Anti-CD38 nano-antibody, encoding gene and application
A nanobody and gene-encoding technology, applied in the field of biomedicine or biopharmaceuticals, can solve problems such as insufficient activity, difficulty in high-affinity antibodies, and high production costs of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction and Screening of Anti-CD38 Nanobody Phage Display Library
[0039] 1.1 Immunization of dromedary camels: select a healthy adult dromedary, mix the ectodomain of CD38 expressed by yeast at a ratio of 10 μg / Kg and Freund’s adjuvant at a ratio of 1:1, and inject it subcutaneously at multiple points on the back. Vaccines were immunized six times with an interval of 4 weeks. Freund's complete adjuvant was used for the first immunization, and Freund's incomplete adjuvant was used for the remaining several immunizations. After the immunization, 100 mL of peripheral blood from the neck of the dromedary camel was collected for the construction of a phage display library.
[0040] 1.2 Separation of camel-derived lymphocytes: analyze lymphocytes from the collected camel-derived anticoagulated whole blood according to the routine procedures in this technical field, every 3×10 7 Add 1mL Trizol RNA separation solution to each cell, take 2mL for RNA extraction...
Embodiment 2
[0068] Example 2 Expression and Purification of Nanobodies in Escherichia coli
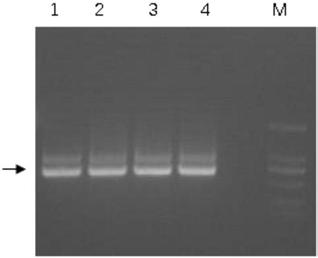
[0069] Select clones with positive phage ELSIA results, extract plasmids and transform into competent cells of strain BL21 DE3, induce nanobody protein expression with 100mM IPTG, collect and break up the bacteria, use Ni-NTA (GE Healthcare) resin for purification, use different concentrations Imidazole was eluted and collected, and the collected samples were subjected to reduced protein electrophoresis analysis. For the results, see Figure 4 , M is the molecular weight marker; 1-5 are nanobody cell disruption solution, penetration solution, low-concentration imidazole elution of impurity protein and elution of target protein, respectively.
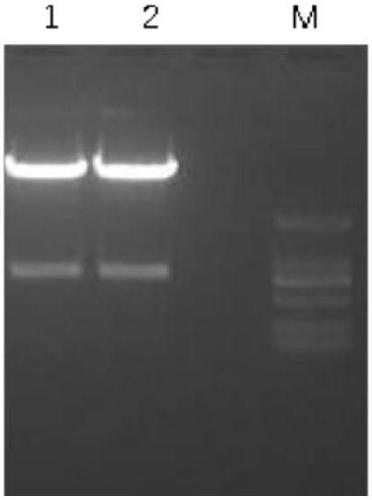
[0070] The recovered target protein was dialyzed into a low-salt solution and subjected to ion exchange chromatography. For the results, see Figure 5 , M is the molecular weight marker; 1-9 are nanobody loading solution, penetration solution, and different...
Embodiment 3
[0072] Example 3 Determination of Nanobody and CD38 Affinity
[0073] Through biofilm interferometry (Octet RED96, PALL Fortebio), we coated the nanobody on the biosensor and measured its affinity with the target antigen CD38, Figure 6 And Table 1 is the measurement result.
[0074] In the present invention, through camel immunization, cell separation, phage library construction, and nanobody screening, a total of one anti-CD38 nanobody was screened out. And analyze the antibody light chain and heavy chain genes to determine the framework regions (framework regions, FR) and complementarity determining regions (complementarity determining regions, CDR) of the variable region, as follows:
[0075] FR1:Gln Val Gln Leu Val Glu Ser Gly Gly Gly Ser Val Gln Ala Gly GlySer Leu Arg Leu Ser Cys Ala Ala Ser Gly
[0076] FR2: Met Ala Trp Phe Arg Gln Ala Pro Gly Lys Glu Arg Glu Val Val Ala
[0077] FR3: Tyr Ala Asp Ser Val Lys Gly Arg Phe Thr Ile Ser Arg Asp Asn AlaGlu Asn Thr Val Tyr ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com