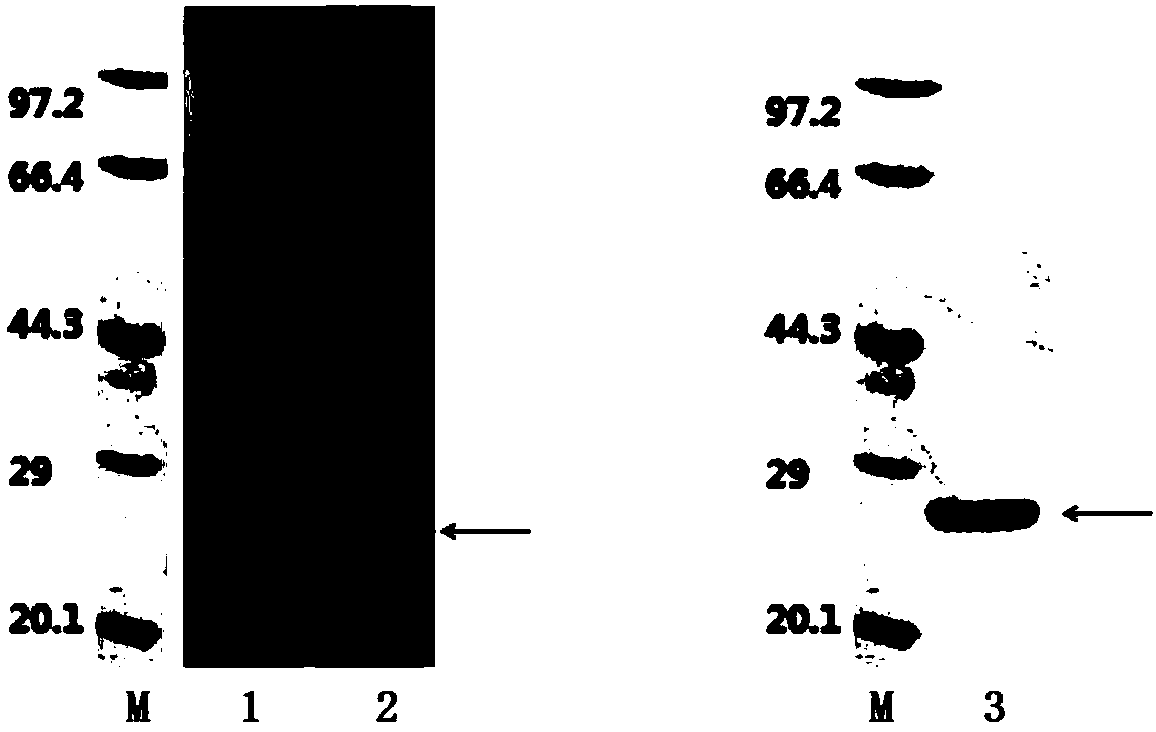
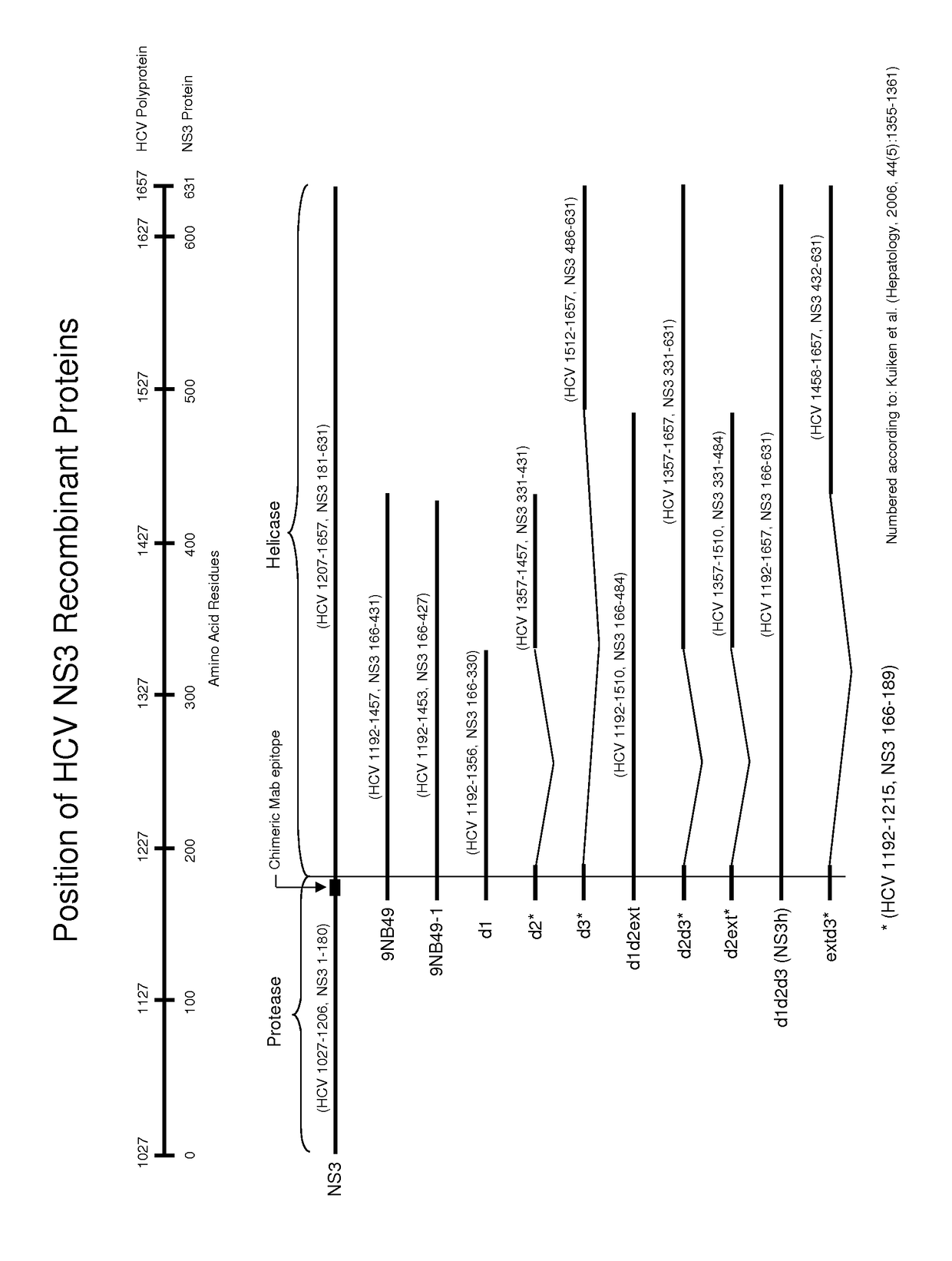
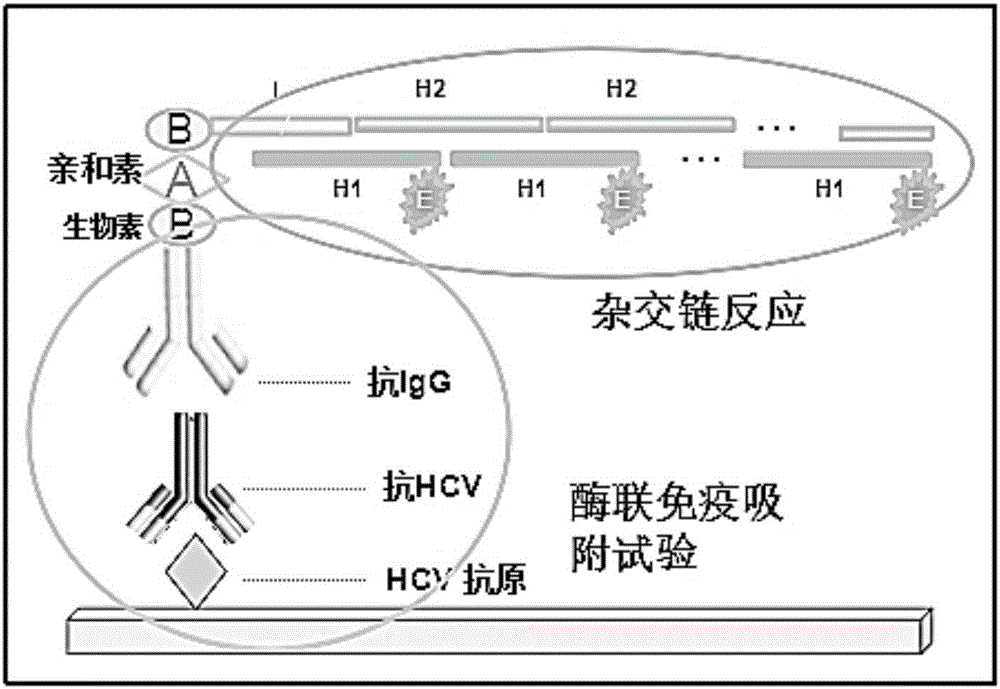
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
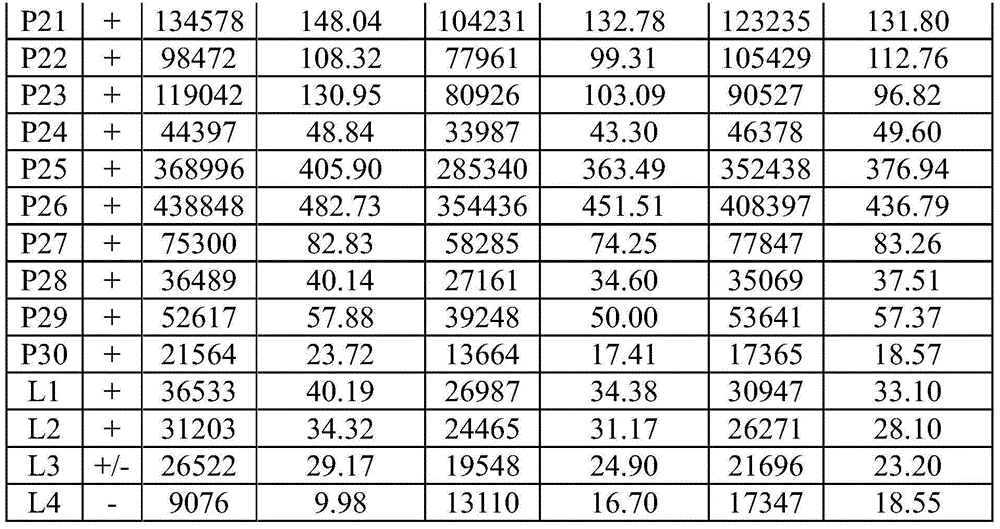
59 results about "HCV Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis C virus antibody quick diagnosis test paper and its preparation method
This invention discloses a kind of immunity chromatography test paper and its preparation method which can diagnose the type-C hepatitis virus fast, the said test paper includes the sampling tray, the gold size tray has labeled HCV mix antigen which links one end of the sampling tray closely, the pyroxylin film which links the other end of the gold size tray closely, and the suction sampling tray which links the other end of the pyroxylin film closely, the film encrusts the test (T) line and the quality control (C) line which are separate with each other, the sampling tray, the gold size tray, the film and the suction sampling tray are affixed in the plastic shoe plate to form the test paper, the said T line is the HCV mix antigen which is entrusted in the NC film, the said gold size tray has HCV recombination mix antigen, the said C line is the antibody of the HCV mix antigen entrusted in the film, it is used to test or clinical diagnosis of the HVC antigen, has the merits that the sensitive is high, the specificity is good, the operation is simple, the reaction is fast, it is fit to test in local and it is economical and useful.
Owner:天津中新科炬生物制药股份有限公司
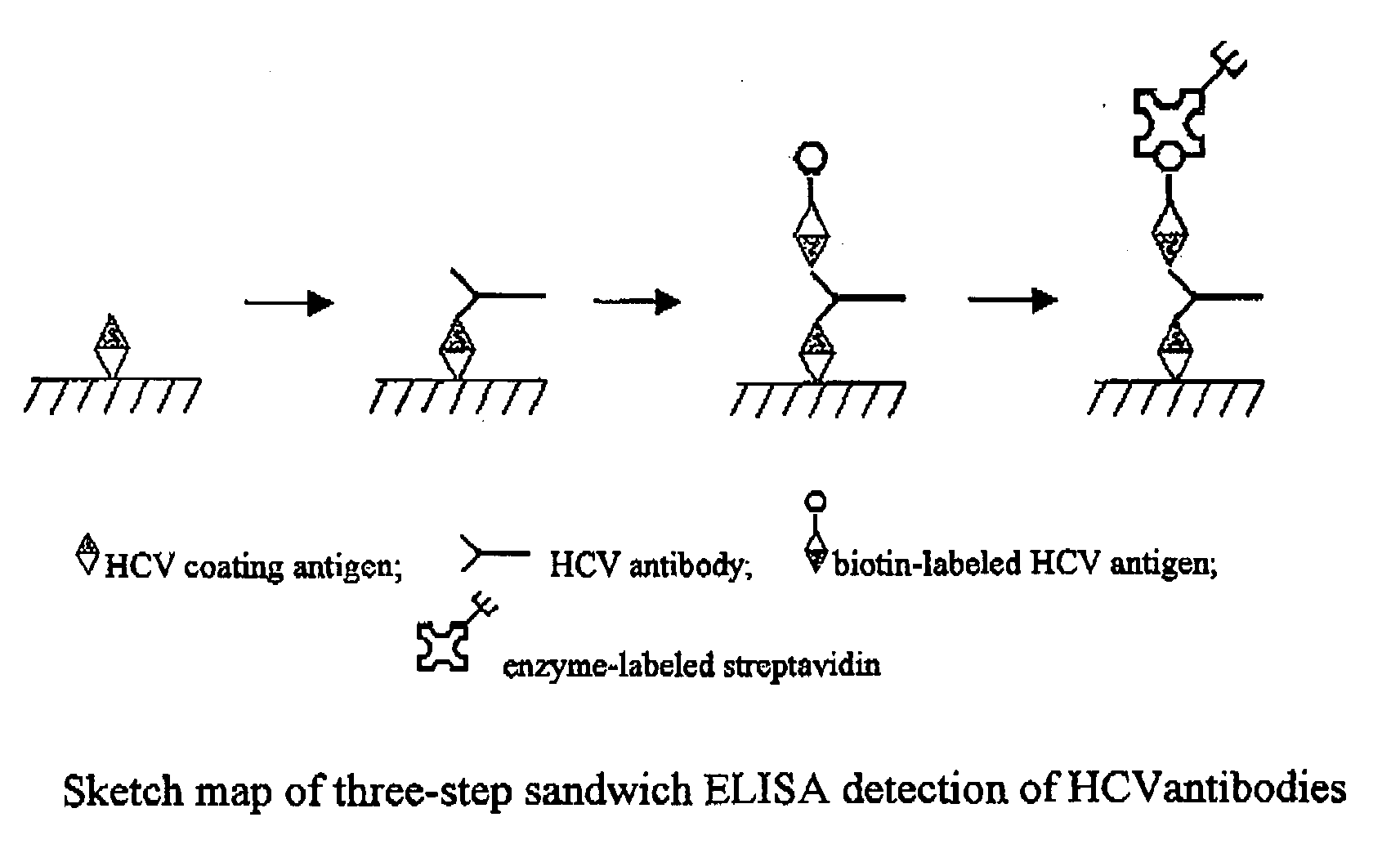
Hepatitis C virus antigen-antibody combined detection method
The invention discloses a method for jointly detecting hepatitis C virus (HCV) antigen-antibody. A monoclonal antibody of an anti-HCV core antigen and a chimeric antigen are coated with enzyme-linked plate to be detected simultaneously, so that the method can shorten the 'window period' of HCV virus detection. Moreover, HCV antibodies in serum and the compatibility with the coated monoclonal antibody can be detected as possible so as to avoid the combination of the chimeric antigen and the monoclonal antibody by modifying HCV overall length core antigen, telescoping non-structural areas and realizing the expression of the chimeric antigen, and the positive detection rate is close to the PCR positive detection rate. The HCV detection method also has the advantages of simple operation, low price, so the method is suitable for promotion and application.
Owner:湖南景达生物工程有限公司
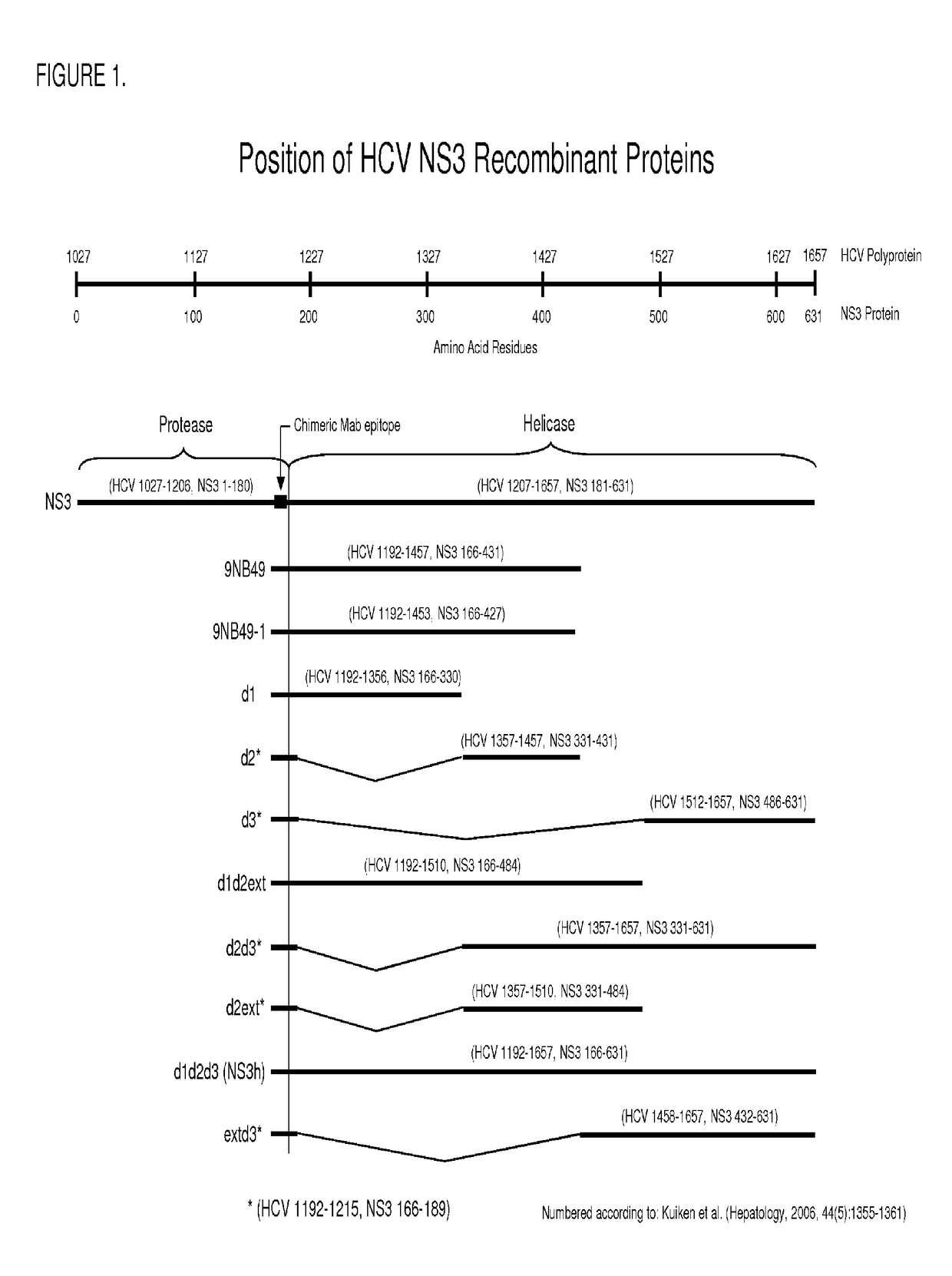
Hcv ns3 recombinant antigens and mutants thereof for improved antibody detection
ActiveUS20140272932A1Excellent redox stabilityImprove stabilitySsRNA viruses positive-senseBacteriaAnti hcv antibodyHCV Antibody
Owner:ABBOTT LAB INC
Hepatitis C virus antibody diagnostic kit and preparation method thereof
ActiveCN102072957AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceSorbentQuality control
The invention belongs to the technical field of immunodiagnosis, in particular relates to a hepatitis C virus (HCV) antibody diagnostic kit adopting a micro particle chemiluminescence method, and a preparation method thereof. The kit consists of anti-HCV detecting magnetic micro particles, an anti-HCV detecting tracer conjugate, anti-HCV sample diluted solution and a quality control material. Theinvention also discloses the preparation method of the diagnostic kit, which adopts micro particle chemiluminescence immunoassay technology, has higher sensitivity and specificity than enzyme-linked immuno sorbent assay (ELISA), is suitable for clinical HCV auxiliary diagnosis and blood donor screening, and makes up the blank of the domestic production of the diagnostic kits for detecting the anti-HCV by the micro particle chemiluminescence method.
Owner:威海威高生物科技有限公司
Kit for detecting hepatitis c virus antibody as well as detection method and application thereof
ActiveCN104697988AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceAntigenMicrosphere
The invention discloses a kit for detecting a hepatitis c virus antibody as well as a detection method and an application thereof, and belongs to the technical field of in vitro diagnosis and detection. The kit consists of the following components: (1) a magnetic microsphere system: including a magnetic microsphere and an HCV antigen in indirect connection by virtue of a first bridged compound; and (2) a marker system: including an HCV fusion antigen and a marking tracer which are in indirect connection by virtue of a second bridged compound, wherein the HCV antigen and the HCV fusion antigen are combined with an HCV antibody on different sites. The kit and the detection method, by detecting the hepatitis c virus antibody through chemiluminescence immunoassay, have the advantages of high sensitivity, good specificity and broad detection scope.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
HCV antigen-antibody combination assay and methods and compositions for use therein
The present invention generally relates to combination immunoassays, reagents and kits for simultaneous detection of HCV antigens and anti-HCV antibodies in a test sample.
Owner:ABBOTT LAB INC
Hepatitis C virus antigen-antibody combined detection kit and detection method
ActiveCN103630690AImprove blood consumptionImprove securityBiological material analysisAntigenTherapy Evaluation
The invention discloses a hepatitis C virus (HCV) antigen-antibody combined detection kit. The kit comprises a microwell plate coated with an HCV chimeric antigen and an HCV monoclonal antibody, a sample diluent, an HCV antigen-antibody combined detection enzyme working fluid, an HCV abzyme working fluid, an HCV antigen enzyme working fluid, a substance A fluid, a substance B fluid, a 20-times concentrated washing fluid and a stopping fluid. The invention also discloses preparation and usage of the key components of the kit, such as the microwell plate of the HCV chimeric antigen and the HCV monoclonal antibody, the sample diluent, and the diluent of enzyme working fluid. The kit and detection method, disclosed by the invention, are able to be used for detecting the HCV antigen and antibody at the same time, or individually detecting the situation of the HCV antigen during the early hepatitis c or the acute infection period, or before the antibody is produced, or when an antigen-antibody compound is produced, or individually detecting the situation of the HCV antibody after the antibody is produced; the kit and detection method can be applied to early HCV detection and therapy evaluation, so as to provide important detection evaluation index for clinical guideline.
Owner:山东莱博生物科技有限公司
Micro-molecule indirectly labeled dual-antigen sandwich method for determining hepatitis C virus total antibody
The invention relates to a method for testing the total antibody of hepatitis C virus (HCV), based on dual-antibody interlayer method of small-molecule indirect mark, wherein said method is characterized in that: it uses small molecule matter as mark; uses dual-antigen interlayer method to test the total antibody of sample; and said method comprises: using small molecule material to mark HCV antigen; using HCV antigen to pack solid material; using single generate material to mark small molecule antibody or other material that combined with small molecule; the HCV antibody of sample can be reacted with HCV antigen of solid material surface and small molecule mark, to form dual-antigen interlayer composite; then reacting the small molecule with single report molecule, to make the composite with detected signal generate material. The invention can keep activity of antigen, with signal amplifying function and better specificity of dual-antigen interlayer mode, while it can detect variable kinds of antibodies, to improve the detecting sensitivity and specificity.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Hepatitis virus type C immune body chemiluminescence method diagnostic reagent kit and its producing method
ActiveCN101196518AWide applicabilityLow costChemiluminescene/bioluminescenceBiological testingAntigenChemiluminescence
The invention relates to a diagnostic reagent kit for testing the hepatitis c virus (HCV) and the preparation and test method, which is to add the HCV recombinant antigen used for peridium into the buffer solution, blend it, move into the luminous microplate, make incubation for 18 hours under 4DEG.C, wash the luminous microplate, add into the confining liquid, leave the liquid after incubation and fully dry the luminous microplate to complete the preparation of the pre-peridium luminous microplate; combine the anti-human IgG used for marking and the horse radish peroxidase by improving the sodium periodate to complete the preparation of the enzyme marker; prepare the chemical luminous substrate solution A with luminal, Tween20 and luminous intensifier and prepare the chemical luminous substrate solution B with the hydrogen peroxide. The reagent kit also comprises the sample diluent and concentrated scrub solution. The negative corresponds to the normal human serum while the positive corresponds to the people with serum of pooled serum with HCV antibody. The reagent kit provided in the invention has much higher detection sensitivity than the ELISA, which is safe and reliable, easy to operate with low cost, and without any expensive full-automatic chemical luminous measuring apparatus required.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Combined detection kit for hepatitis c virus (HCV) antigen-antibody
InactiveCN106093402AImprove specific recognition abilityReduce non-specific bindingMaterial analysisHCV AntibodyHcv core antigen
The invention discloses a combined detection kit for HCV antigen-antibody. The kit comprises a first monoclonal antibody against HCV core, a first HCV recombinant chimeric antigen, a first diluted sample solution, a second diluted sample solution, a first enzyme-labeled second monoclonal antibody against HCV core antigen, a first ligand-labeled second HCV recombinant chimeric antigen, a second enzyme-labeled second ligand, a developer and a stopping solution, wherein the first diluted sample solution comprises a reducing agent, a first surfactant and a first denaturant; and the second diluted sample solution comprises a second surfactant and a second denaturant. The combined detection kit for the HCV antigen-antibody opens mismatched disulfide bonds in coating antigen and in an antigen-antibody complex of a sample in virtue of the first diluted sample solution, and destroys HCV core antigen and HCV antibody compounds in virtue of the second diluted sample solution, so more antigen is released; and thus, detection sensitivity is improved.
Owner:HUNAN KANGRUN PHARMA
Antibody diagnosing reagent kit for detecting HepatitisType C virus, its preparation and method for detection
InactiveCN1670532AHigh detection activitySolve the shortcomings of only detecting IgG antibodiesMaterial analysis by observing effect on chemical indicatorAntigenIodic acid
This invention relates to hepatitis C virus diagnose agent box and its process method, which comprises the following steps: joining HCV regroup antigen to the buffer liquid into the enzyme yoke plate; hatching overnight for buffer liquid washing enzyme yoke plate; then joining buffer liquid and after hatching to abandon hole liquid for total enzyme yoke plate to complete the process of yoke plate; combining the marked HCV regroup antigen and horse radish peroxides enzyme through improved iodic acid method to complete the enzyme marked HCV antigen. The agent box has color liquid A and B, terminal liquid, sample diluent, negative comparing normal serum, positive comparing human serum with HCV antibody.
Owner:北京科卫临床诊断试剂有限公司
Hepatitis c antibodies and uses thereof
InactiveUS20120039846A1Reduce loadReduce in quantityPeptide/protein ingredientsDigestive systemConformational epitopeHCV Antibody
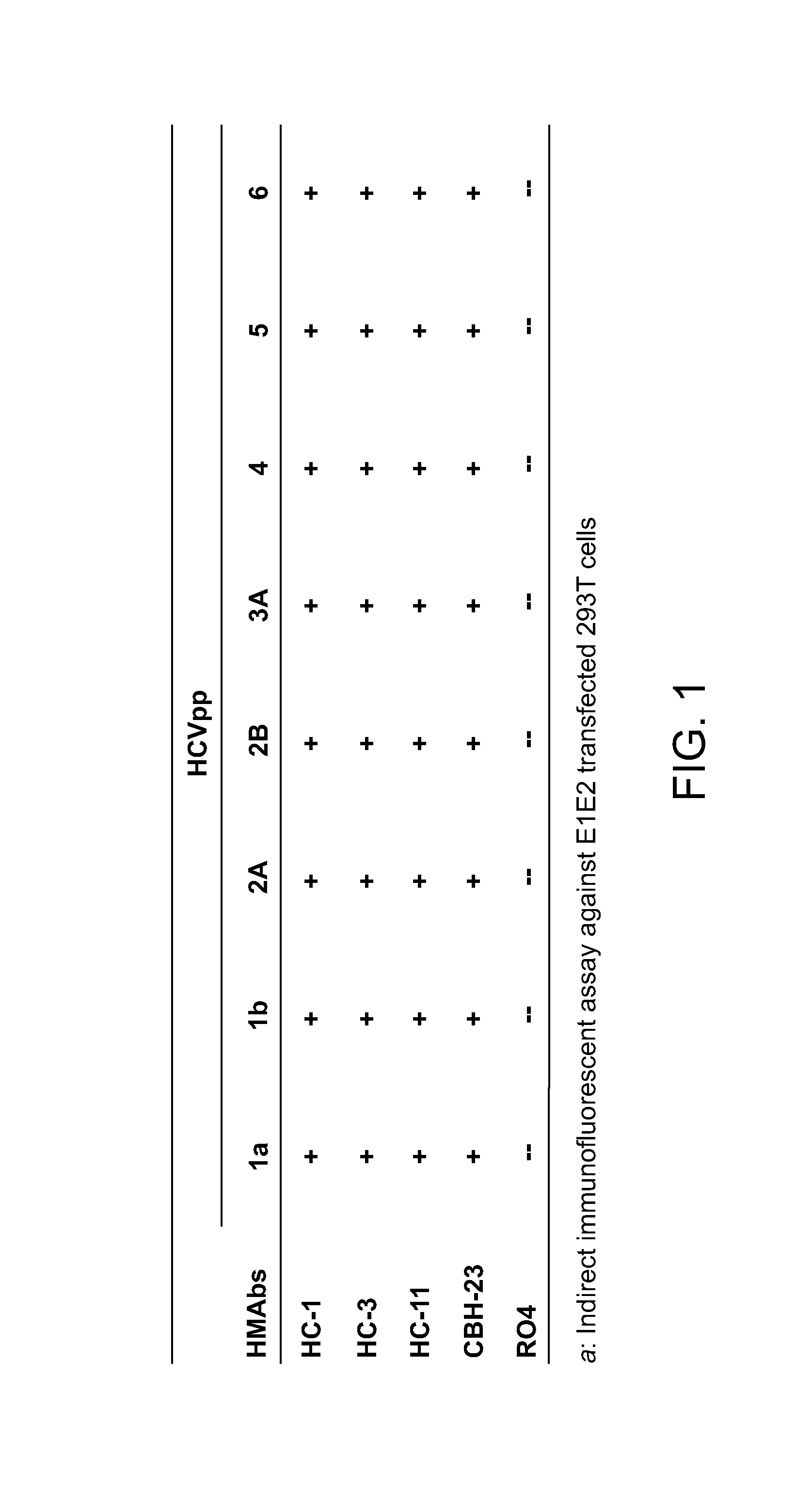
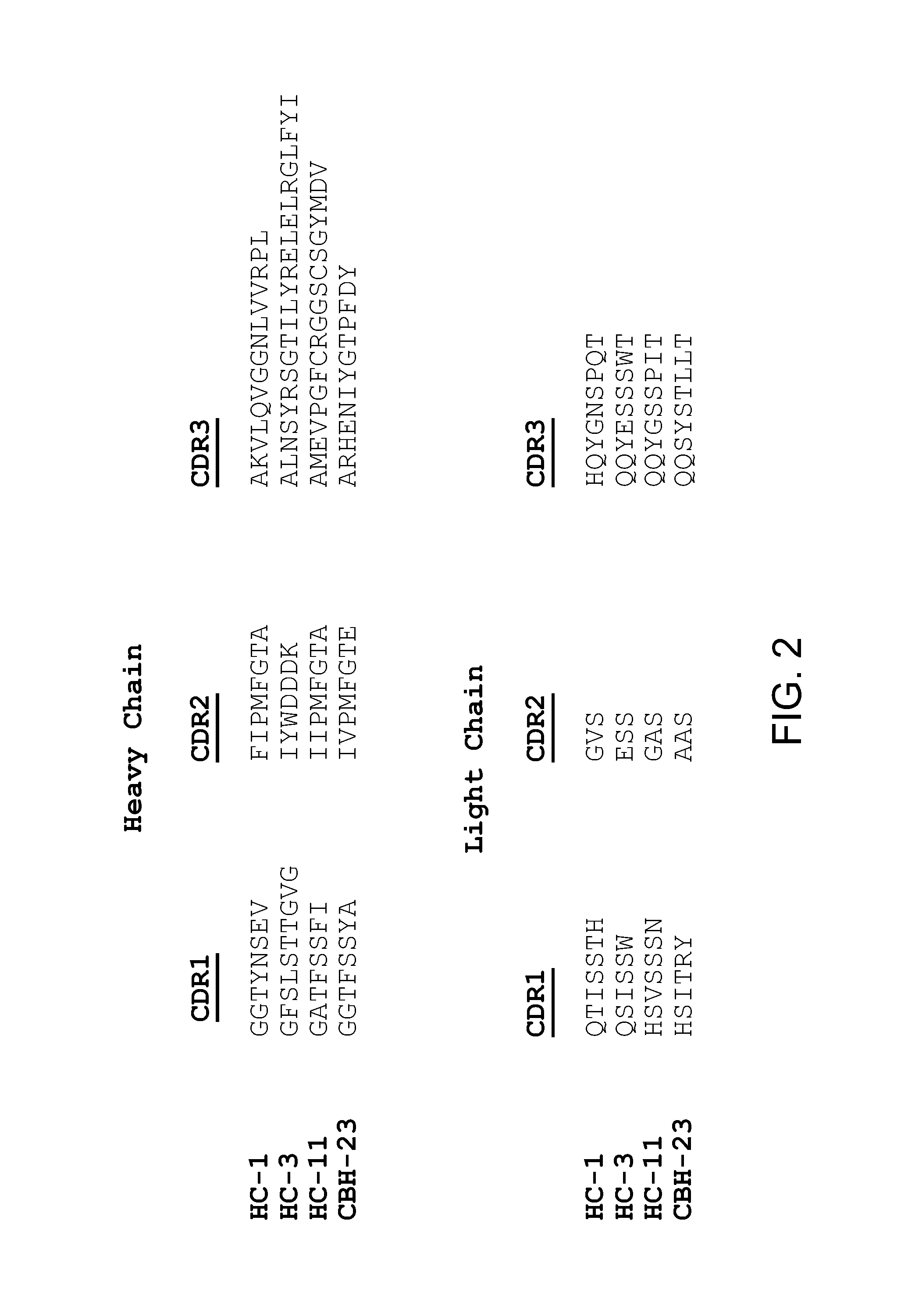
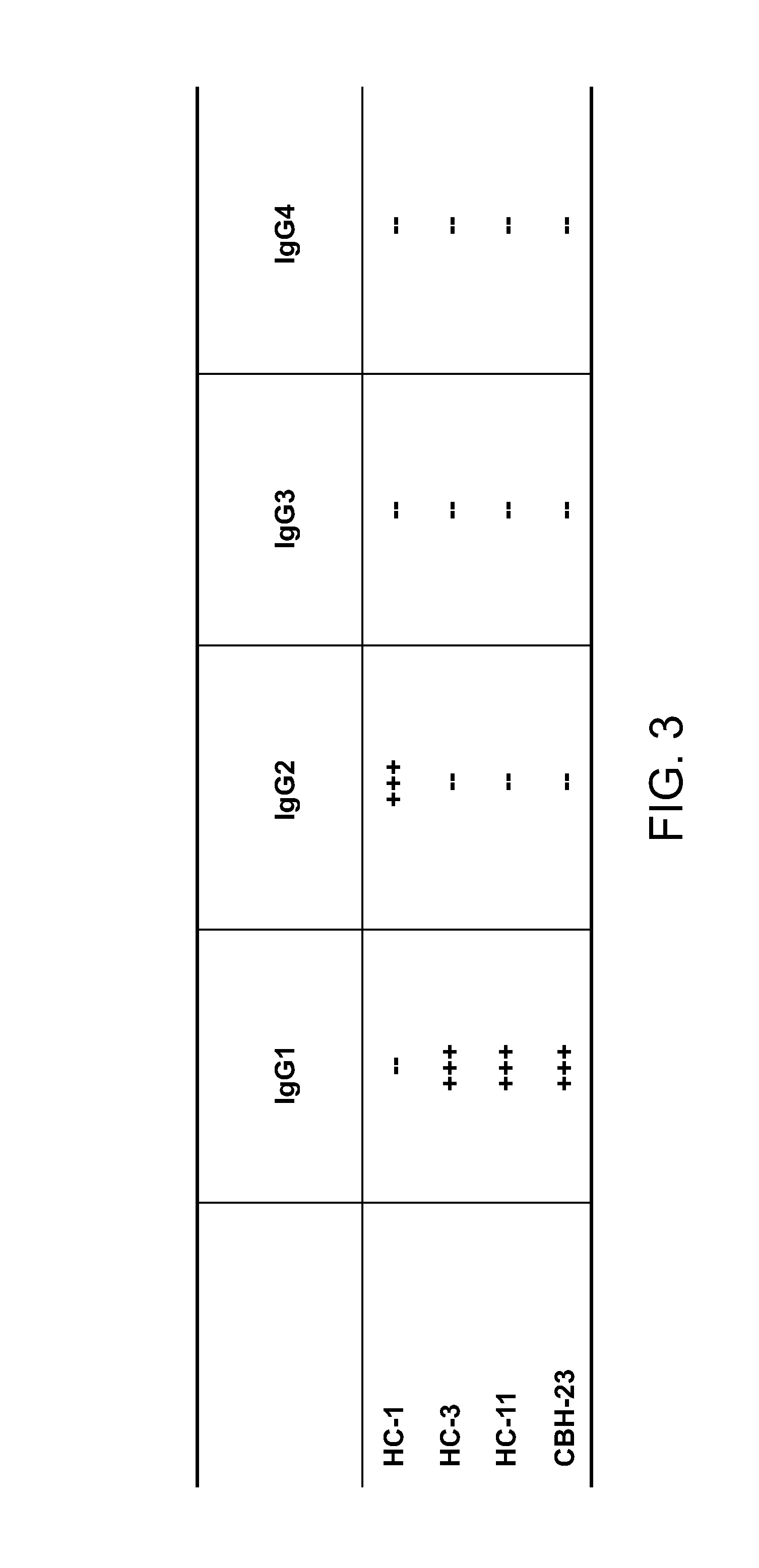
The present invention provides identification and characterization of conformational epitopes of the envelope protein E2 of the Hepatitis C virus (HCV). The present invention provides a panel of human monoclonal antibodies that recognize conformational epitopes of E2. The antibodies are derived from patients infected with HCV. The present invention provides methods for utilizing HCV antibodies as therapeutic, diagnostic, and / or prophylactic agents. The present invention provides mimotopes with conformational epitopes intact and methods of using mimotopes. The present invention provides methods of stratifying patients based on their response to HCV. The present invention provides pharmaceutical compositions for prevention and treatment of HCV comprising one or more HCV antibodies.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
HCV (Hepatitis C Virus) antibody detection kit as well as preparation method and detection method thereof
The invention relates to a HCV (Hepatitis C Virus) antibody detection kit based on a flowing microsphere carrier technology as well as a preparation method and a detection method thereof. The preparation method and the detection method comprise the following steps of: coating activated high molecular polymer microspheres on HCV recombinant antigen, enclosing blank combination points by using bovine serum albumin, and preparing specific HCV probe-high molecular polymer microspheres; co-culturing with a specimen to be tested and capturing HCV antibodies to the tested; washing and centrifugally removing combined HCV antibodies, and then adding fluorescently-labeled anti-human IgG or IgM antibodies; detecting the fluorescence intensity of the microspheres by using a flow cytometry and indirectly carrying out qualitative and quantitative analysis on the tested antibodies. The kit has high sensitivity, good specificity and good stability and can carry out multivalence detections including HCV antibodies simultaneously.
Owner:NANJING UNIV OF TECH
Kit and Method for the Detection of Anti-Hepatitis C Virus (Hcv) Antibodies
The present invention provides a kit for the detection of anti-hepatitis C virus (HCV) antibodies using a double-antigen sandwich technique, said kit carries out the detection by the process ‘solid support-first antigen-HCV antibody to be detected-second antigen-enzyme linked substance-recognizable signal’, wherein one or more coupling reactions between the second antigen and the enzyme linked substance occur, and the diluent for the second antigen and that for the enzyme linked substance are respectively placed into two container, they are added in order in two steps to reaction system and incubated respectively. The present invention can solve the following problems encountered by prior art: 1) the influence of the enzyme linked substance on the activity of the second antigen; 2) the mismatch of disulfide bonds in second antigen, thereby enhance the activity of the second antigen and obviate loss of enzyme activity, therefore, the present kit highly improves the sensitivity and specificity of the detection.
Owner:CUI PENG
HCV/BVDV chimeric genomes and uses thereof
InactiveUS7009044B1SsRNA viruses positive-senseSugar derivativesBovine Viral Diarrhea VirusesNucleic acid sequencing
The present invention relates to molecular approaches to the production of nucleic acid sequences which comprise the genomes of chimeric hepatitis C virus-bovine viral diarrhea viruses (HCV / BVDV). The invention also relates to the use of these chimeric nucleic acid sequences to produce chimeric virions in cells and the use of these chimeric virions in HCV antibody neutralization assays, and for the development of vaccines and therapeutics for HCV.
Owner:UNITED STATES OF AMERICA
Micro-molecule indirectly labeled dual-antigen sandwich method for determining hepatitis C virus total antibody
The invention relates to a method for testing the total antibody of hepatitis C virus (HCV), based on dual-antibody interlayer method of small-molecule indirect mark, wherein said method is characterized in that: it uses small molecule matter as mark; uses dual-antigen interlayer method to test the total antibody of sample; and said method comprises: using small molecule material to mark HCV antigen; using HCV antigen to pack solid material; using single generate material to mark small molecule antibody or other material that combined with small molecule; the HCV antibody of sample can be reacted with HCV antigen of solid material surface and small molecule mark, to form dual-antigen interlayer composite; then reacting the small molecule with single report molecule, to make the composite with detected signal generate material. The invention can keep activity of antigen, with signal amplifying function and better specificity of dual-antigen interlayer mode, while it can detect variable kinds of antibodies, to improve the detecting sensitivity and specificity.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
Method for detecting hepatitis C virus antibody in serum
InactiveCN102305862ARealize quantitative detectionIncreased sensitivityBiological testingAntigenReporter gene
The invention belongs to the field of biotechnology and in particular relates to a method for detecting hepatitis C virus (HCV) antibody in serum, which comprises the following steps of: constructing an HCV gene and reporter gene recombinant plasmid provided with tagged protein gene, introducing the recombinant plasmid into a mammal cell line for expression, purifying fusion protein by using the tagged protein antibody, incubating the fusion protein and the serum, capturing an antigen-antibody complex by using immunomagnetic beads, and adding a reporter gene substrate to detect reporter gene activation level so as to reflect the content of the HCV antibody.
Owner:方辉
Multi-mer peptides derived from hepatitis C virus envelope proteins for diagnostic use and vaccination purposes
Multimer peptides (e.g. 30- to 45-mer peptides) derived from hepatitis C virus envelope proteins reacting with the majority of anti-HCV antibodies present in patient sera are described. The usage of the latter peptides to diagnose, and to vaccinate against, an infection with hepatitis C virus is also disclosed.
Owner:INNOGENETICS NV
Truncated type hepatitis c virus HCV NS3 antigen and preparation method and application thereof
ActiveCN105254724AEfficient developmentHigh positive serum coverageBacteriaVirus peptidesAntigenEscherichia coli
The invention discloses a truncated type hepatitis c virus HCV NS3 antigen which is a nonstructural protein amino acid sequence of the NS3 full-length gene encoded 1252th-1526aath segment in an HCV genome. The amino acid sequence is shown as SEQ ID NO.1, and a corresponding nucleotide sequence is shown as SEQ ID NO.2. A preparation method of HCV NS3 recombinant antigen protein comprises the steps that an optimized escherichia coli codon for expressing the truncated type hepatitis c virus HCV NS3 antigen are established, the nucleotide sequence of the codon is shown as SEQ ID NO.3, the gene segment of the codon undergoes double enzyme digestion and then is connected with an expression vector undergoing the enzyme digestion as well to obtain a recombinant expression plasmid, the antigen can perform expression by adopting a conventional method, and the antigen protein is obtained through purification. The antigen protein has high sensitivity and good specificity and is used for detecting an HCV antibody, and the coincidence rate of a detection result of a patient with positive hepatitis c virus RNA is as high as 97%. It is indicated that the antigen can be applied to polyclonal or monoclonal antibodies and preparation of hepatitis c virus detection kits and has good prospect of clinical application.
Owner:山东莱博生物科技有限公司
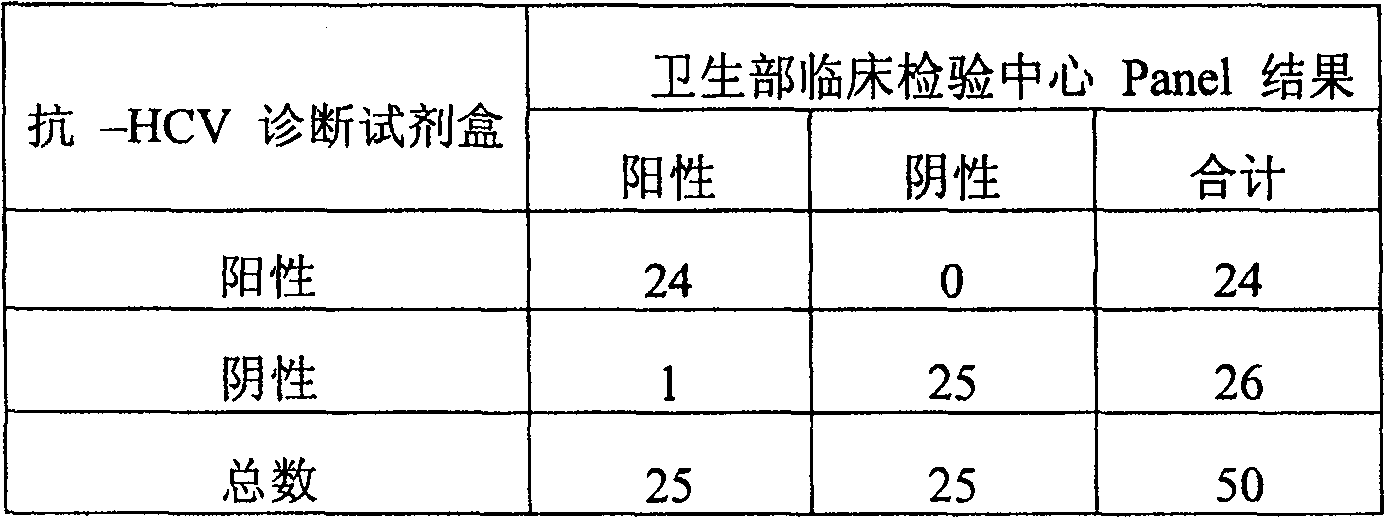
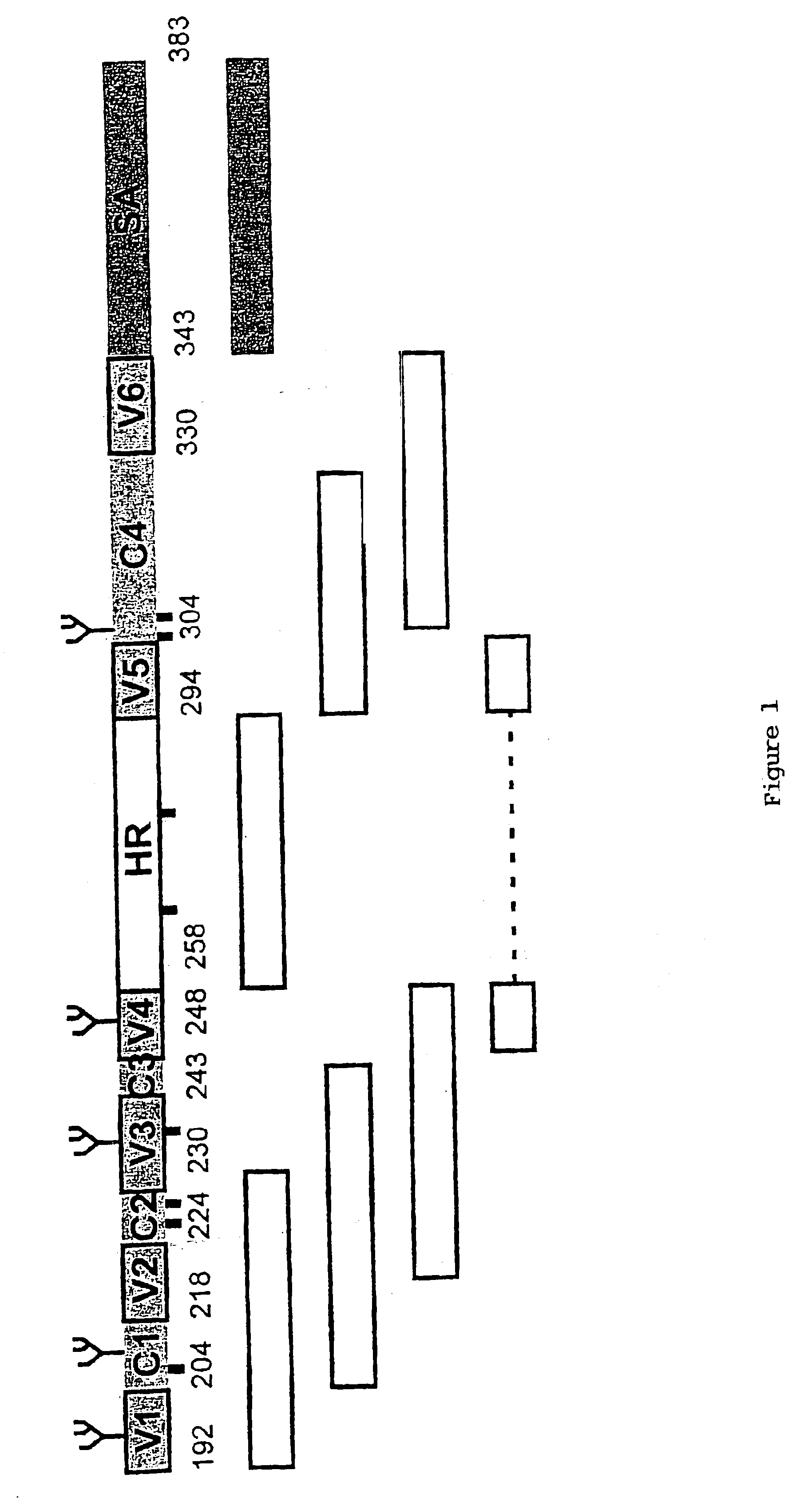
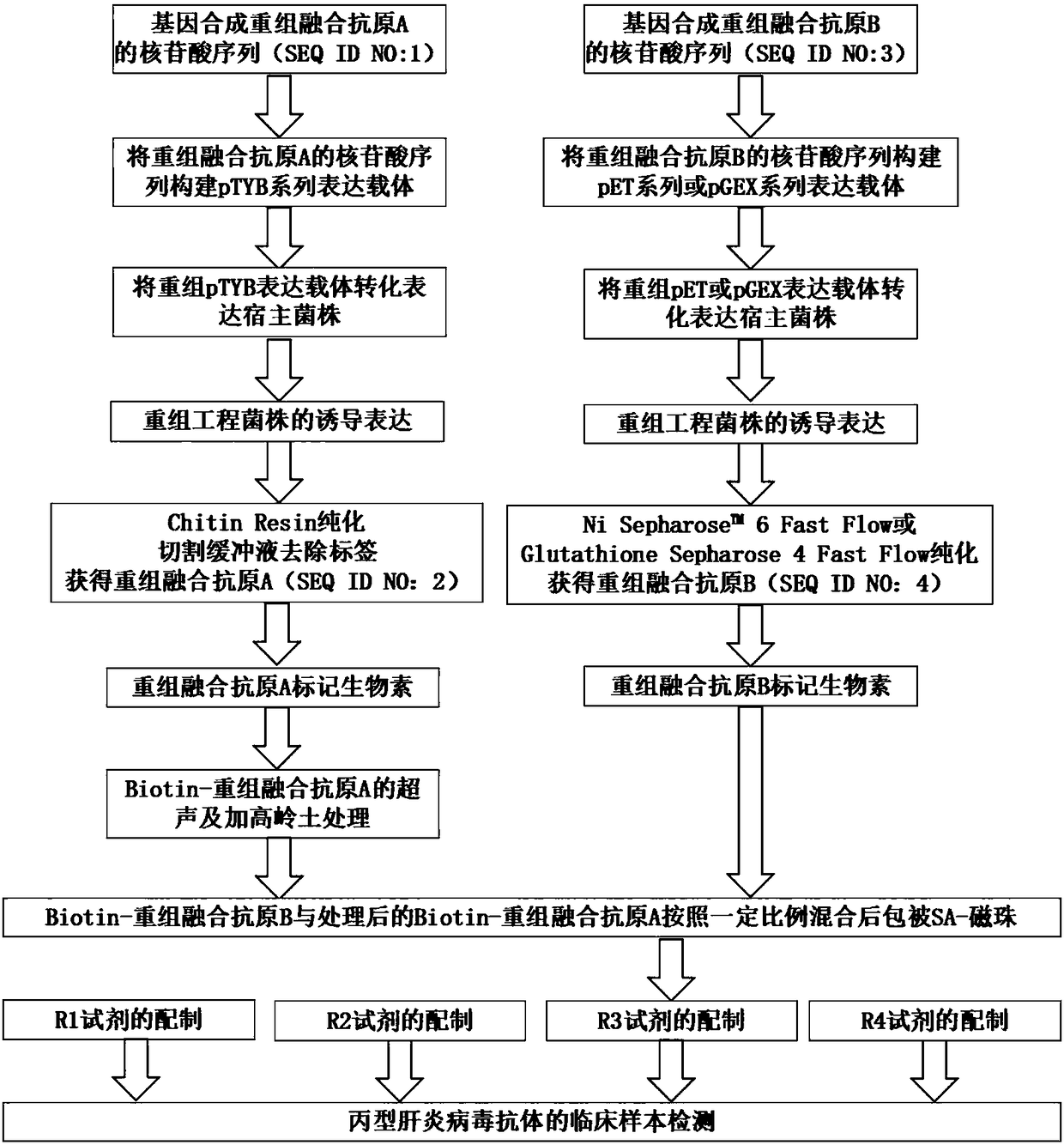
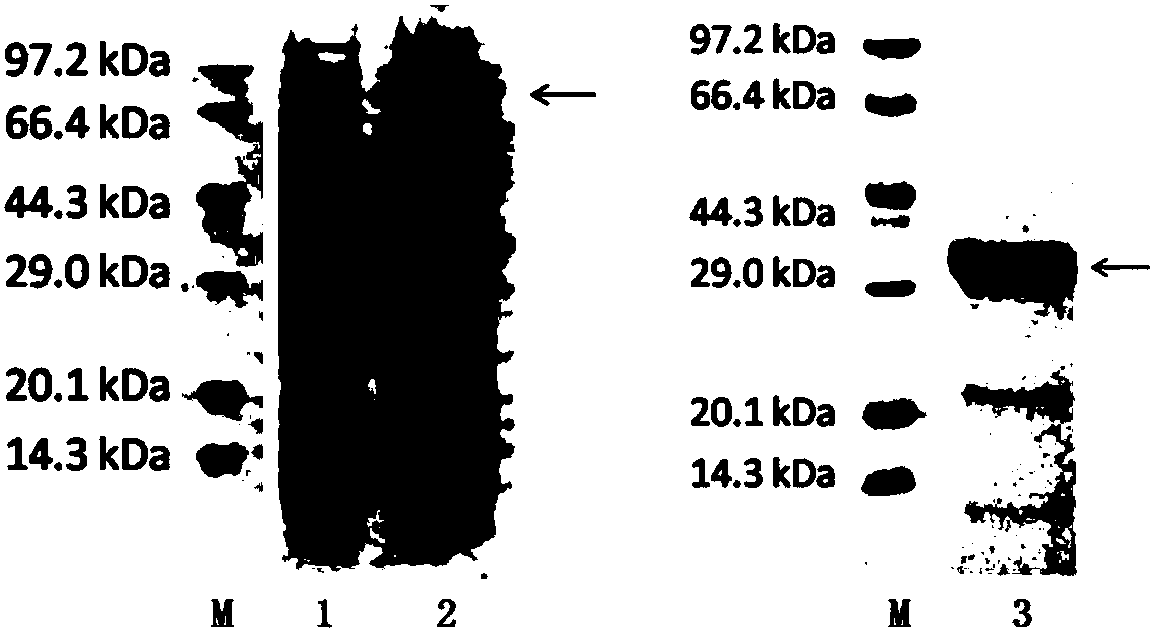
HCV (hepatitis c virus) antibody detection reagent containing recombinant fusion antigens A and B, application and recombinant fusion antigens A and B
ActiveCN108196069AImprove solubilityFold preciselySsRNA viruses positive-senseAntibody mimetics/scaffoldsChylomicronHCV Antibody
The invention relates to an HCV (hepatitis c virus) antibody detection reagent containing recombinant fusion antigens A and B, an application and the recombinant fusion antigens A and B, and belongs to the technical field of bioengineering. According to the HCV antibody detection reagent containing the recombinant fusion antigens A and B, the recombinant fusion antigen A contains an amino acid sequence as shown in SEQ ID NO: 2; the recombinant fusion antigen B contains an amino acid sequence as shown in SEQ ID NO: 4; the detection reagent comprises a reagent R1, a reagent R2, a reagent R3 anda reagent R4. The detection reagent has the following advantages: the two recombinant fusion antigens have the characteristics of high specificity and good stability and can be used for preparing thehepatitis C virus antibody detecting reagent by mixing in a certain ratio, and the clinical detection effect of the reagent is remarkable. Meanwhile, the detection reagent has high anti-interference capacity to chylomicron, bilirubin and hemoglobin, and can greatly meet requirements of clinical diagnosis of HCV.
Owner:深圳德睿生物科技有限公司
HCV NS3 recombinant antigens and mutants thereof for improved antibody detection
ActiveUS9790478B2Excellent redox stabilityStimulate immune responseSsRNA viruses positive-senseHydrolasesAnti hcv antibodyHCV Antibody
Owner:ABBOTT LAB INC
Detection kit for detecting HCV (Hepatitis C) antibody in saliva and preparation method of detection kit
The invention relates to a detection kit for detecting an HCV (Hepatitis C) antibody in saliva and a preparation method of the detection kit, and belongs to the technical field of biological detection. The detection kit comprises a clamp casing, a saliva pad and a test strip, wherein the test strip comprises a base plate, a sample pad, a gold-labelled pad, an NC membrane and absorbent paper, and the sample pad, the gold-labelled pad, the NC membrane and the absorbent paper are stuck on the base plate in sequence; the gold-labelled pad is coated with a colloidal gold marker; the NC membrane iscoated with an HCV recombinant antigen and a second antibody; the saliva pad and the sample pad are connected; the colloidal gold marker is a mixture of an HCV recombinant antigen-colloidal gold marker and a mouse IgG-colloidal gold marker or a binding protein proteinG-colloidal gold marker. According to the detection kit, saliva is acquired by the saliva pad, conveyed to the sample pad and subjected to immunoreaction with the colloidal gold marker on the gold-labelled pad and the recombinant antigen on the NC membrane by lateral chromatography, and a result visible to naked eyes is displayed.
Owner:江苏维尔生物科技有限公司
Nondiagnostic HCV (hepatitis c virus) antibody immunodetection method and kit
InactiveCN106771174AEnables ultra-sensitive detectionGuaranteed Assay SpecificityMaterial analysisBiotin-streptavidin complexAntigen
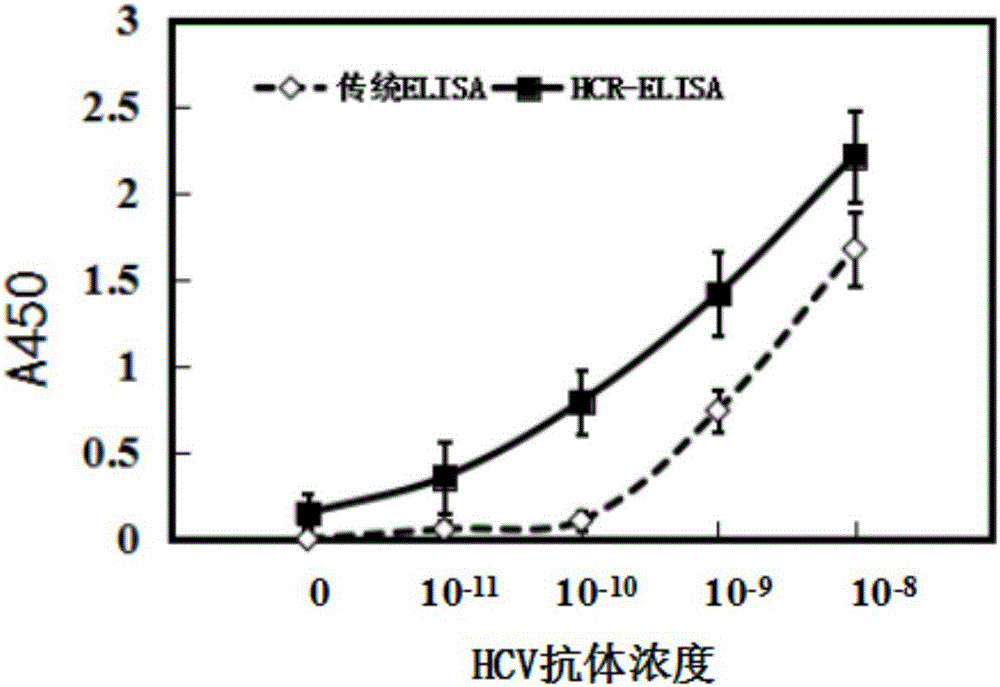
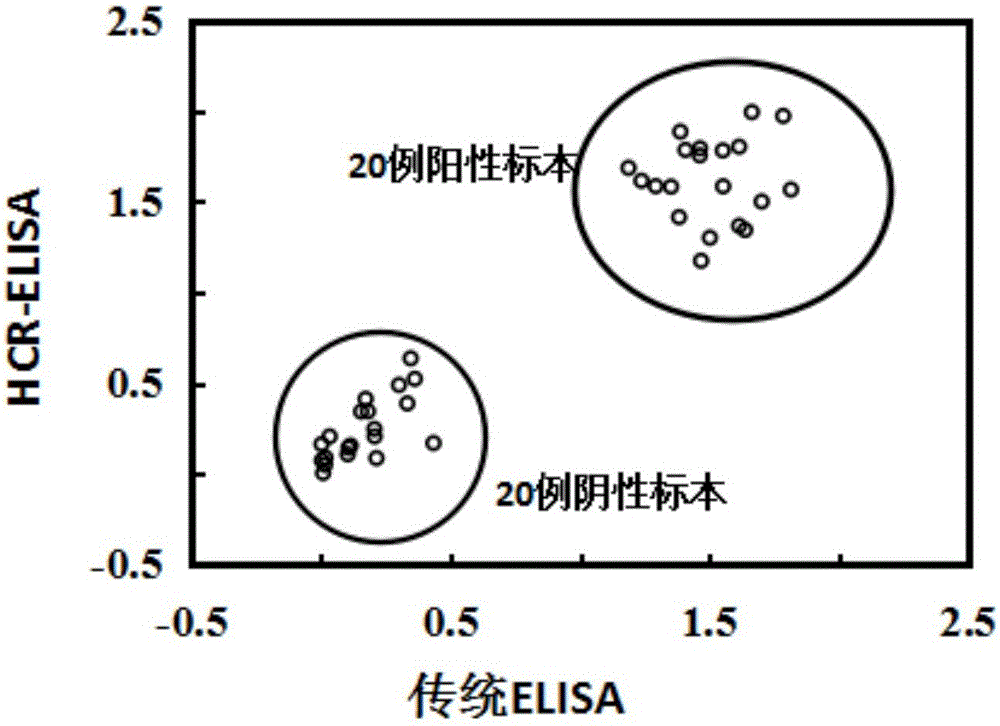
The invention relates to a nondiagnostic HCV (hepatitis c virus) antibody immunodetection method and a kit. The method comprises steps as follows: an HCV antigen specifically captures an HCV antibody in a detection sample, a biotin-labeled second antibody is added, and an antigen-antibody-biotin-labeled second antibody compound is formed after incubation; in the presence of streptavidin, a biotin primer Biotin-I is added, incubation is performed, so that the compound and the Biotin-I are bound, and then, a hair grip chain H1 and a hairpin chain Biotin-H2 are added for a hybridization reaction; avidin-labeled horseradish peroxidase is added, a substrate solution is catalyzed to develop, and quantitative detection of the HCV antibody is performed by measuring the absorbance. ELISA (enzyme-linked immuno sorbent assay) is introduced into an HCR (hybridization chain reaction), HCR is used for signal amplification, ultra-sensitive detection of the HCV antibody is realized, and the lower limit of detection is lower than 10 pg / mL and is improved by at least two orders of magnitude as compared with conventional ELISA.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV
Kit for Detecting the Antibody of HCV and Its Preparing Method
ActiveUS20090029348A1High sensitivityStrong specificityMicrobiological testing/measurementSnake antigen ingredientsAntigenHCV Antibody
Owner:FAPON BIOTECH INC
Hcv antigen-antibody combination assay and methods and compositions for use therein
The present invention generally relates to combination immunoassays, reagents and kits for simultaneous detection of HCV antigens and anti-HCV antibodies in a test sample.
Owner:ABBOTT LAB INC
Subject anti-HCV antibody detection assays employing NS3 capture peptides
The present disclosure provides methods, kits, and compositions for detecting subject anti-HCV antibodies in a sample using NS3 capture peptides. In certain embodiments, at least two NS3 helicase (NS3h) capture peptides and at least two conjugate peptides (e.g., NS3h conjugate peptides) are employed together, which allows for a broad dynamic range of subject antibody detection in a one-step type assay. In other embodiments, methods are provided of detecting NS3-specific subject antibodies without the use of a reducing agent. In some embodiments, NS3-specific subject antibodies are detected with a ‘double shot’ of NS3 conjugate peptide (e.g., conjugate peptide added to a sample both before and after washing).
Owner:ABBOTT LAB INC
Multi sub-gene resistant type HCV (Hepatitis C Virus) antibody gene R3-19 and application thereof
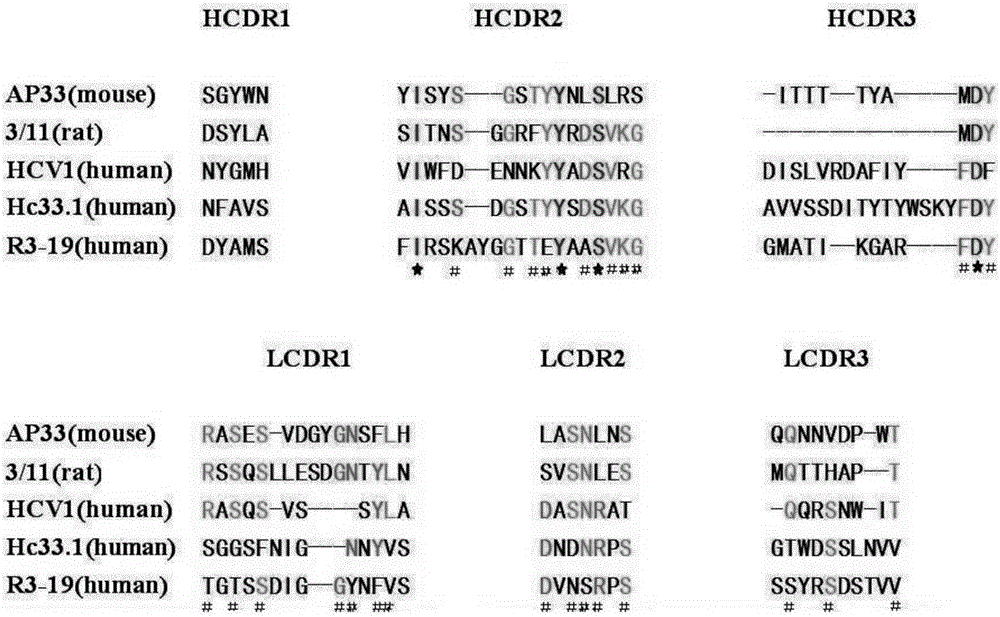
InactiveCN105219780AExtensive featuresBroad neutralization capacityImmunoglobulins against virusesAntiviralsLinear epitopeScFv Antibodies
The invention discloses a multi sub-gene resistant type HCV (Hepatitis C Virus) antibody gene R3-19, wherein the heavy chain amino acid sequence thereof is shown by SEQ ID NO.38, and the light chain amino acid sequence is shown by SEQ ID NO.39, the antibody gene is obtained by vanning in an independently-constructed anti-HCV scFv antibody library through E2 region aa412-423 linear epitope (an HCV CD81 receptor binding epitope) synthetic peptide which is highly conservative in various subtype HCVs; the antibody library is formed by 39 parts of anti-HCV antibody positive peripheral blood samples of Chinese patients, the HCVs infected by the patients cover the main HCV popular subtypes in China, therefore, the R3-19 is an anti-HCV antibody which is typical in China and has wide binding characteristic, and has excellent application value in the fields of HCV diagnosis, HCV treatment, vaccine research and development and the like in China.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
HCV NS3 recombinant antigens and mutants thereof for improved antibody detection
InactiveUS20170327803A1Excellent redox stabilityStimulate immune responseSsRNA viruses positive-senseHydrolasesAnti hcv antibodyHCV Antibody
Owner:ABBOTT LAB INC
Preparation method of hepatitis c virus antibody detection test paper
The invention discloses a preparation method of a HCV (hepatitis c virus) antibody detection test paper. The preparation method comprises the following steps of using a mixed liquid labeled by HCV labeled monoclonal antibody and HCV labeled antigen to spray gold to a fiber cellulose membrane by membrane dotting and gold spraying equipment, so as to obtain a gold pad; using a HCV-coated antigen detection line coating liquid and a contrast line coating liquid to respectively score on a nitrate cellulose membrane, treating, and adhering with a polyvinyl chloride substrate, so as to obtain the membrane-dotted polyvinyl chloride substrate; assembling a sample pad, the gold pad, the membrane-dotted polyvinyl chloride substrate and water absorbing paper, and cutting into the detection test paper. By adopting the type, the prepared detection test paper has the advantages that on the basis of immune lateral flow chromatography technique, the containing of HCV antibody in a blood sample can be judged by manual operation and naked eye reading, the diagnosis speed is quick, the result is accurate, and the injury to a body of the detected person is avoided.
Owner:SUZHOU WANMUCHUN BIOLOGICAL TECH
Kit for detecting hepatitis C virus antibody, detection method and application thereof
ActiveCN104697988BHigh sensitivityStrong specificityChemiluminescene/bioluminescenceAntigenMicrosphere
The invention discloses a kit for detecting a hepatitis c virus antibody as well as a detection method and an application thereof, and belongs to the technical field of in vitro diagnosis and detection. The kit consists of the following components: (1) a magnetic microsphere system: including a magnetic microsphere and an HCV antigen in indirect connection by virtue of a first bridged compound; and (2) a marker system: including an HCV fusion antigen and a marking tracer which are in indirect connection by virtue of a second bridged compound, wherein the HCV antigen and the HCV fusion antigen are combined with an HCV antibody on different sites. The kit and the detection method, by detecting the hepatitis c virus antibody through chemiluminescence immunoassay, have the advantages of high sensitivity, good specificity and broad detection scope.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com