Preparation combination of multivalence pneumococcal conjugate vaccine and application thereof
A pneumococcal and conjugated vaccine technology, used in multivalent vaccines, vaccines, medical preparations containing active ingredients, etc., can solve the problems of no GSK development and increased antigen content.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 1. Differences in molecular weight of conjugates prepared with different carrier proteins
[0080] Select pneumonia capsular polysaccharides of different serotypes and couple them with CRM197 and TT respectively. The coupling method of polysaccharides and proteins adopts the commonly used experimental method in the industry—CDAP or reducing amine conjugation chemical reaction (see patent WO95 / 08348 for details ), the molecular weight of the conjugate was detected after purification, and the results are shown in Table 1.
[0081] Table 1 Molecular weight of different carrier conjugates
[0082]
[0083]
[0084] Remarks: The molecular weight of the conjugate is represented by the recovery rate of KD<0.35
[0085] The results showed that the molecular weights of the conjugates of different carrier proteins were different for the same serotype, and generally the molecular weight of TT as carrier protein was higher than that of CRM197 as carrier protein. The molecul...
Embodiment 2
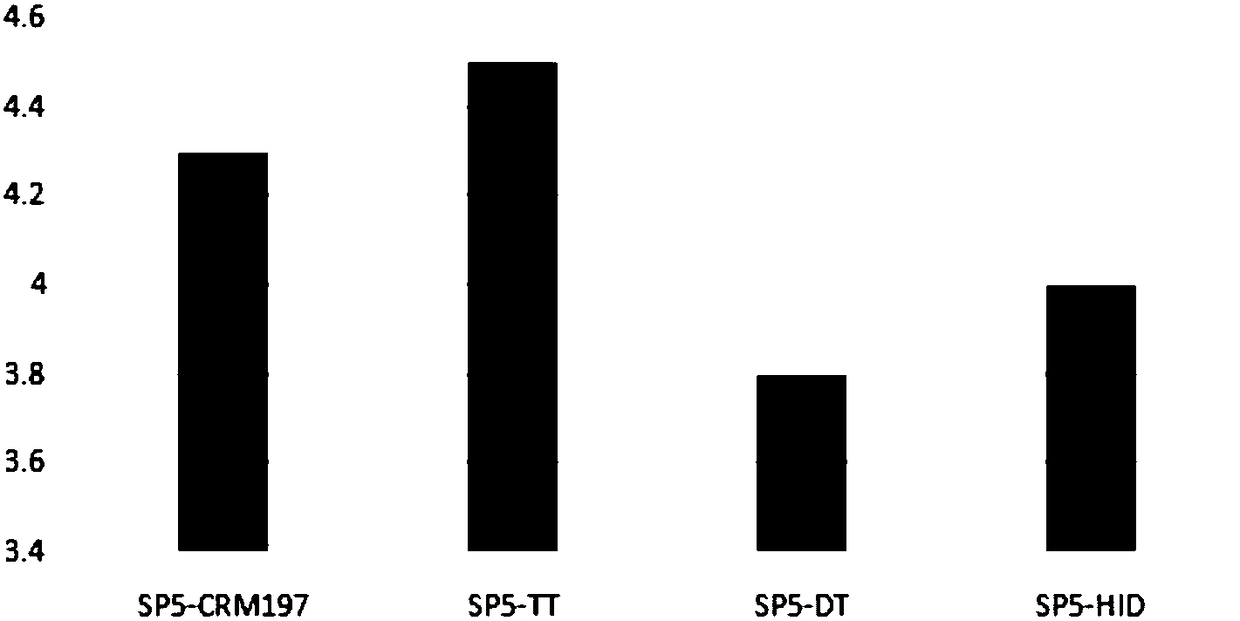
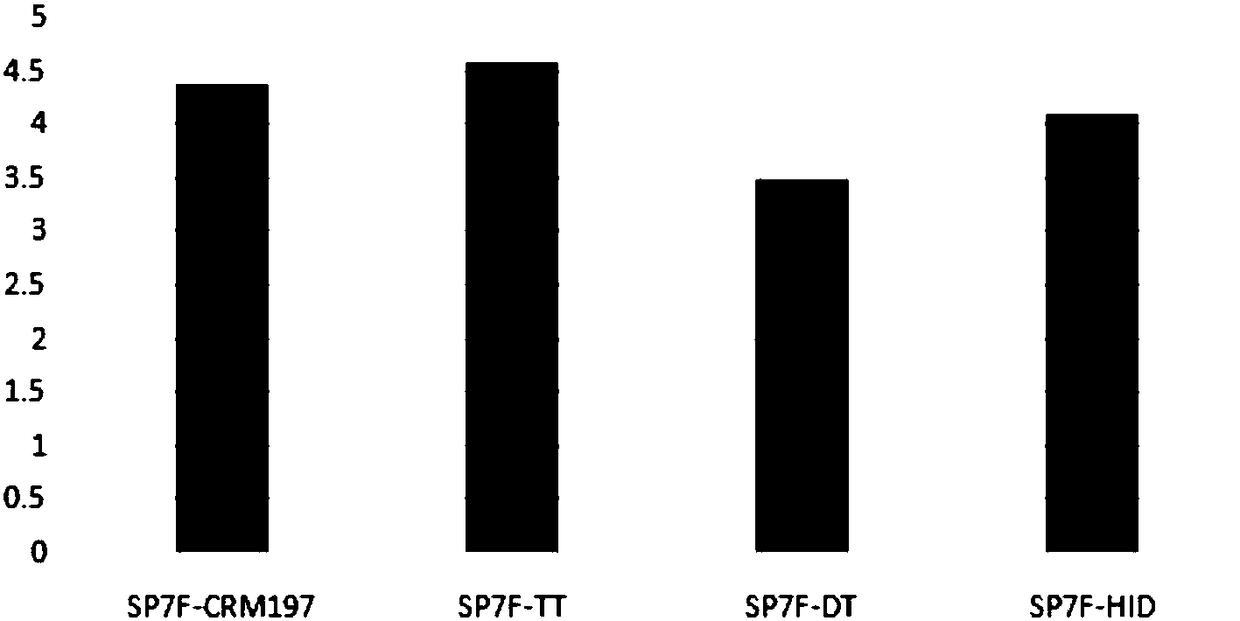
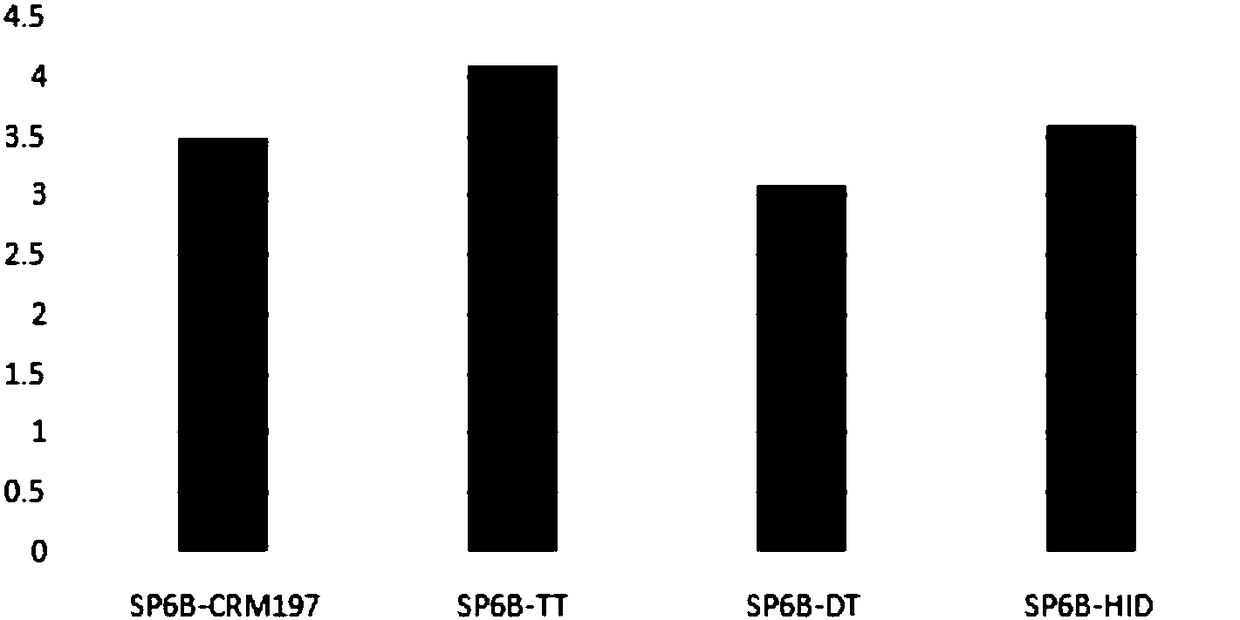
[0102] 1. Immunogenicity of different carrier 13-valent pneumonia conjugate vaccines
[0103] The experimental method of capsular polysaccharide and carrier protein coupling is the same as in Example 1, prepares 13-valent pneumococcal conjugates (Table 6) of different carriers, and measures the immunogenicity in the mouse model (see Figure 5 ).
[0104] Table 6 Summary of carrier protein information for 13-valent conjugates
[0105]
[0106]
[0107] Note: All samples have the same polysaccharide antigen content of the same serotype
[0108] From Figure 5 It can be seen that the immunogenicity of some serotypes has little correlation with the carrier protein, such as SP1, 3, and 18C, but the immunogenicity of some serotypes has a great correlation with the carrier protein. On the whole, the immunogenicity of samples 4 and 5 is better than that of samples 1-3.
[0109] In short, the use of dual-carrier conjugate vaccines reduces the immune interference between diffe...
Embodiment 3
[0118] 1. Comparison of the immunogenicity of the new 13-valent pneumonia conjugate vaccine
[0119] The experimental method of capsular polysaccharide and carrier protein coupling is the same as that of Example 1, and different quantities of dual-carrier conjugate vaccines are prepared. All pneumonia serotypes are divided into two categories, one with CRM197 as the carrier protein, and the other with TT / HID as the carrier protein, choose one of them (SP7F or SP19F), two (SP7F and SP19F) , and all (SP1, SP4, SP5, SP19F, SP7F) coupled with TT or HID, the sample information is shown in Table 8, and the immune mice were studied for immunogenicity (see Figure 7 ).
[0120] Table 8 New 13-valent conjugate vaccine
[0121]
[0122] Remarks: All samples have the same polysaccharide antigen content of the same serotype, and the classification of samples coupled with HID carrier protein is the same as TT
[0123] From Figure 7It can be seen that the immunogenicity of sample 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com