Hepatitis virus type C immune body chemiluminescence method diagnostic reagent kit and its producing method
A technology of hepatitis C virus and diagnostic kit, which is applied in chemiluminescence/bioluminescence, analysis through chemical reaction of materials, scientific instruments, etc., can solve the problem of inability to effectively and widely apply clinical diagnosis and scientific research work, and limit promotion Use and operation are complicated, etc., to achieve the effect of simple operation, wide applicability and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0043] 1. The present invention detects the hepatitis C virus antibody diagnostic kit component embodiment:
[0044] (1), pre-coated luminescent plate coated with HCV antigen
[0045] (2), enzyme-labeled anti-human IgG conjugate labeled with horseradish peroxidase
[0046] (3), HCV sample diluent containing indicator
[0047] (4), washing solution containing Tween20 phosphate buffer
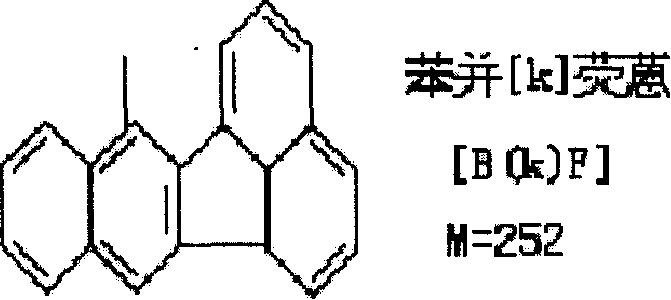
[0048] (5), chemiluminescent substrate solution A containing luminol, Tween20 and its enhancer benzofluoranthene
[0049] (6), chemiluminescent substrate solution B containing hydrogen peroxide
[0050] (7) Negative control for normal human serum
[0051] (8), the positive control that is the newborn bovine serum dilution HCV antibody positive human serum
[0052] 2, the present invention detects the preparation method embodiment of hepatitis C virus antibody diagnostic kit:
[0053] I. Preparation of pre-coated chemiluminescent plates coated with HCV antigens
[0054] Add HCV recombinant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com