Drug-loaded polymer vesicle with asymmetric membrane structure, preparation method and application in preparation of drug for treating acute myeloid leukemia
A kind of drug-loaded polymer and polymer technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of acute myeloid leukemia not making great progress and avoid losses And toxic and side effects, ensure long cycle, efficient and stable loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
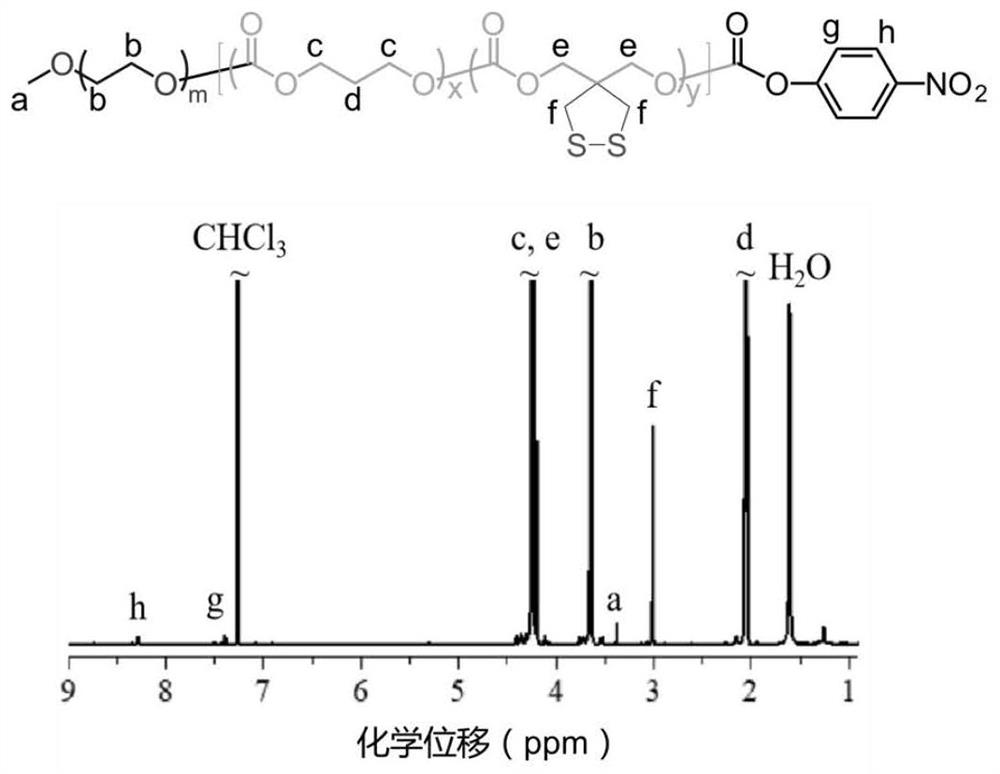
[0065] Example 1 Synthesis of amphiphilic triblock polymer
[0066] Firstly, amphiphilic block polymers PEG-P(TMC-DTC), PEG-P(CL-DTC) and PEG-P(LA-DTC) were synthesized by ring-opening polymerization. Then, after the terminal hydroxyl groups of the above three amphiphilic block polymers were activated by p-nitrophenyl chloroformate (p-NPC), the amphiphilic triblock polymer was prepared by reacting with PAsp. Specifically, taking the synthesis of PEG-P(TMC-DTC)-PAsp as an example, the synthesis route is as follows:
[0067]
[0068] Wherein, in step (i), the reaction conditions are anhydrous dichloromethane (DCM), pyridine, 25 ºC, 24 hours; in step (ii), the reaction conditions are anhydrous dimethyl sulfoxide (DMSO), PAsp , triethylamine, 30 ºC, 48 hours.
[0069] Concrete synthetic steps are as follows:
[0070]The synthesis of PEG-P(TMC-DTC)-PAsp is divided into two steps, that is, after the terminal hydroxyl group of PEG-P(TMC-DTC) (5.0-(15.0-2.0) kg / mol) is activated...
Embodiment 2
[0071] Example 2 Synthesis of targeted amphiphilic block polymers
[0072] The preparation of targeting amphiphilic block polymer is divided into two steps. Firstly, functionalized amphiphilic block polymers with Mal functional groups and NHS functional groups were synthesized, and then targeted amphiphilic block polymers were obtained by reacting targeting polypeptides with functionalized amphiphilic block polymers. Specifically, take A6-PEG-P (TMC-DTC) as an example. First, Mal-PEG-P(TMC-DTC) (7.5-(14.9-2.1) kg / mol) was synthesized by ring-opening polymerization, and then the Michael addition reaction of the sulfhydryl group of A6 with Mal-PEG-P(TMC-DTC) A6-PEG-P(TMC-DTC) was obtained. Under nitrogen atmosphere, 1 mL of Mal-PEG-P(TMC-DTC) (100 mg, 4.1 µmol) in anhydrous DMSO was added dropwise to 2 mL of A6 (7.47 mg, 8.2 µmol) solution at room temperature for 48 hours. After the reaction, the reaction solution was first dialyzed with DMSO for 36 hours (replace the medium...
Embodiment 3
[0074] Example 3 Preparation of non-targeted drug-loaded polymersomes
[0075] The non-targeted drug-loaded polymersomes were prepared by a solvent displacement method, and encapsulated by the electrostatic interaction between the drug and the PAsp segment in the amphiphilic triblock polymer. Specifically, the amphiphilic triblock polymer is PEG-P(TMC-DTC)-PAsp as an example. Dissolve PEG-P(TMC-DTC)-PAsp in DMSO (40 mg / mL), and inject 100 µL into 900 µL HEPES (pH 6.8, 10 mM) containing small molecule drugs, at 300 rpm After stirring for 3 minutes, the mixture was incubated at 37°C for 8 hours. The non-targeted drug-loaded polymersome cPS-VCR was obtained by dialysis with HEPES (pH 7.4, 10 mM) for 8 hours.
[0076] Among them, the theoretical drug loading of the small molecule drug VCR was set at 4.8-9.1 wt.%, and the study found that the particle size of the obtained cPS-VCR was about 30 nm, and the particle size distribution was about 0.1 (Table 1). The drug-loading capaci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com