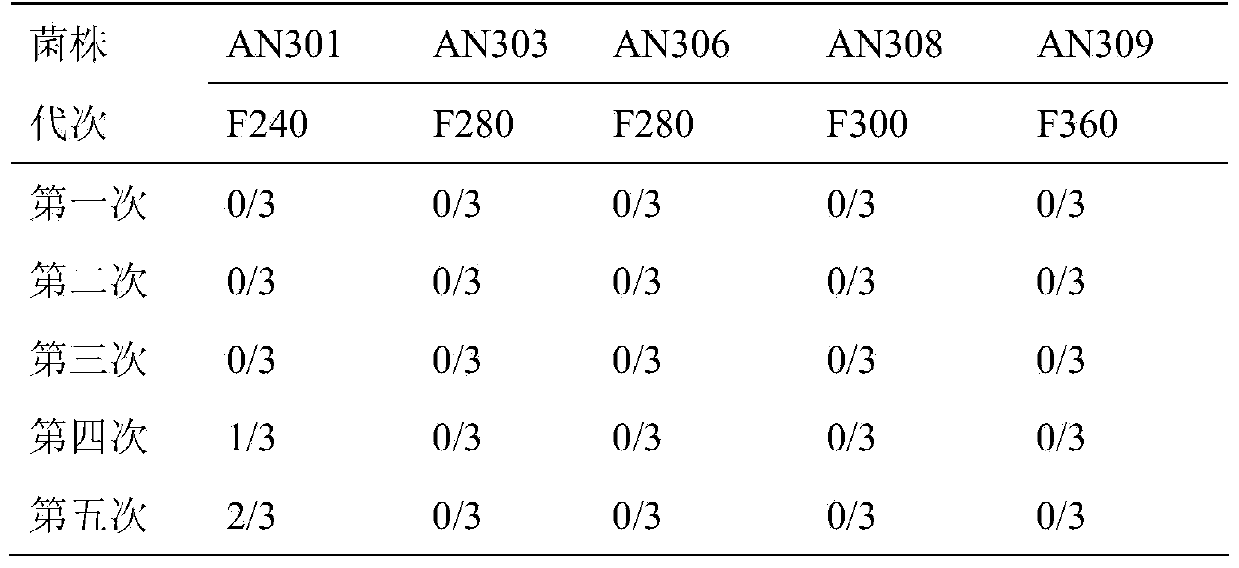
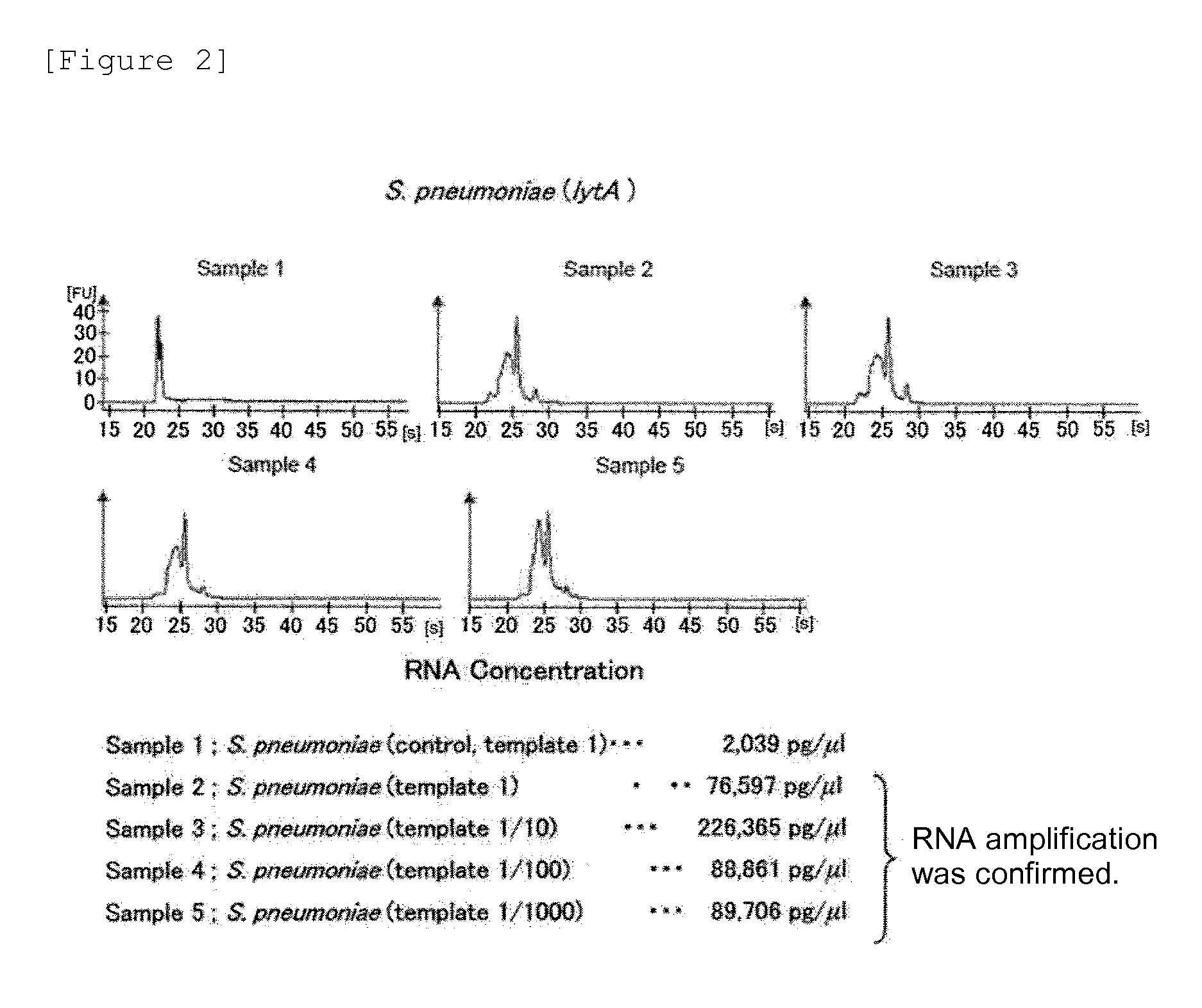
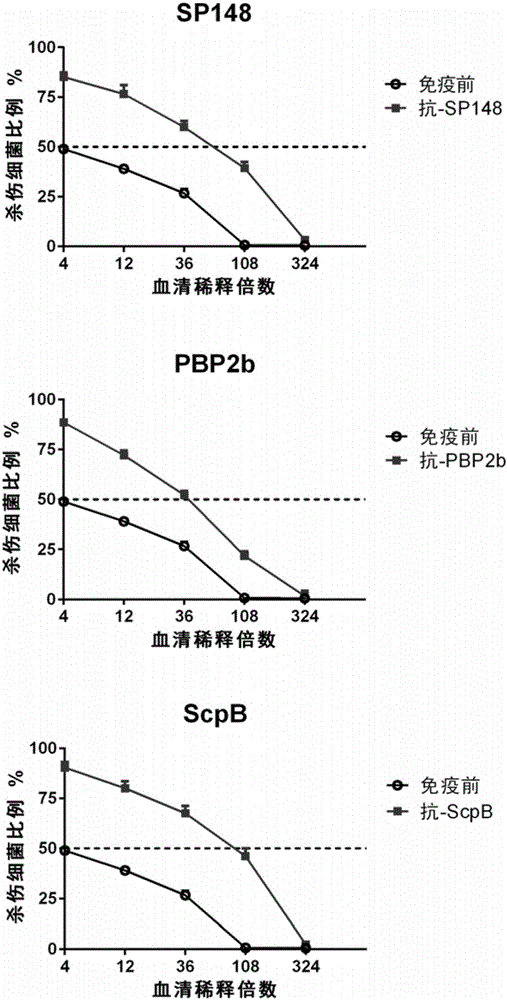
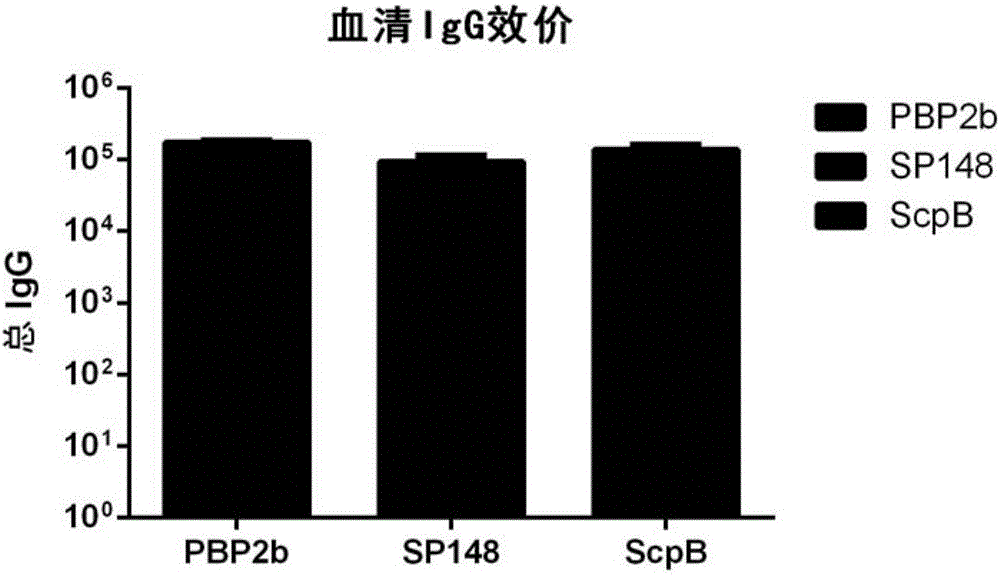
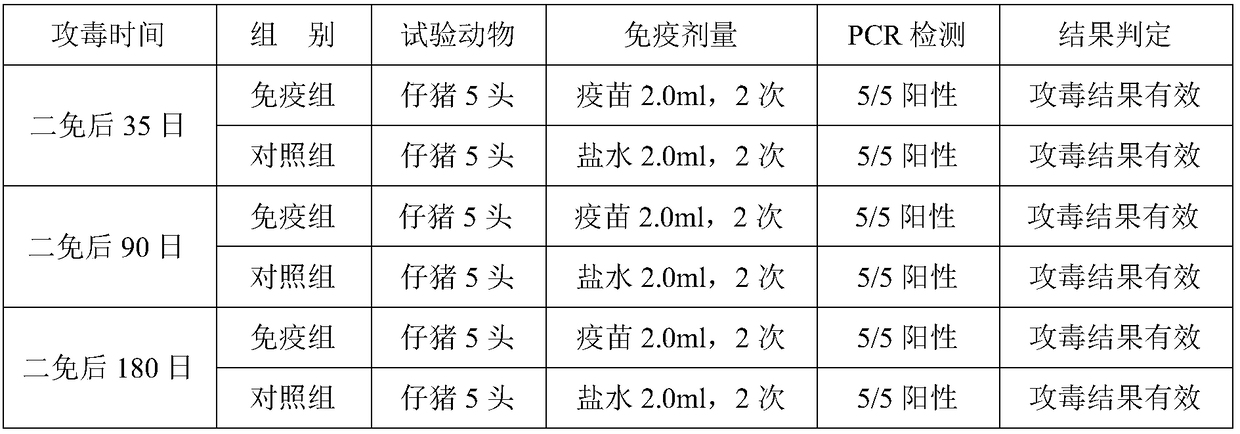
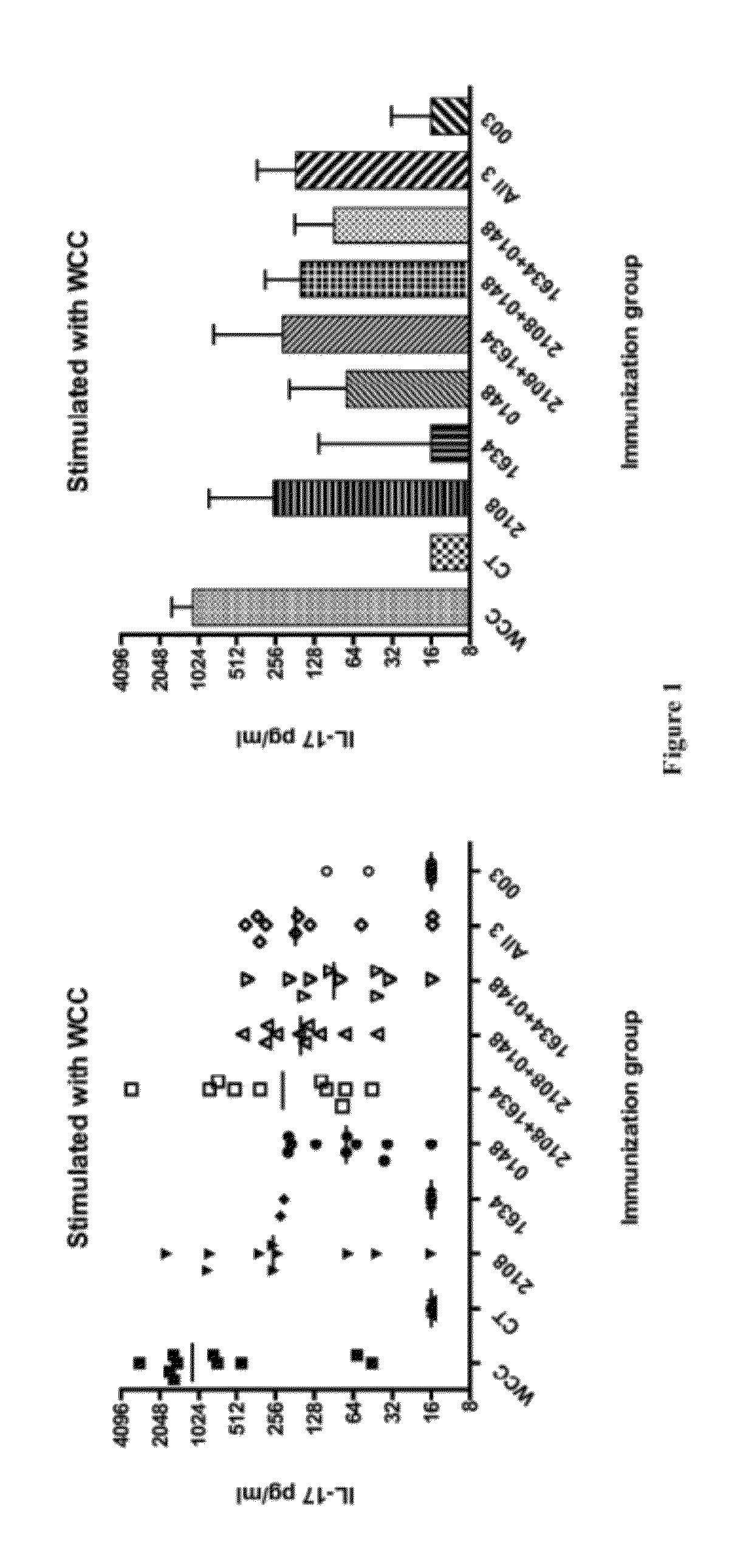
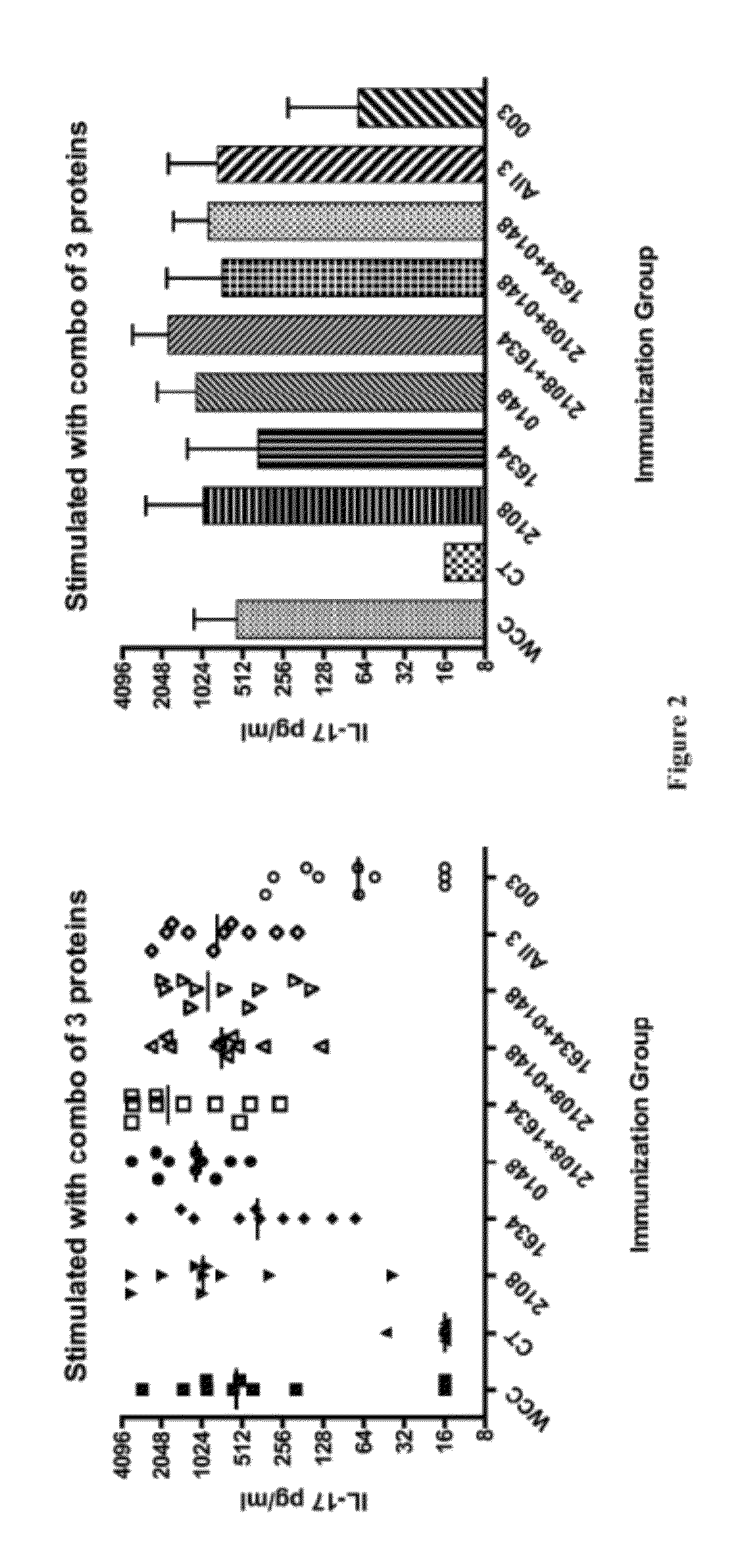
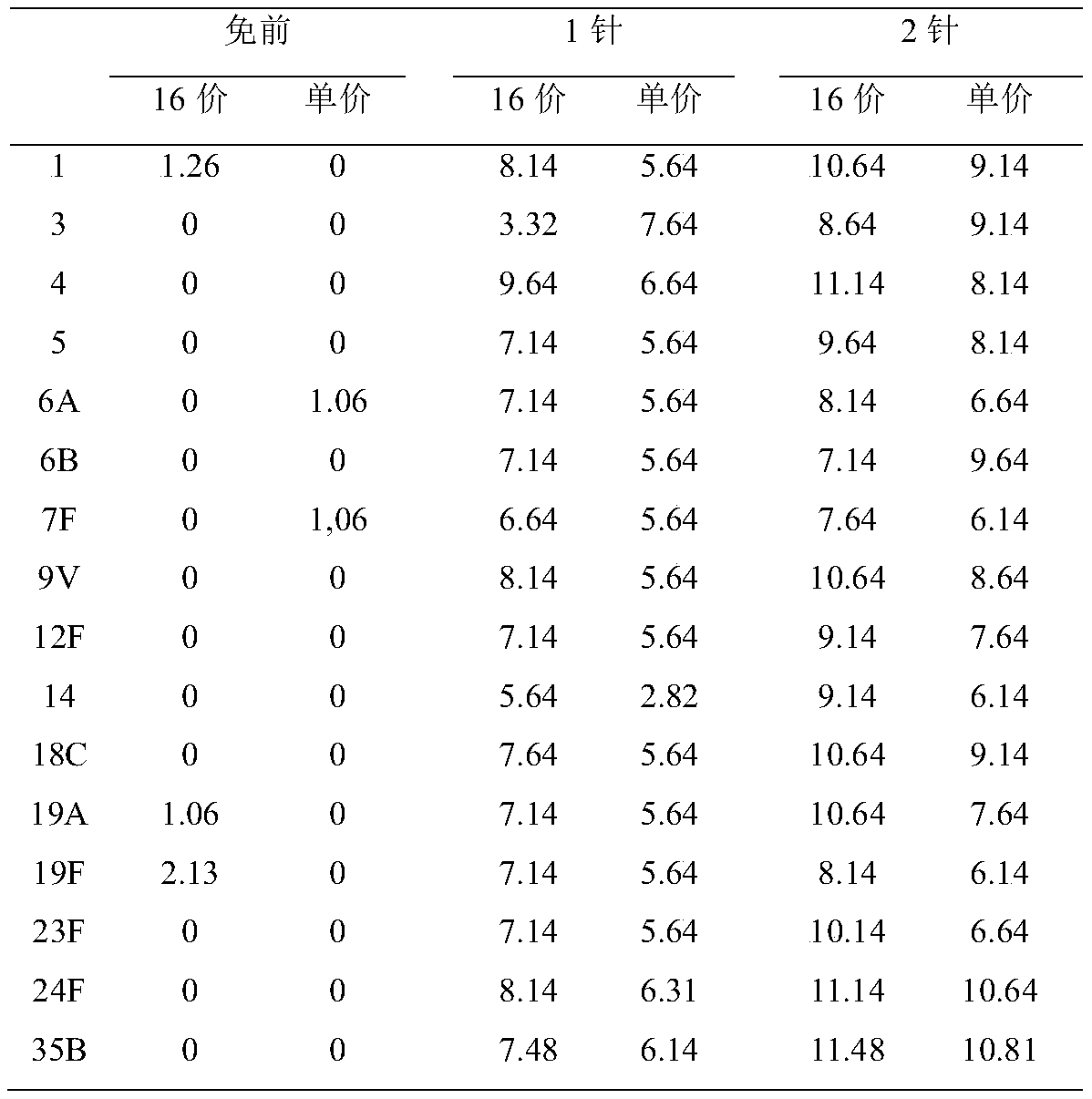
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Pneumonia mrsa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
MRSA Pneumonia Symptoms. In general, symptoms of MRSA are confined to the skin, appearing as a red bump, or several red bumps, that are often swollen, painful, tender, and warm. There may or may not be pus. In fact, these bumps are often mistaken for a pimple, pustule, or even a spider bite.
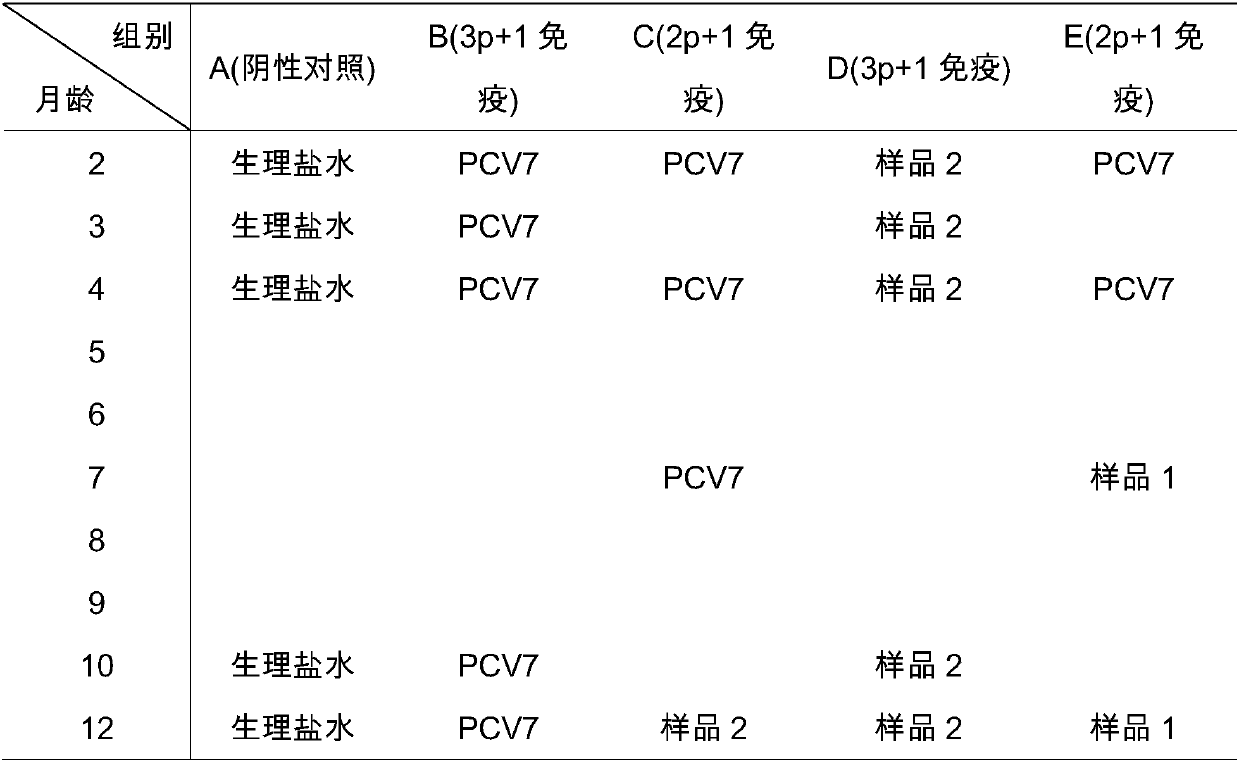
Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
ActiveCN103031258AEffective preventionEffective therapeuticAntibacterial agentsBacterial antigen ingredientsCircovirusPneumonia mrsa
The invention provides a mycoplasma hyopneumoniae strain HN0613 which is isolated and identified to have better immunogenicity, and further provides a mycoplasma hyopneumoniae antigen prepared from the mycoplasma hyopneumoniae strain HN0613 and mycoplasma pneumonia pneumovax containing the mycoplasma hyopneumoniae antigen. The invention further provides a bivalent combined vaccine of porcine circovirus II and mycoplasma hyopneumoniae. The duplex combination vaccine comprises PCV (Porcine Circovirus) antigen II (inactivated porcine circovirus antigen II or PCV20 RF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant. The combination vaccine can achieve the purpose of preventing the porcine circovirus disease and the mycoplasma pneumonia swine by injection once, and has a protective effect of preventing mycoplasma hyopneumoniae infection.
Owner:PU LIKE BIO ENG
Pig mycoplasma pneumonia live attenuated vaccine and application thereof
ActiveCN103740625AAvoid infectionSimple and fast operationAntibacterial agentsBacterial antigen ingredientsAdjuvantPneumonia mrsa
The invention discloses a pig mycoplasma pneumonia live attenuated vaccine and application thereof. A pig lung disease material infected by typical pig mycoplasma hyopneumoniae and not infected by other pathogens is screened and then is subcultured to the 100th generation through the lung of a baby rabbit, then pig mycoplasma hyopneumoniae strains are separated and are continuously subcultured through a culture medium; meanwhile, the mycoplasma hyopneumoniae AN306 is obtained through screening of a plurality of strains, and the preservation number of the mycoplasma hyopneumoniae AN306 is CCTCC M2012431. The invention also relates to a pig mycoplasma pneumonia live vaccine preparation based on the preparation of the attenuated vaccine strain. The pig mycoplasma pneumonia live vaccine preparation comprises a live attenuated vaccine strain, a pharmaceutically acceotable carrier or auxiliary ingredient and an adjuvant, and further comprises immunogens of other pathogens. The pig mycoplasma pneumonia live vaccine disclosed by the invention can be immunized in multiple ways and can enable animals to gain protection ability for resisting pig mycoplasma hyopneumoniae infection.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Mink hemorrhagic pneumonia bivalent inactivated vaccine and preparation method thereof
The invention relates to the technical field of biology, disclosing two pseudomonas aeruginosa virulent strains and a mink hemorrhagic pneumonia divalent inactivated vaccine prepared from the two strains. The pseudomonas aeruginosa virulent strains are inoculated in a culture medium to be amplified and cultured by ventilation; after the obtained cultured object is inactivated, the products are mixed at the ratio of 1-2:1; and adjuvant is added to prepare the mink hemorrhagic pneumonia divalent inactivated vaccine. The divalent inactivated vaccine disclosed by the invention has good immunity effect on mink hemorrhagic pneumonia, at least five-month protection periods can be obtained, the protection ratio is above 80%, the mink hemorrhagic pneumonia can be prevented, and the vaccine has good application prospect.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
New klebsiella pneumoniae phage and application thereof
ActiveCN110438091AImprove disinfection effectBroad spectrum antibacterialAntibacterial agentsBiocideK pneumoniaeMultidrug resistant Klebsiella pneumoniae
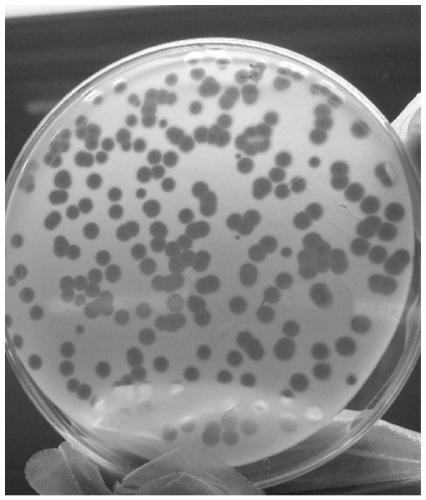
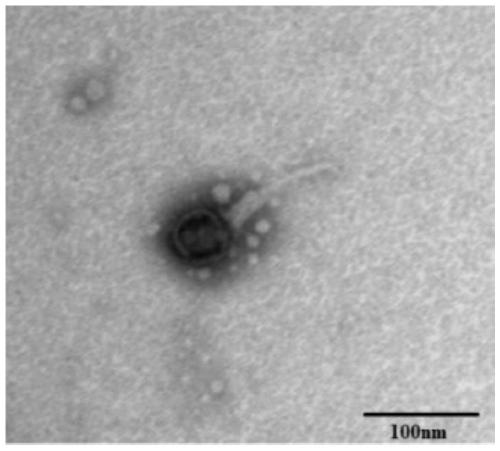
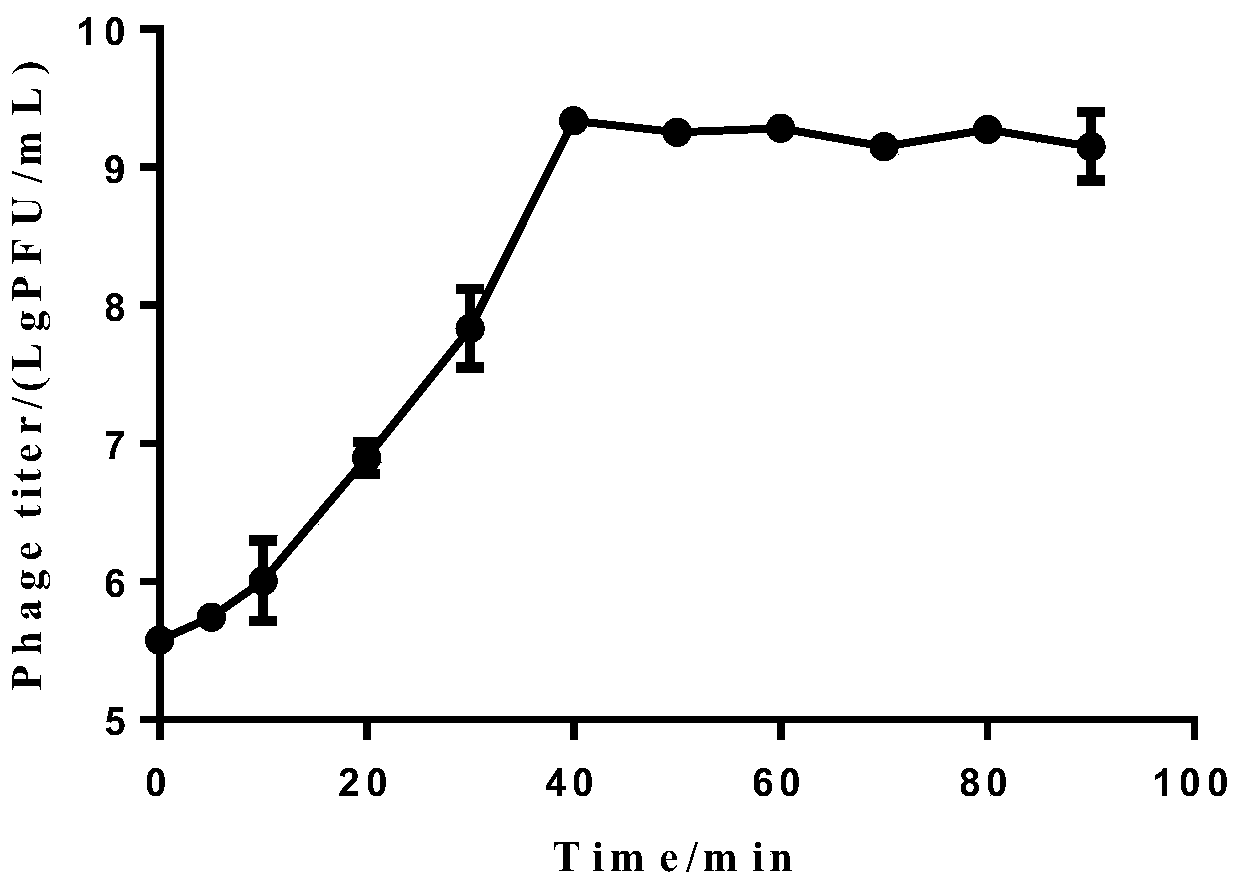
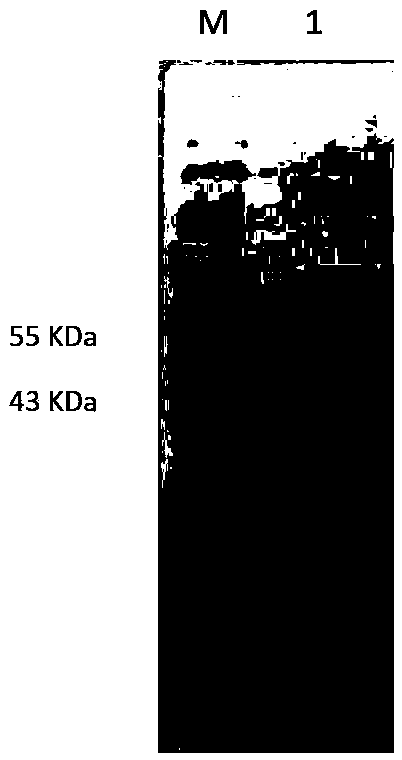
The invention discloses new klebsiella pneumoniae phage vB_KpnM_Bp5. The klebsiella pneumoniae phage (klebsiella pneumoniae phage) phage ) vB_KpnM_Bp5 is preserved in the China Center for Type CultureCollection (CCTCC) on June 13, 2019, and the preservation number is CCTCC NO:M 2019452. The phage has better disinfecting and sterilizing effects on host multidrug resistant klebsiella pneumoniae separated from piggery sewage, also has good disinfecting and sterilizing effects on other two multidrug resistant klebsiella pneumoniae separated from hospitals, is wide in antimicrobial spectrum, and can be applied to preparation of medicines for preventing and treating multidrug resistant klebsiella pneumoniae.
Owner:南宁鑫创生物科技有限公司
Anti-mycoplasma bovis monoclonal antibody, hybridoma cell strain secreting monoclonal antibody and application
InactiveCN103436496AQuick checkRapid and Specific DetectionMicroorganism based processesTissue cultureMycoproteinSpecific immunity
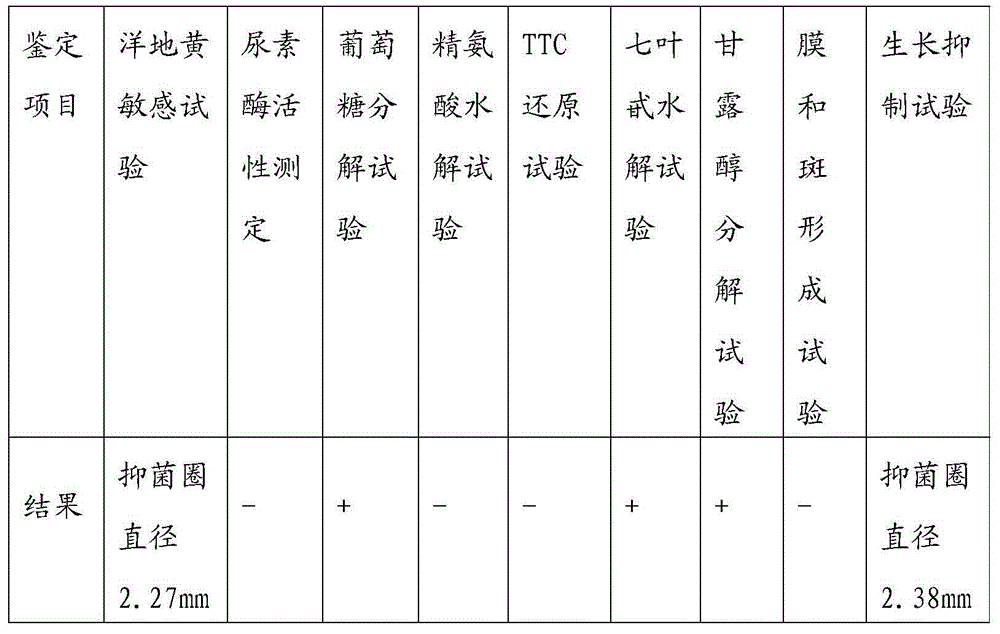
The invention discloses an anti-mycoplasma bovis monoclonal antibody, a hybridoma cell strain secreting the monoclonal antibody and an application. When the monoclonal antibody reacts with different mycoplasma strain mycoproteins, Western Blotting results indicate that the monoclonal antibody 3G11 only performs specific immune reaction with a mycoplasma bovis isolate, and does not react with mycoplasma mycoides SC (MmmSC), mycoplasma mycoides LC (MmmLC), mycoplasma capricolum pneumonia (Mccp), M.agalactiae, and M.ovipneumoniase. Therefore, the anti-mycoplasma bovis monoclonal antibody has good specificity, can be used for diagnosing and testing mycoplasma bovis infection, and provides an effective approach to prevent and control the disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Primer set for respiratory tract infection pathogen detection, rapid diagnostic kit and detection method
InactiveCN108300803AImmediate rapid diagnosisMultiplexingMicrobiological testing/measurementAgainst vector-borne diseasesPolymerase LRecombinase
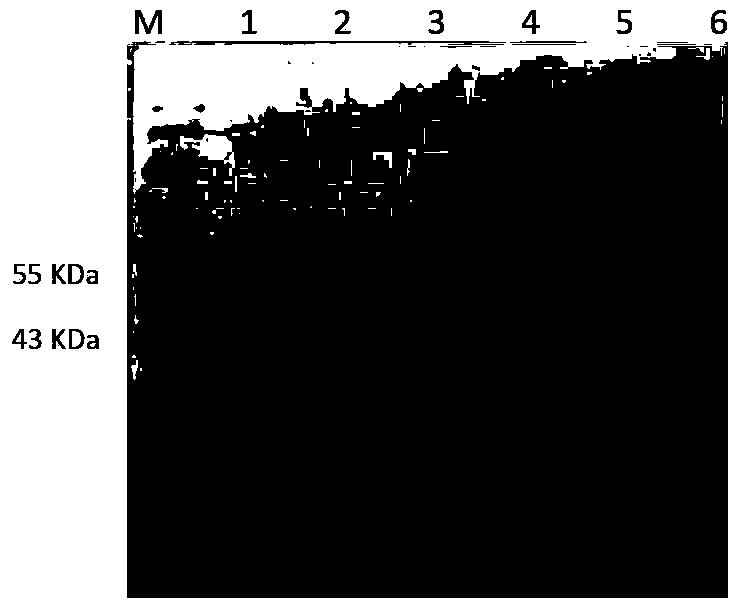
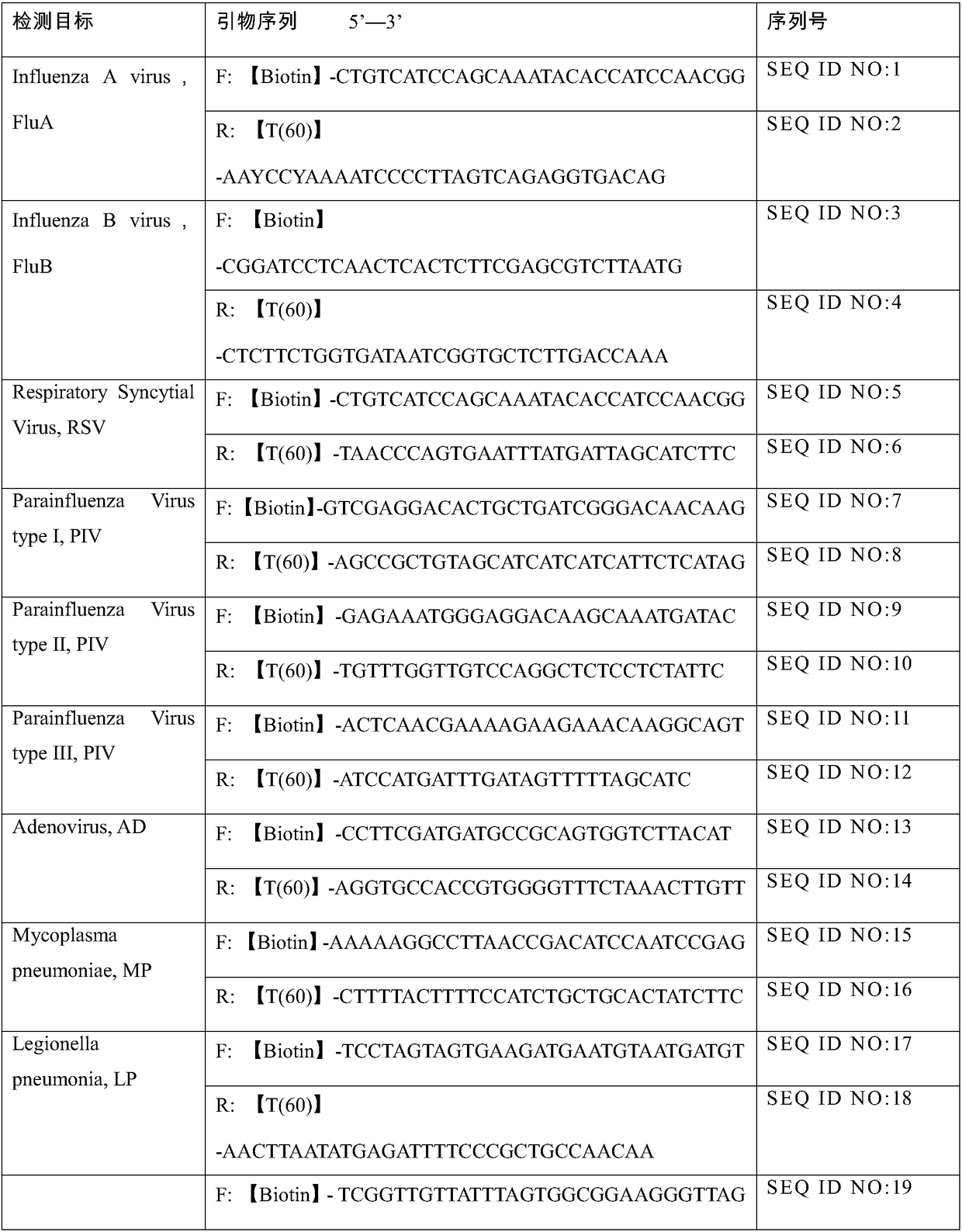
Belonging to the molecular biology field, the invention relates to a primer set for respiratory tract infection pathogen detection, a rapid diagnostic kit and a detection method. The primer set can achieve one-time detection of the following 9 respiratory tract infection pathogens: influenza A virus, influenza B virus, respiratory syncytial virus, parainfluenza virus type I, parainfluenza virus type II and parainfluenza virus type III, adenovirus, mycoplasma pneumonia, legionella pneumonia, chlamydia pneumonia and human rhinovirus. The invention adopts solid phase recombinase-polymerase constant temperature gene amplification method to detect respiratory multiple infection pathogens. The primer set adopted by the invention for respiratory tract infection pathogen detection has strong specificity and high amplification efficiency, can effectively and rapidly detect the 9 pathogens simultaneously, and realizes multiplex detection.
Owner:博迪泰(厦门)生物科技有限公司
Chinese medicine for adjuvant therapy of infant bronchitis or pneumonia
InactiveCN102512579AReduce casesShort healing timeRespiratory disorderPlant ingredientsSide effectTherapeutic effect
The invention discloses a Chinese medicine for adjuvant therapy of infant bronchitis or pneumonia. The Chinese medicine is characterized by being prepared from the following Chinese medicine raw materials in percentage by weight: 7 to 10 percent of ephedra, 13 to 15 percent of almond, 8 to 11 percent of Chinese magnoliavine fruit, 3 to 5 percent of manchurian wild ginger, 8 to 10 percent of perilla fruit, 10 to 12 percent of radish seed, 13 to 15 percent of white mulberry root-bark, 7 to 9 percent of unibract fritillary bulb, 7 to 9 percent of officinal Magnolia bark and 13 to 15 percent of liquorice root. Compared with western medicines for treating infant bronchitis or pneumonia only by the conventional intravenous injection, the Chinese medicine for the adjuvant therapy of infant bronchitis or pneumonia has the advantages that: cases of injecting hormone are remarkably reduced, and the healing time is remarkably shortened; and compared with Chinese medicines only for treating infant bronchitis or pneumonia, the invention has the advantages that: the therapeutic effect is improved, and the Chinese medicine has no toxic or side effect and is easily accepted and adopted by children and parents. The Chinese medicine can be widely used for the treatment of infant bronchitis or pneumonia.
Owner:彭建
New mycoplasma hyopneumoniae strain and vaccine composite of new mycoplasma hyopneumoniae
ActiveCN104450559AImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaMycoplasma
The invention provides a mycoplasma hyopneumoniae strain HN0613 which has better immunogenicity proved through isolation and identification, also provides a mycoplasma hyopneumoniae antigen prepared by using the mycoplasma hyopneumoniae strain HN0613 and a mycoplasma hyopneumoniae vaccine containing the mycoplasma hyopneumoniae antigen. The invention further provides a porcine circovirus type 2 / mycoplasma hyopneumoniae bicombinant vaccine, containing a PCV2 antigen (inactivated PCV2 antigen or PCV2ORF2 protein), an inactivated mycoplasma hyopneumoniae and vaccine adjuvants. Through injection of the bicombinant vaccine, the aim of preventing PCV and mycoplasma hyopneumoniae can be achieved and the effect of prevention and protection from mycoplasma hyopneumoniae infection can be achieved further.
Owner:PU LIKE BIO ENG +1
Method for preparing actinobacillus pleuropneumoniae (App) bacterial ghost and method for preparing subunit vaccine by loading pasteurella antigen with App bacterial ghost
InactiveCN101934072APrevention of swine pleuropneumoniaPrevention of PasteurellosisAntibacterial agentsBacterial antigen ingredientsAntigenPleuronectes pinnifasciatus
The invention discloses a method for preparing an actinobacillus pleuropneumoniae (App) bacterial ghost and a method for preparing a subunit vaccine by loading a pasteurella antigen with the App bacterial ghost. A recombinant swine App bacterial ghost is prepared by controllable double-cracking technology and a pasteurella protection gene is introduced into an App bacterial ghost carrier, so that swine pleuropneumonia and a pasteurella bigeminal gene vaccine for preventing and treating swine pasteurellosis and swine pleuropneumonia are obtained. The preparation of the bacterial ghost carrier and the application of the bacterial ghost carrier to the prevention and treatment of important animal epidemic diseases are realized and a method is provided for the research of a multi-geminal gene vaccine at the same time. An animal experiment indicates that the protection rates of the bigeminal vaccine on infectious swine pleuropneumonia and pasteurellosis are up to 99 percent and 99.2 percent respectively.
Owner:TIANJIN AGRICULTURE COLLEGE
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Application of streptococcus pneumoniae protein to resisting infection of S. pneumoniae
ActiveCN109456393AIncrease infectionReduced Colonization Protection ExperimentAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaStreptococcus mitis
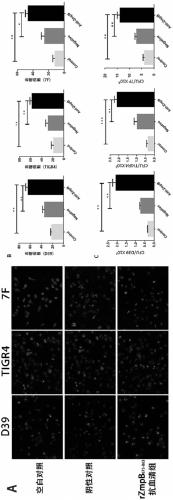
The invention provides application of S. pneumoniae protein to resisting infection of S. pneumoniae. The endopeptidase O (PepO) of S. pneumoniae is a subcutaneous immunologic adjuvant, and the prepared S. pneumoniae protein vaccines have the good protection effects on resisting infection of S. pneumoniae through mixing and fusing expression of the subcutaneous immunologic adjuvant and 673rd to 863rd amino acid peptide fragment of zinc metal protease B (ZmpB).
Owner:CHONGQING MEDICAL UNIVERSITY
Rapid-recognition gene chip for pathogenic bacteria of pneumonia
The invention discloses a rapid-recognition gene chip for pathogenic bacteria of pneumonia. The gene chip can detect 15 pathogenic bacteria including streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, acinetobacter baumannii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae, and clinically common and difficult-to-culture pathogenic bacteria are contained. In a preparation process of the gene chip, design, screening and verification of probes are performed by adopting 16S rDNA and a specific gene sequence corresponding to each of the pathogenic bacteria, and types of the bacteria in a to-be-detected sample are identified from levels of genus and species simultaneously and respectively. The gene chip has the advantages that the defect that clinical detection of the pathogenic bacteria of pneumonia is not timely and comprehensive at present is overcome, and one novel detection way is provided for early diagnosis and early treatment of patients with pneumonia.
Owner:GENERAL HOSPITAL OF PLA +1
Method for detecting pneumonia causative bacteria using nucleic acid chromatography
InactiveUS20130023443A1Quick and accurate identificationNucleotide librariesMicrobiological testing/measurementBacteroidesStaphylococcus aureus
Provided are a method and a kit for accurately and rapidly detecting ten types of targeting pneumonia bacteria: Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Klebsiella pneumoniae, Pseudomonas aeruginosa, Moraxella catarrhalis, methicillin-resistant Staphylococcus aureus (MRSA), and Staphylococcus aureus. A set of primer pairs directed to their respective target regions contained in the DnaJ gene, etc., of the ten types of pneumonia causative bacteria is designed for the ten bacterial strains and used to amplify gene products. A set of bacterial strain-specific probe pairs is further designed for the ten bacterial strains such that the probe pairs hybridize with the amplification products via sequences in the respective target regions differing from the sequences hybridized by the set of primer pairs. A first probe-bound labeled high molecular carrier in which plural types of first probes for the pneumonia bacteria are bound to a labeled high molecular carrier and a solid-phase second probe-carrying developing support are used as the set of probe pairs to perform nucleic acid chromatography.
Owner:YAMAGUCHI TECH LICENSING ORG
Streptococcus pneumoniae vaccine
ActiveCN106822885AAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaStreptococcus mitis
The invention brings forward streptococcus pneumoniae vaccine, a method for preparing an antibody, the antibody, an artificial antibody, application of a preparation in the preparation of a medicine, and application of a reagent in the preparation of a kit. The streptococcus pneumoniae vaccine can activate the immune system, generate an antibody and phagocytose and kill streptococcus pneumoniae so as to achieve a good effect of treating / preventing streptococcus pneumoniae-related diseases.
Owner:TSINGHUA UNIV
Pig mycoplasma pneumonia inactivated vaccine and preparation method thereof
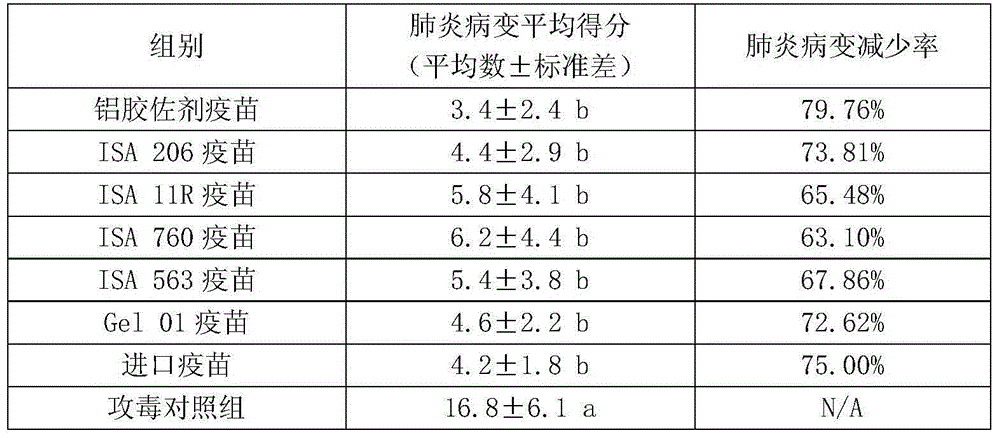
The invention relates to a production method for pig mycoplasma pneumonia inactivated vaccine. The method takes an isolated mycoplasma hyopneumoniae (Mycoplasma hyopneumoniae Mhp) ZY strain with strong poison immunity and good epidemic focus as a production bacterial strain, and efficient culture medium, appropriate cultural method, inactivation technology and appropriate immunologic adjuvant areused to prepare the mycoplasma hyopneumoniae inactivated vaccine. The production method for the pig mycoplasma pneumonia inactivated vaccine has the advantages that the content CUU of inactivated antigens is no less than 1.5*108 per dose in the prepared vaccine, the use of the vaccine is safe, healthful 2-3-week-old piglets easy to be infected do not have local or whole-length untoward effect after inoculation and all live healthily; the vaccine comprises an effectiveness testing result with a matched group by using an assay of vaccination and challenge method, and the decrement rate of pneumonia of tested pigs in the matched group is no less than 75%; the vaccine is still safe and effective after being stored at 2-8 DEG C for 27 months; and the effectiveness testing of the vaccine also can be performed by using an IHA method to detect the titer of a rabbit serum mycoplasma hyopneumoniae antibody, which has great benefit for the effectiveness testing of the vaccine.
Owner:北京万牧源生物科技有限公司
Porcine mycoplasma pneumoniae and application of porcine mycoplasma pneumoniae
ActiveCN107488612AHigh proliferative titerStable biological propertiesAntibacterial agentsBacterial antigen ingredientsImmunogenicityMicrobiological culture
The invention discloses porcine mycoplasma pneumoniae and an application of the porcine mycoplasma pneumoniae. The porcine mycoplasma pneumoniae is mycoplasma bovis HNMhy1, has a collection number of CGMCC (China General Microbiological Culture Collection Center) NO: 13858 and is collected in China General Microbiological Culture Collection Center in Beijing, China on May 26, 2017. The porcine mycoplasma pneumoniae strain HNMhy1 is separated from a nursery pig having typical dyspnea and lung consolidation, has stable biological characteristics, and higher pathogenicity on the nursery pig, can cause a typical deep breathing symptom of the nursery pig and has good immunogenicity. A vaccine prepared from the porcine mycoplasma pneumoniae strain HNMhy1 is safe and reliable and has a better protection effect on the porcine primary atypical pneumonia.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
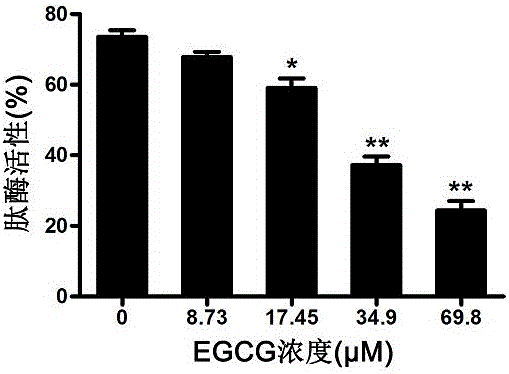
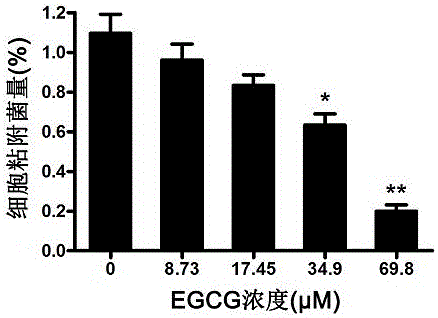
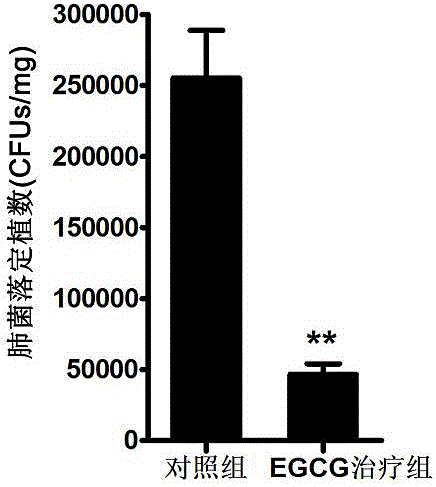
EGCG with inhibitory effect on streptococcus pneumoniae pathogenicity and application
InactiveCN106667997AInhibition of pathogenicityLess likely to induce drug resistanceAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaePneumonia mrsa
Owner:JILIN UNIV
Chinese medicine preparation capable of curing pediatric asthmatic pneumonia
The invention discloses a Chinese medicine preparation capable of curing pediatric asthmatic pneumonia. The asthmatic pneumonia belongs to the category of Chinese medicine 'pneumonia with dyspnea and cough', the pathogenesis includes that wheezing and coughing are probably caused by pathogen blocked in a lung, lung infection, heat transmission, phlegm production and reversed flow of phlegm along with air. Therefore, by means of the Chinese medicine preparation capable of curing the pediatric asthmatic pneumonia, the Chinese medicine of marsdenia tenacissima, callianthemus, small lilac, crepis phoenix, radix tetrastigme, vernonia cinerea, ardisa japonica blume, gynostemma pentaphyllum, cortex mori radicis, eriobotrya seguinii, ephedra, lanjiang lily bulb, aster, perny false fairybells rhizome, lotus seeds and gordon euryale seeds, and water are decocted into soup decoction to be drunk by a patient. By means of the clinical test of the hospital, the overall effective rate reaches 98.3%, the curing effect is stable, and the side effect is small.
Owner:南通东湖国际旅行社有限公司
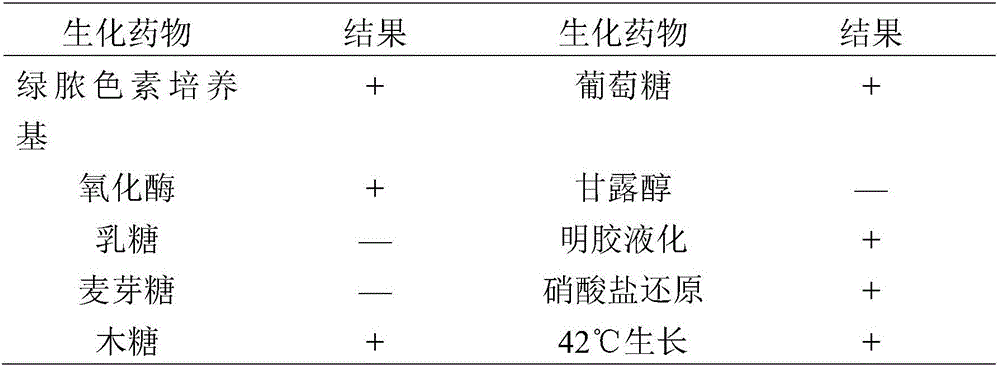
Pseudomonas aeruginosa of minks and application of pseudomonas aeruginosa
InactiveCN105754905AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacteriaPneumonia mrsaPathogenicity
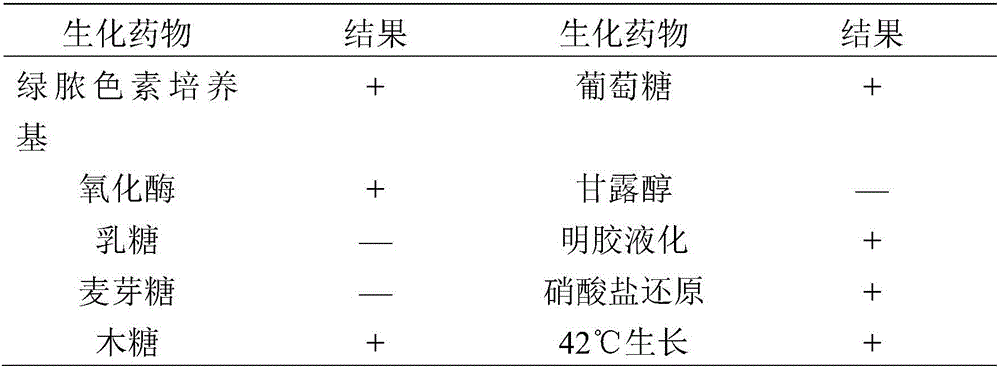
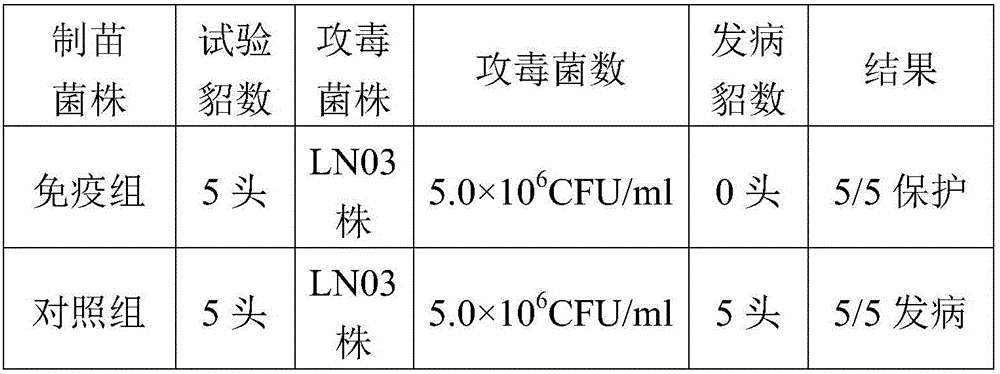
The invention provides pseudomonas aeruginosa of minks and application of the pseudomonas aeruginosa.A preservation number of a type-B pseudomonas aeruginosa strain LN03 of the minks is CCTCC NO.M2016068.The type-B pseudomonas aeruginosa strain LN03 of the minks further can be used for preparing inactivated vaccine of the pseudomonas aeruginosa of the minks.The pseudomonas aeruginosa and the application have the advantages that the type-B pseudomonas aeruginosa strain LN03 is high in pathogenicity for the minks and is excellent in immunogenicity, the inactivated vaccine prepared by the aid of the type-B pseudomonas aeruginosa strain LN03 is safe and reliable, accordingly, homologous attacking protection effects can be realized, the minks are high in immunity after being immunized, the morbidity and the mortality of vaccinated mink groups can be obviously reduced, the epidemiology and propagation of hemorrhagic pneumonia of the minks can be effectively prevented, economic loss due to the hemorrhagic pneumonia can be reduced for the fur animal breeding industries, and the pseudomonas aeruginosa has a broad application prospect.
Owner:QINGDAO AGRI UNIV
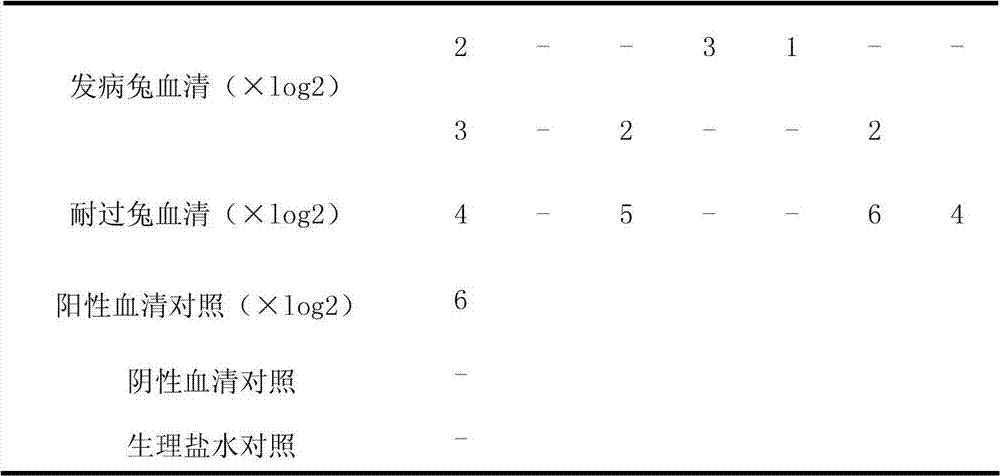
Rabbit Klebsiella pneumoniae agglutinogen and application of rabbit Klebsiella pneumoniae agglutinogen
The invention relates to the technical field of organisms, in particular to a rabbit Klebsiella pneumoniae agglutination reaction experimental technique. A rabbit Klebsiella pneumoniae agglutinogen is prepared from rabbit Klebsiella pneumoniae liquid dyed by fuchsin dye liquid. An application method concretely belongs to an application method in agglutination reaction experiments: the rabbit Klebsiella pneumoniae agglutination is combined with agglutinin, and the agglutination result is judged. The rabbit Klebsiella pneumoniae agglutinogen has the advantages that expensive instruments and reagents are not needed, the cost is low, the speed is high, the operation is simple and convenient, the technical requirements on operators are low, the result is intuitional, the rabbit Klebsiella pneumoniae agglutinogen is applied to basic level site detection application, the detection antigen of a rabbit Klebsiella pneumoniae microagglutination reagent kit is dyed, agglutination particles or agglutination blocks are red, the observation is easier, and the detecting method can be used for quantificationally detecting the rabbit serum antibody valence, can also be used for qualitatively detecting the rabbit Klebsiella pneumoniae antigen and is particularly suitable for on-site rabbit Klebsiella pneumoniae diagnosis for basic level veterinary stations and warrens.
Owner:CHONGQING ACAD OF ANIMAL SCI
Vaccines and compositions against Streptococcus pneumoniae
Streptococcus pneumoniae is a major health concern, especially in very young, elderly, or immunocompromized patients. The present disclosure provides, inter alia, certain highly effective vaccines and pharmaceutical compositions in Streptococcus pneumoniae. The antigens may be used therapeutically or prophylactically.
Owner:GENOCEA BIOSCI +1
Polyvalent pneumococci conjugate vaccine
InactiveCN109771640AAntibacterial agentsBacterial antigen ingredientsConjugate vaccinePneumonia mrsa
The invention provides a serotype combination of a polyvalent pneumococci conjugate vaccine. Pneumococci serotypes 24 and 35 (24F and 35B) contained in the serotype combination of the polyvalent pneumococci conjugate vaccine are used for preventing the infection caused by the pneumococci which contains the serotypes.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Monoclonal antibody against mycoplasma bovis, hybridoma cell line secreting the monoclonal antibody and application
InactiveCN103436496BQuick checkRapid and Specific DetectionMicroorganism based processesTissue cultureMycoproteinSpecific immunity
The invention discloses an anti-mycoplasma bovis monoclonal antibody, a hybridoma cell strain secreting the monoclonal antibody and an application. When the monoclonal antibody reacts with different mycoplasma strain mycoproteins, Western Blotting results indicate that the monoclonal antibody 3G11 only performs specific immune reaction with a mycoplasma bovis isolate, and does not react with mycoplasma mycoides SC (MmmSC), mycoplasma mycoides LC (MmmLC), mycoplasma capricolum pneumonia (Mccp), M.agalactiae, and M.ovipneumoniase. Therefore, the anti-mycoplasma bovis monoclonal antibody has good specificity, can be used for diagnosing and testing mycoplasma bovis infection, and provides an effective approach to prevent and control the disease.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Application of swine enzootic hyopneumoniae vaccine strain
InactiveCN104645324AImproving immunogenicityGuaranteed immune efficiencyAntibacterial agentsBacterial antigen ingredientsVeterinary DrugsImmunogenicity
The invention relates to a novel application of a swine enzootic hyopneumoniae vaccine strain, in particular to an application of the swine enzootic hyopneumoniae vaccine strain in preparing a veterinary medicine for treating swine enzootic hyopneumoniae. The swine enzootic hyopneumoniae vaccine strain is named and classified as Mycoplasma hyopneumoniae and CJ, and is preserved in China General Microbiological Culture Collection Center with the preservation Number of CGMCC NO:9909 on November 6, 2014. The strain for an inactivated vaccine is separated and obtained from a commercial swine in China, and has high growth titer and good immunogenicigy, and the inactivated vaccine has a simple preparation process, is safe and effective, and can effectively prevent the swine enzootic hyopneumoniae.
Owner:TECON BIOLOGY CO LTD
Inactivated vaccine prepared through mink pseudomonas aeruginosa strain SD17
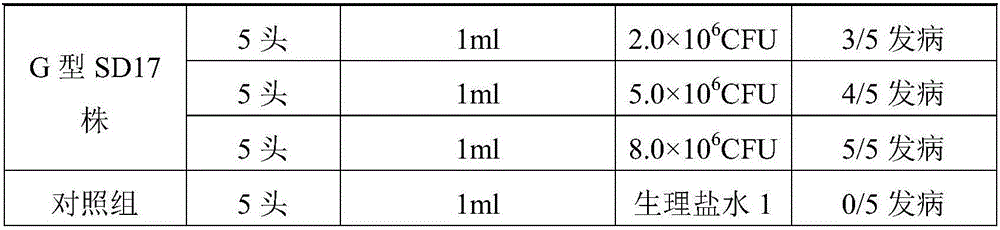
InactiveCN105727273AReduce morbidityReduce mortalityAntibacterial agentsRespiratory disorderDiseaseMortality rate
The invention provides an inactivated vaccine prepared through a mink pseudomonas aeruginosa strain SD17. The inactivated vaccine comprises antigen and vaccine adjuvant, wherein the antigen is an inactivated pseudomonas aeruginosa strain SD17 and has the preservation number of CCTCC NO:M2016069. The prepared inactivated vaccine is safe and reliable, can provide effective homologous challenge protection and can produce strong immunity after immunity, the morbidity and death rate of vaccinated mink groups are both remarkably decreased, the prevalence and transmission of mink hemorrhagic pneumonia can be effectively prevented, the economic loss caused by the disease to the fur-bearing animal breeding industry is reduced, and wide application prospects are achieved.
Owner:QINGDAO AGRI UNIV
Streptococcus pneumoniae protein vaccine and preparation method thereof
InactiveCN107929728ANasal administration is convenient and quickReduce the chance of vaccination failureAntibacterial agentsBacterial antigen ingredientsIntramuscular injectionCoccidia
The invention discloses a Streptococcus pneumoniae protein vaccine, which is a Streptococcus pneumoniae capsule saccharide-protein conjugate obtained by covalently linking one or a variety of Streptococcus pneumoniae capsule saccharides and a carrier protein, wherein the carrier protein is lectin. Compared to the Streptococcus pneumoniae protein vaccine in the prior art, the Streptococcus pneumoniae protein vaccine of the present invention has the following advantages that the administration mode of the Streptococcus pneumoniae protein vaccine is intranasal administration, wherein the intranasal administration is convenient and rapid compared to intramuscular injection; the Streptococcus pneumoniae protein vaccine can be used as the commercially available Streptococcus pneumoniae supplementary immunization vaccine; and when the large-scale epidemic situation occurs, the Streptococcus pneumoniae protein vaccine can be temporarily used as the emergency vaccine, and can provide the immunization foundation for the subsequent vaccination of the commercially available Streptococcus pneumoniae vaccine.
Owner:BRAVOVAX
14 type pneumococcal neutralizing monoclonal antibody and application
InactiveCN104250635AImproving immunogenicityMeet the integration needsImmunoglobulins against bacteriaMicroorganism based processesBacteroidesPneumonia mrsa
The invention relates to an anti 14 type pneumococcal capsular polysaccharide neutralizing monoclonal antibody and a hybridoma cell strain for producing the anti 14 type pneumococcal capsular polysaccharide neutralizing monoclonal antibody, and the accession number of the hybridoma cell strain is CGMCC No.9455. The monoclonal antibody is high in titer and good in specificity, can be effective in vitro neutralization of 14 type pneumococcal infection, can effectively remove bacteria, has wide application prospect in the aspects of pneumonia diagnosis and treatment, can be applied to the pneumonia vaccine polysaccharide content detection, can be used for the polysaccharide content detection by enzyme linked immunosorbent assay, and breaks through the current detection methods.
Owner:SINOVAC RES & DEV
Inactivated vaccine for mink pseudomonas aeruginosa
InactiveCN105727274AReduce morbidityReduce mortalityAntibacterial agentsRespiratory disorderMinkMortality rate
The invention provides an inactivated mink Pseudomonas aeruginosa vaccine, which includes an antigen and a vaccine adjuvant, wherein the antigen used is type B mink Pseudomonas aeruginosa LN03 strain, and the preservation number is CCTCC NO: M2016068. The inactivated vaccine prepared by the invention is safe and reliable, can provide effective homologous virus attack protection, can produce strong immunity after immunization, significantly reduces the morbidity and mortality of inoculated mink groups, and can effectively prevent the prevalence of mink hemorrhagic pneumonia It can reduce the economic loss caused by the disease to the fur animal breeding industry, and has broad application prospects.
Owner:QINGDAO AGRI UNIV
Glyceroglycolipid antigen of Mycoplasma pneumoniae
ActiveUS8367076B2Highly-sensitivelyAccurate detectionBacterial antigen ingredientsSugar derivativesAntigenPneumonia mrsa
Owner:M BIO TECH
Bivalent inactivated vaccine to bacterium pyocyaneum for mink
InactiveCN105641691AReduce morbidityReduce mortalityAntibacterial agentsBacterial antigen ingredientsBacterium pyocyaneumMink
The invention provides a bivalent inactivated vaccine to bacterium pyocyaneum for mink. The vaccine comprises an antigen and a vaccine adjuvant, wherein the antigen is inactivated mink bacterium pyocyaneum LN03 strain having a preservation number of CCTCC NO:M2016068 and inactivated pyocyaneum SD17 having a preservation number of CCTCC NO:M2016069. The inactivated vaccine is safe and reliable and can provide effective homologous attacking protection, strong immunity can be generated after immunization, the incidence and death rate of a vaccinated mink group can be remarkably reduced, prevalence and transmission of hemorrhagic pneumonia of mink can be effectively prevented, and economic loss of hemorrhagic pneumonia of mink for fur-bearing animal breeding can be reduced. The bivalent inactivated vaccine to bacterium pyocyaneum for mink has a wide application prospect.
Owner:QINGDAO AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com