Hepatitis C virus antigen-antibody combined detection kit and detection method
A combined detection technology for hepatitis C virus, applied in the field of immune detection, can solve problems such as weakened capture ability, high quality control requirements, and errors, and achieve the effects of improving blood use and blood transfusion safety, improving work efficiency, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Microwell Plates of Hepatitis C Virus Chimeric Antigen and Hepatitis C Virus Monoclonal Antibody
[0038] (1) Through the sequence analysis of HCV and the design of chimeras, reconstitute the chimeric antigens of HCV core antigen and other non-structural proteins, and clone monoclonal antibodies with different epitopes, so that the two cannot combine with each other when they are coated with antigens and antibodies at the same time , to avoid affecting the combination of the corresponding HCV antibody and HCV-cAg in the sample to be tested. After sequence analysis and chimera design, the preparation methods of antigens and monoclonal antibodies were operated according to the conventional methods in "Molecular Cloning".
[0039] A. Antigen preparation and purification identification
[0040] ① Extract the total RNA of HCV virus;
[0041] ② RT-PCR amplified and cloned the corresponding gene fragments of NS-3, NS-4 and Core;
[0042] ③ cloned in...
Embodiment 2
[0069] Embodiment 2 Preparation of sample diluent
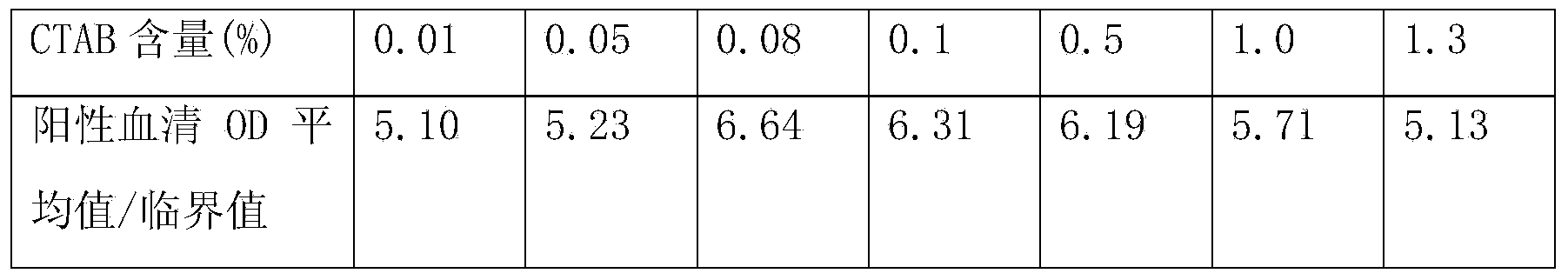
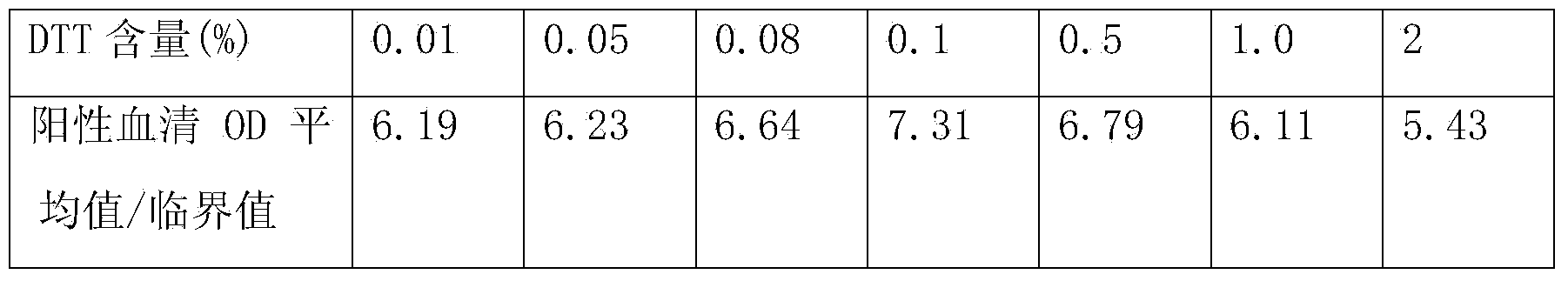
[0070] The content of hepatitis C antibody is high in the blood, and the core region antigen used for detection of hepatitis C antibody has strong homologous and heterologous binding ability in vivo or in vitro, which can easily cause high background or even false positive, so it is used to detect hepatitis C antibody The required sample size is very small. Currently, the sample size of common hepatitis C antibody detection kits is generally 10ul. However, the content of hepatitis C antigen in the blood is very small, and the detection sample requires a lot of sample size. Currently, the commercialized hepatitis C antigen The sample volume requirement in the test kit is 100ul. Therefore, there is a certain contradiction in the sample size requirements when the two are tested at the same time.
[0071] In the present invention, by adding protein sedimentation agent, reducing agent, detergent, etc. to the sample diluent, the h...
Embodiment 3
[0090] Example 3 Preparation of Enzyme Working Solution and Detection Operation Process
[0091] (1) Preparation of enzyme-labeled protein: take an appropriate amount of horseradish peroxidase (according to 1mg protein plus 1mg HRP), dissolve 5mg HRP in 1.0ml NaHCO 3 solution to fully dissolve the HRP. Add appropriate amount of 0.06M NaIO 4 Stir at room temperature in the dark for 30 minutes. Transfer to a dialysis bag and dialyze overnight with 0.01M pH9.6 carbonate buffer. Take an appropriate amount of purified protein to be labeled, and use 0.01M NaHCO 3 The solution was fully dissolved, mixed with the enzyme solution, put into a dialysis bag, and dialyzed with 0.01M pH9.6 carbonate buffer solution at 4°C in the dark for 20 hours, and the medium was changed 3 times in the middle. Take the combined solution after dialysis, add 0.4% NaBH 4 (Based on the basis of 5mg HRP dissolved in 0.2ml NaBH4 solution) after stirring for 30 minutes, stand at 4°C for 4 hours. The enzym...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com