Primer set for respiratory tract infection pathogen detection, rapid diagnostic kit and detection method
A rapid diagnosis and respiratory technology, applied in the field of molecular biology, can solve the problems of slow development and no mature technology for pathogen detection of multiple respiratory infections, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
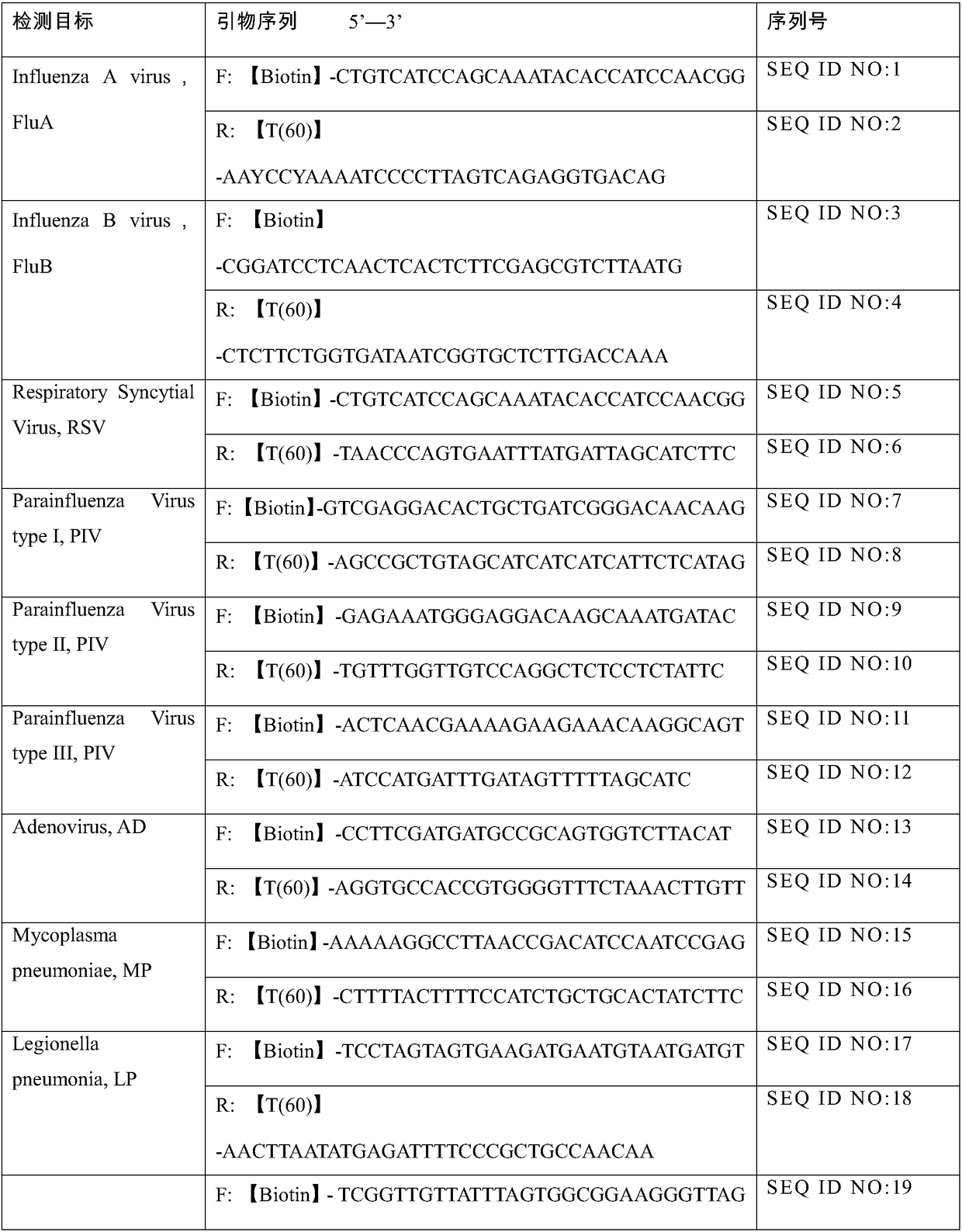
[0037] Embodiment 1: primer and test kit
[0038] 1. Primer design:
[0039] The primers were designed according to the pathogenic gene sequence of respiratory tract infection in the NCBI gene database, referring to the TwistDx instructionmanual and Primer-BLAST primer design principles. The primer length is about 30-35nt. Since there is no primer design software for RPA at present, a large number of primers were designed and synthesized in the previous work of the present invention, and the primers with high sensitivity and good specificity were screened out for use in the present invention (Table 1).
[0040] 2. Primer synthesis:
[0041] According to the primer sequences shown in Table 1, sequence synthesis was carried out. The 5' end of the forward primer was modified by adding Biotin; the 5' end of the reverse primer was modified by adding 60 T bases.
[0042] Table 1
[0043]
[0044]
[0045] 3. Kit
[0046] The kit (nine-in-one) for the rapid diagnosis of mu...
Embodiment 2
[0048] Embodiment 2: detect multiple respiratory infection pathogens
[0049] The present embodiment adopts the kit described in Example 1 to detect multiple respiratory tract infection pathogens, and the method used in the detection is the solid-phase recombinase polymerase constant temperature gene amplification method, and the specific steps are as follows:
[0050] 1. Clinical Sample Preparation
[0051] Collect the remaining samples after routine examinations of the respiratory tract (throat swabs, nasopharyngeal swabs, etc.), and the collection conditions are: positive clinical samples confirmed to have respiratory pathogenic infection after laboratory examinations (culture method or molecular biology examination method), And negative clinical samples confirmed to be free of respiratory pathogen infection after inspection. The remaining samples collected will be deactivated by heating at 95°C for 10 minutes to avoid the possibility of infection during transportation. C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com