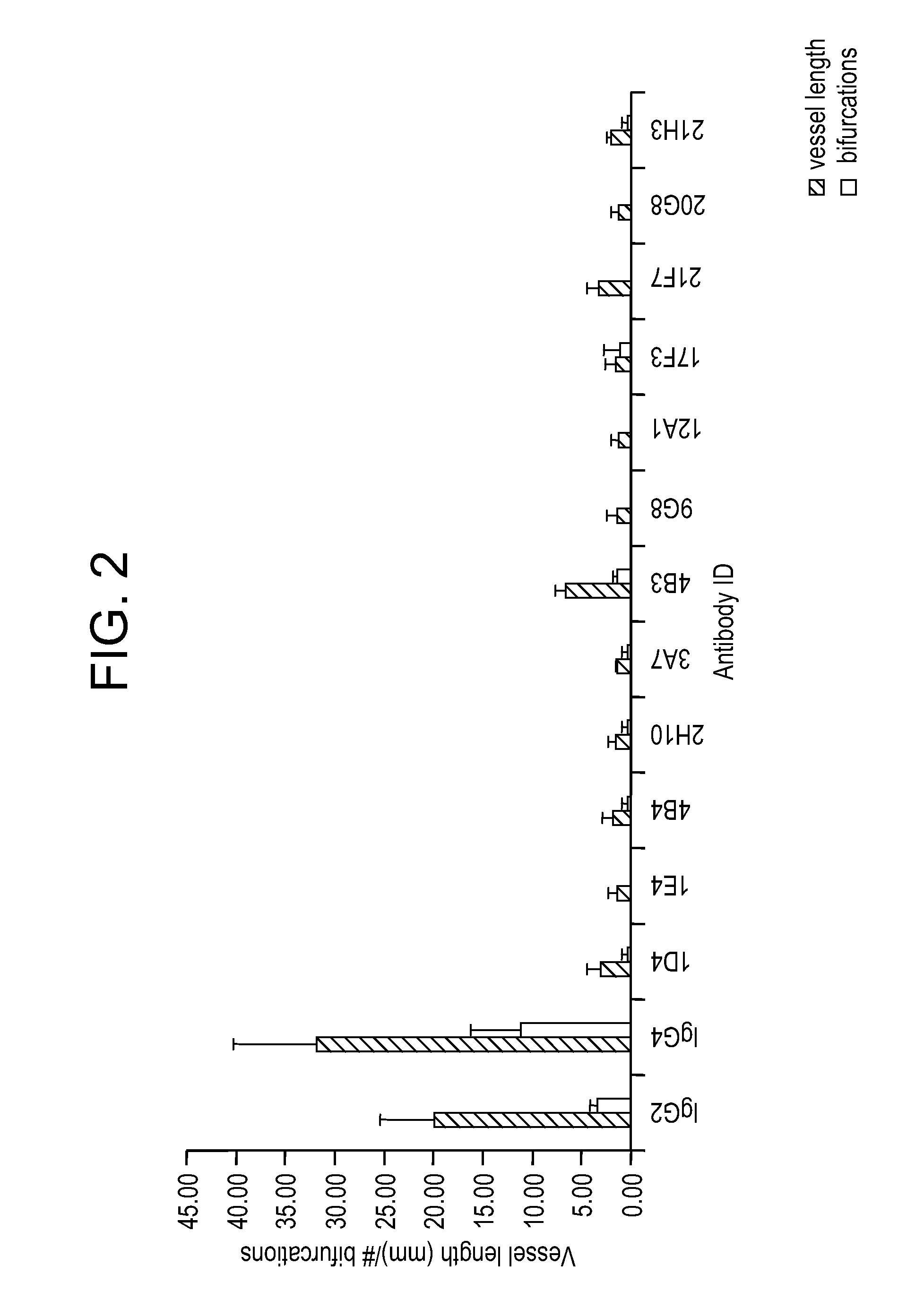
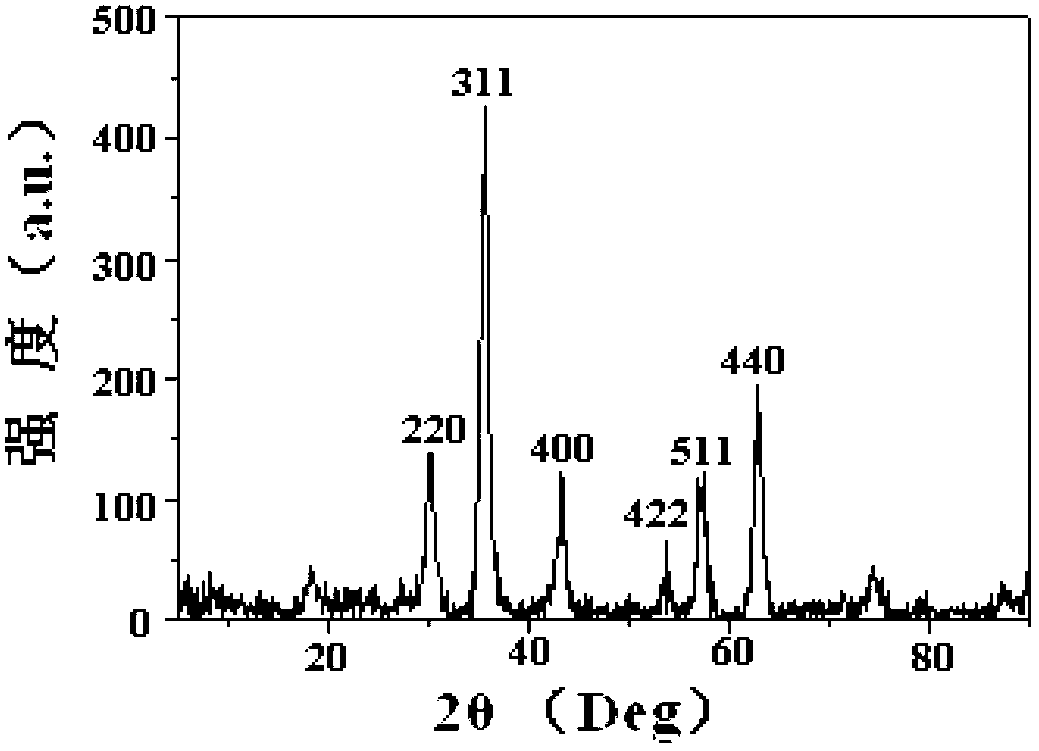
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
754 results about "Monoclonal antibody agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monoclonal antibody: An antibody produced by a single clone of cells. A monoclonal antibody is therefore a single pure type of antibody. Monoclonal antibodies can be made in large quantities in the laboratory and are a cornerstone of immunology. Monoclonal antibodies are increasingly coming into use as therapeutic agents.
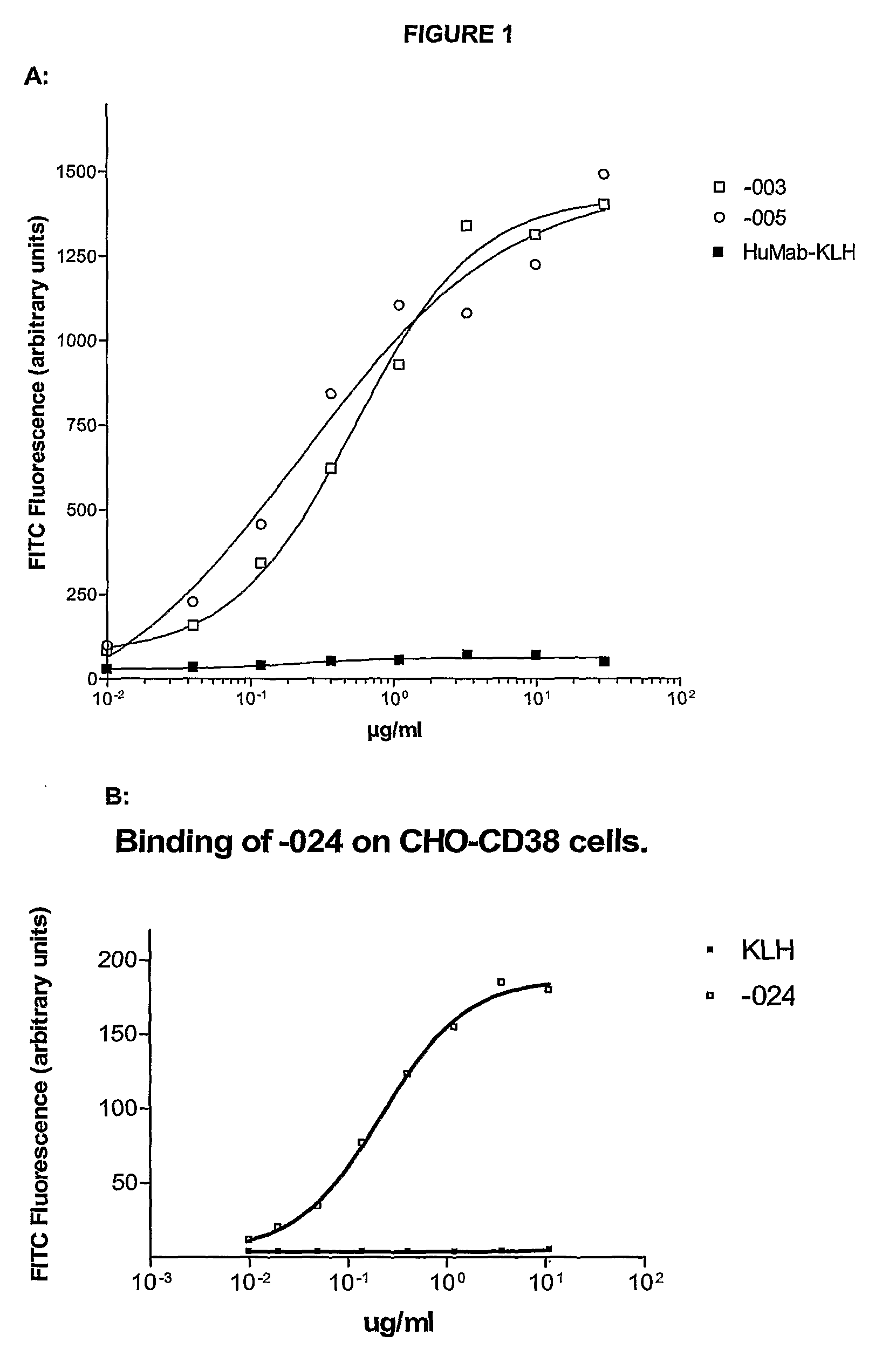
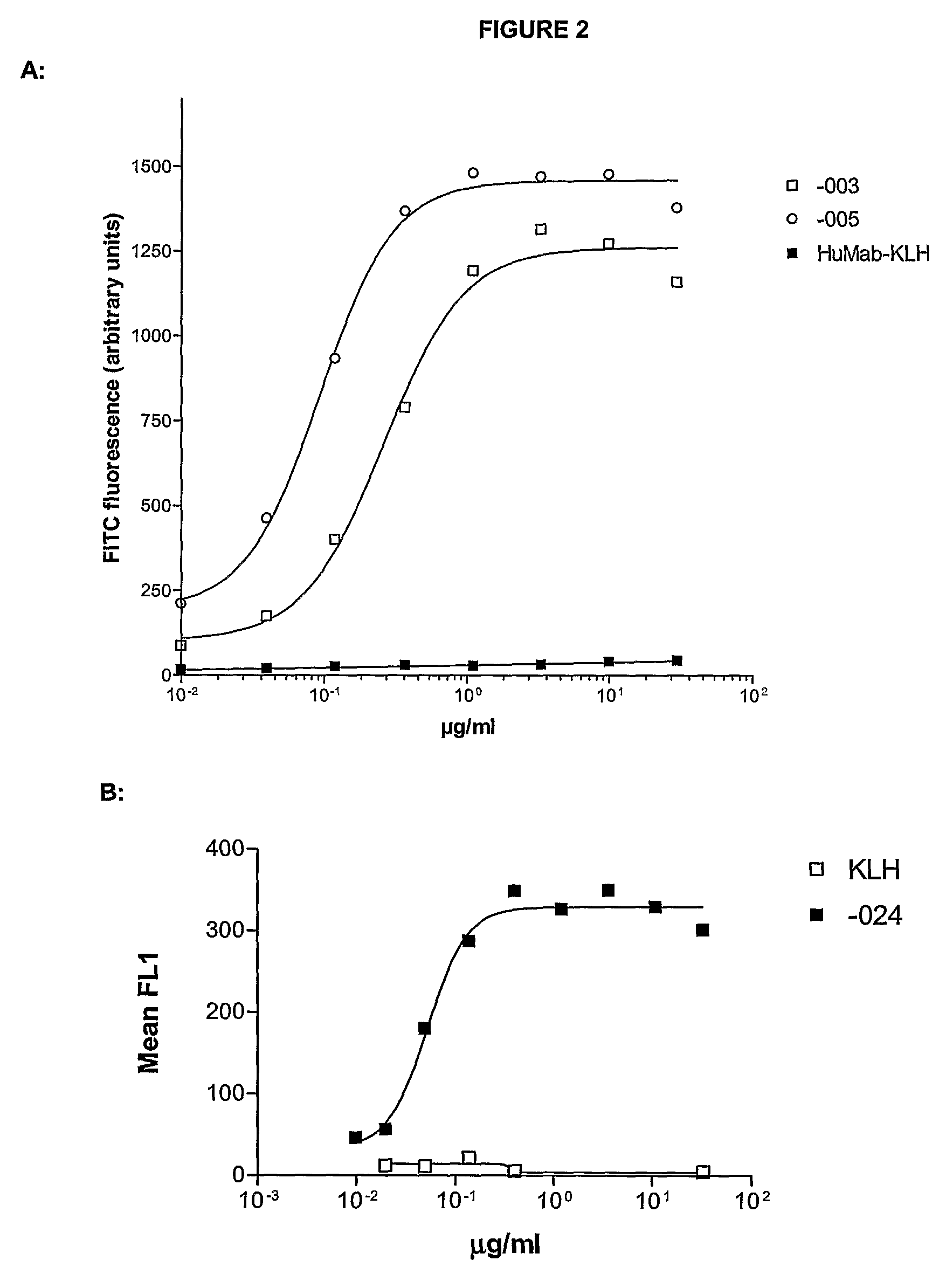
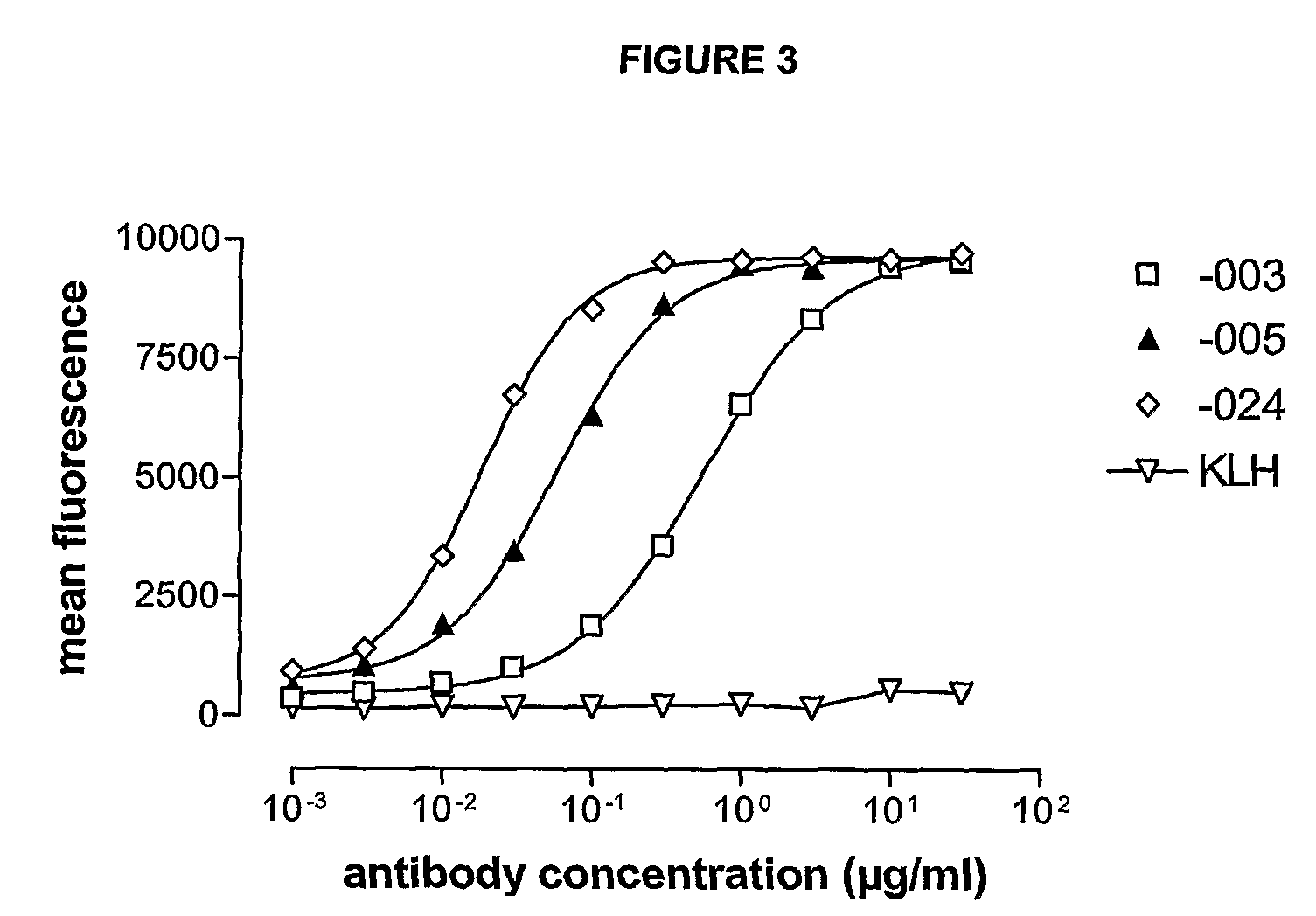
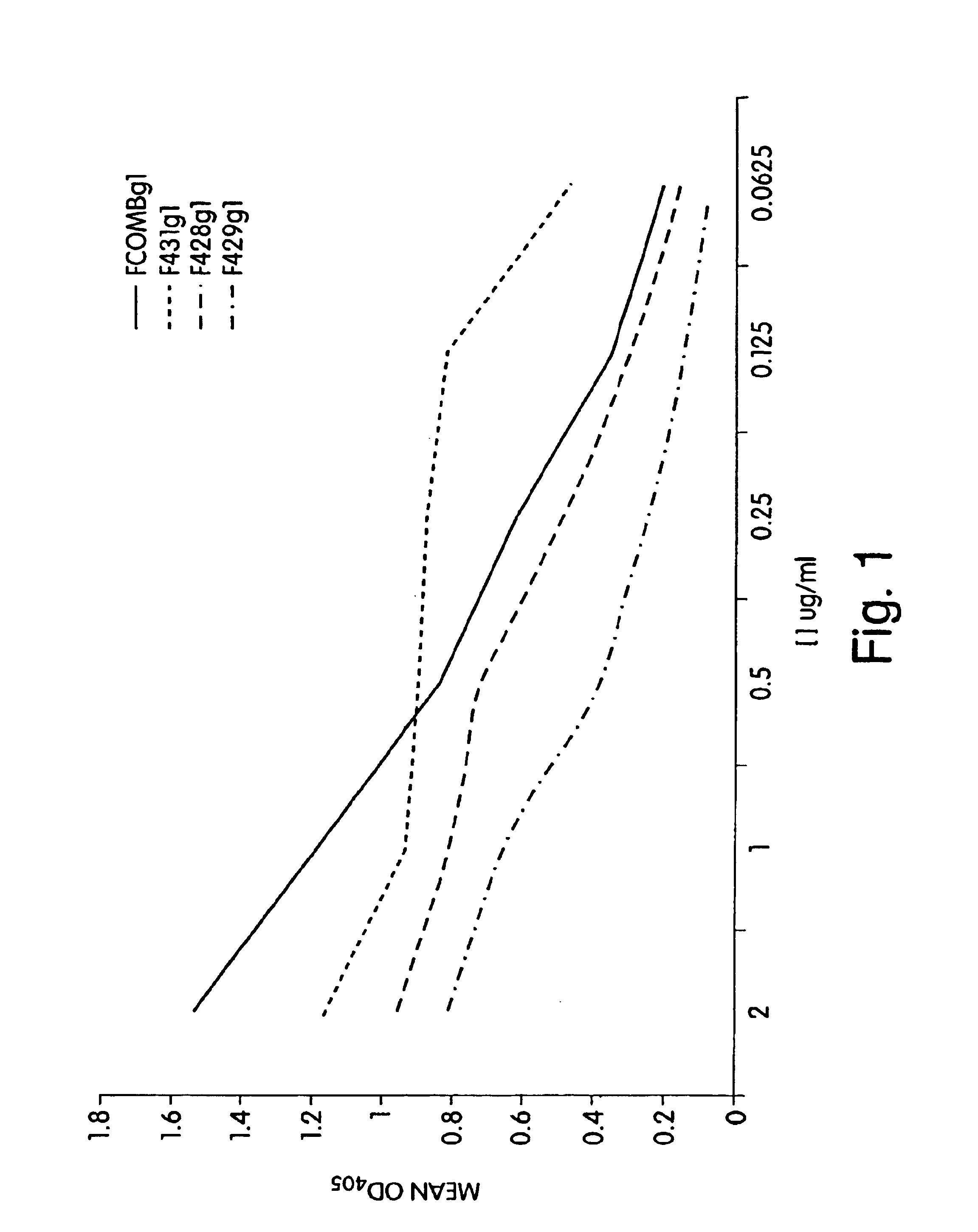
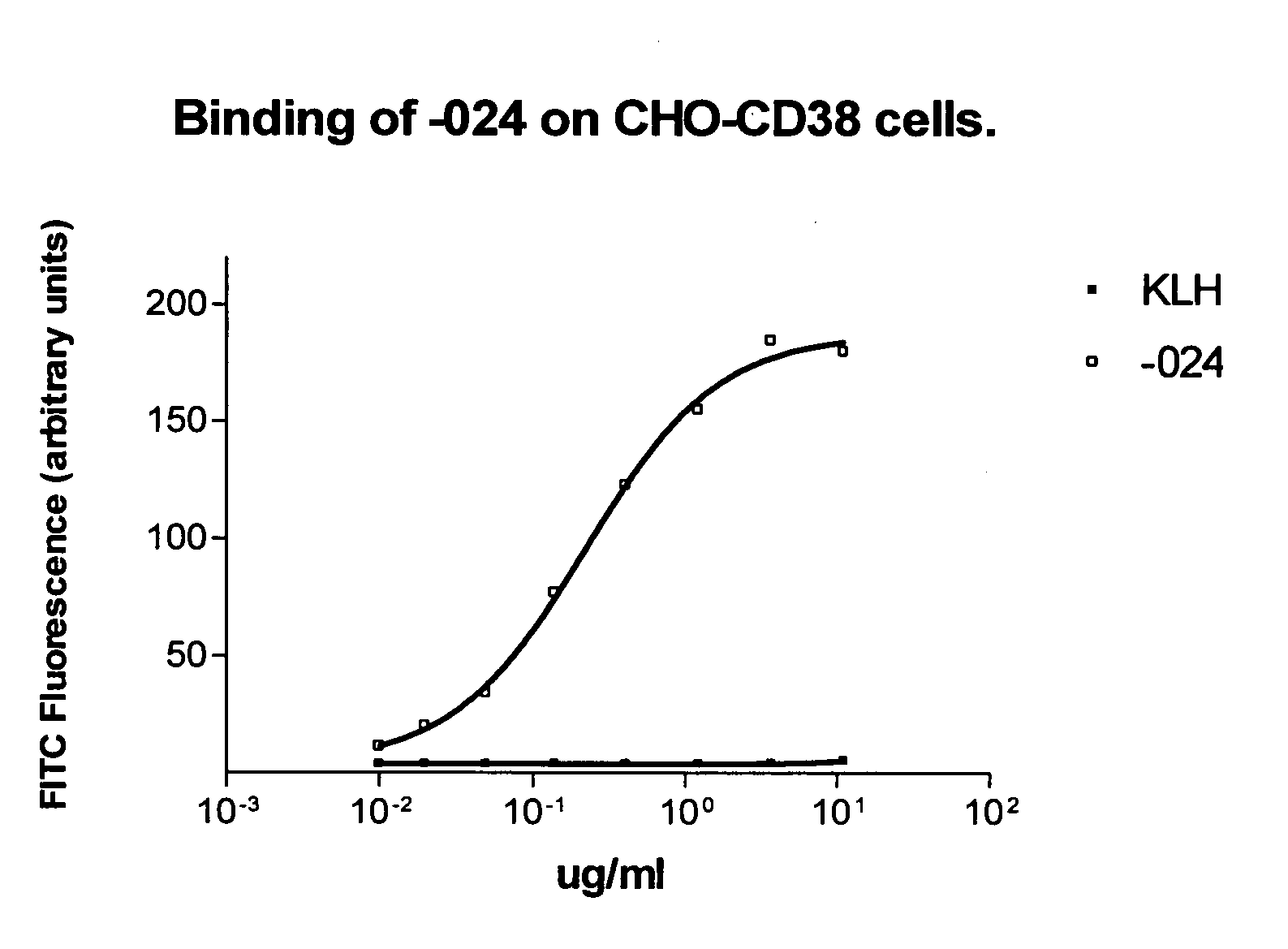
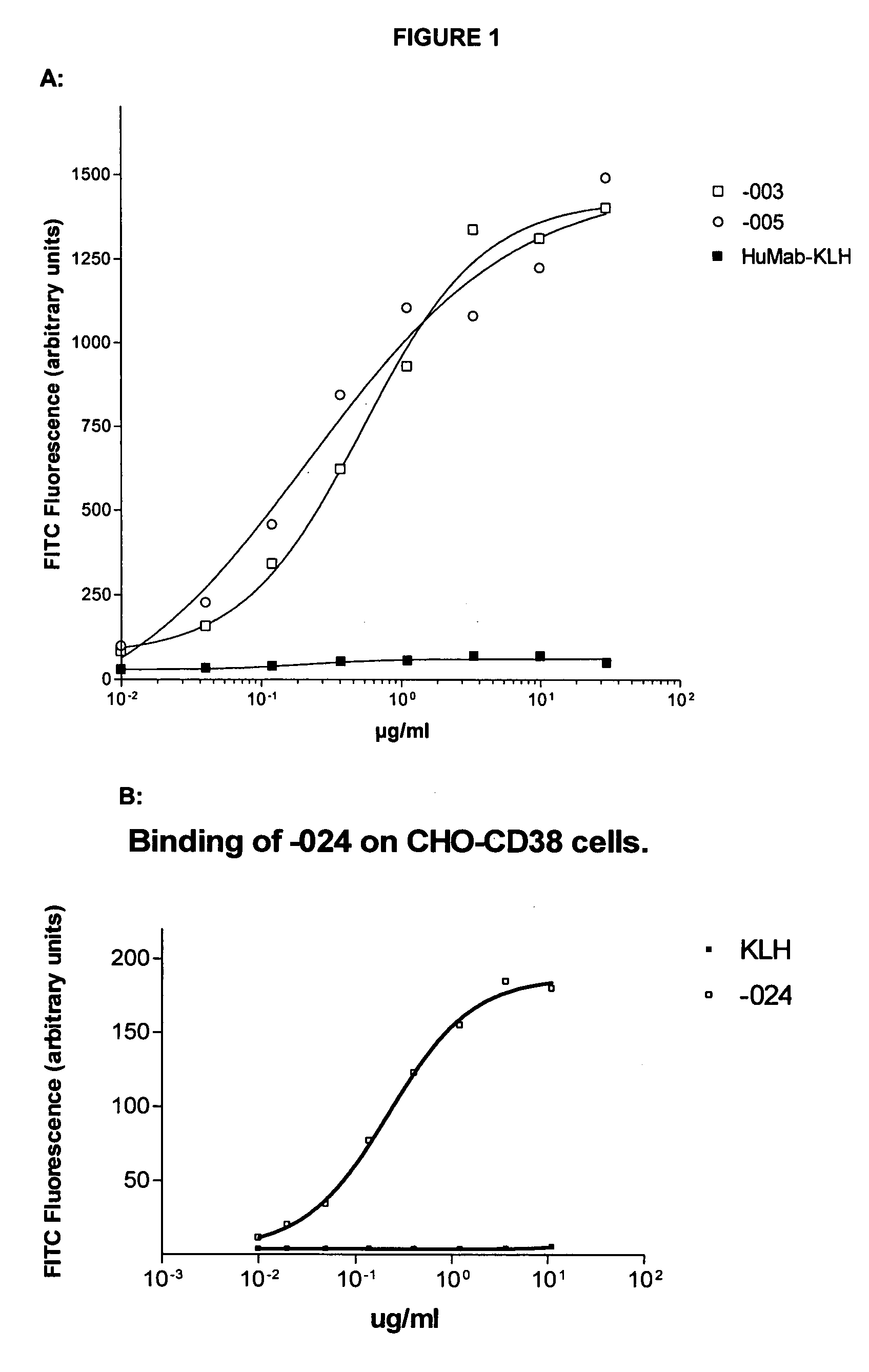
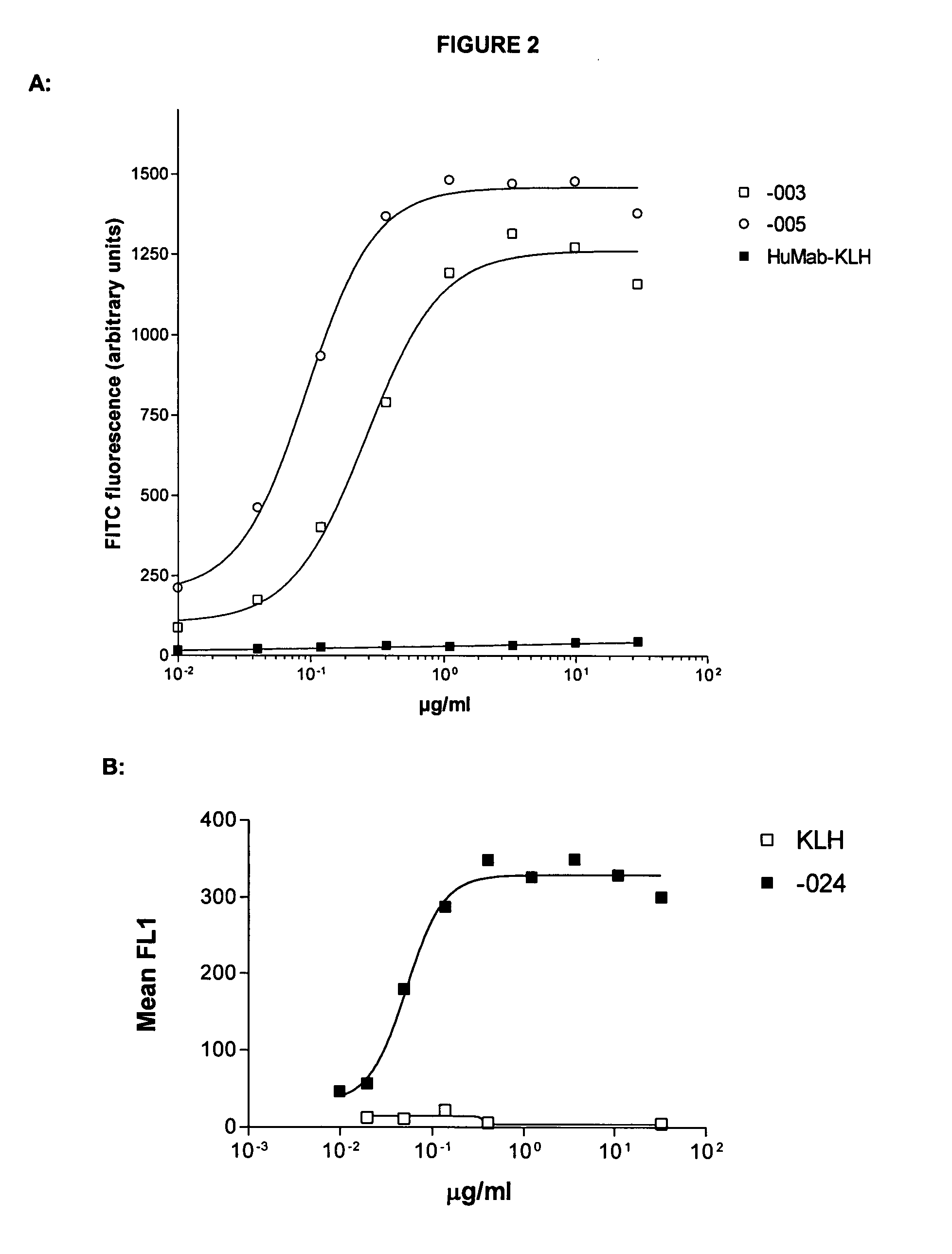
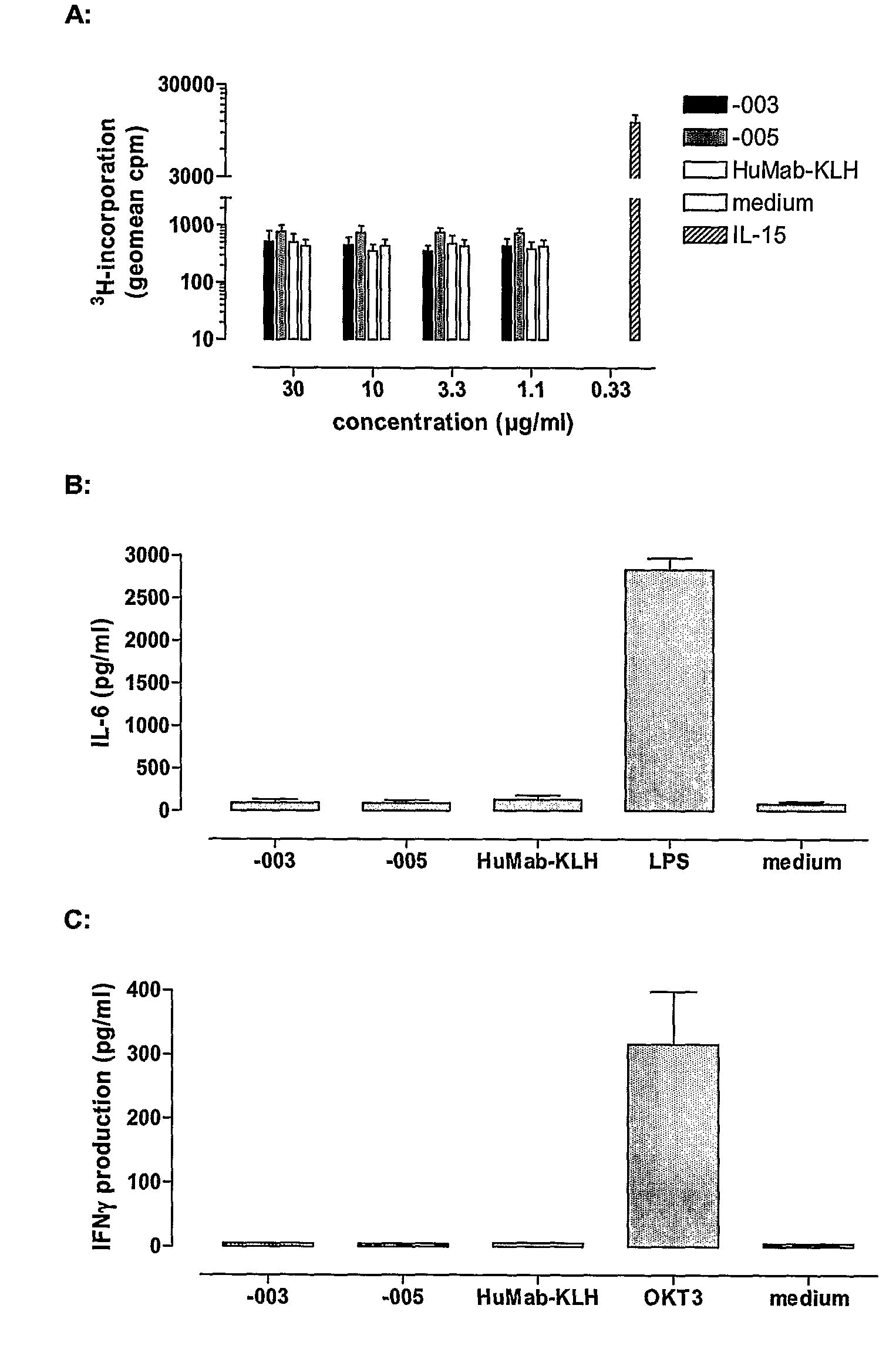
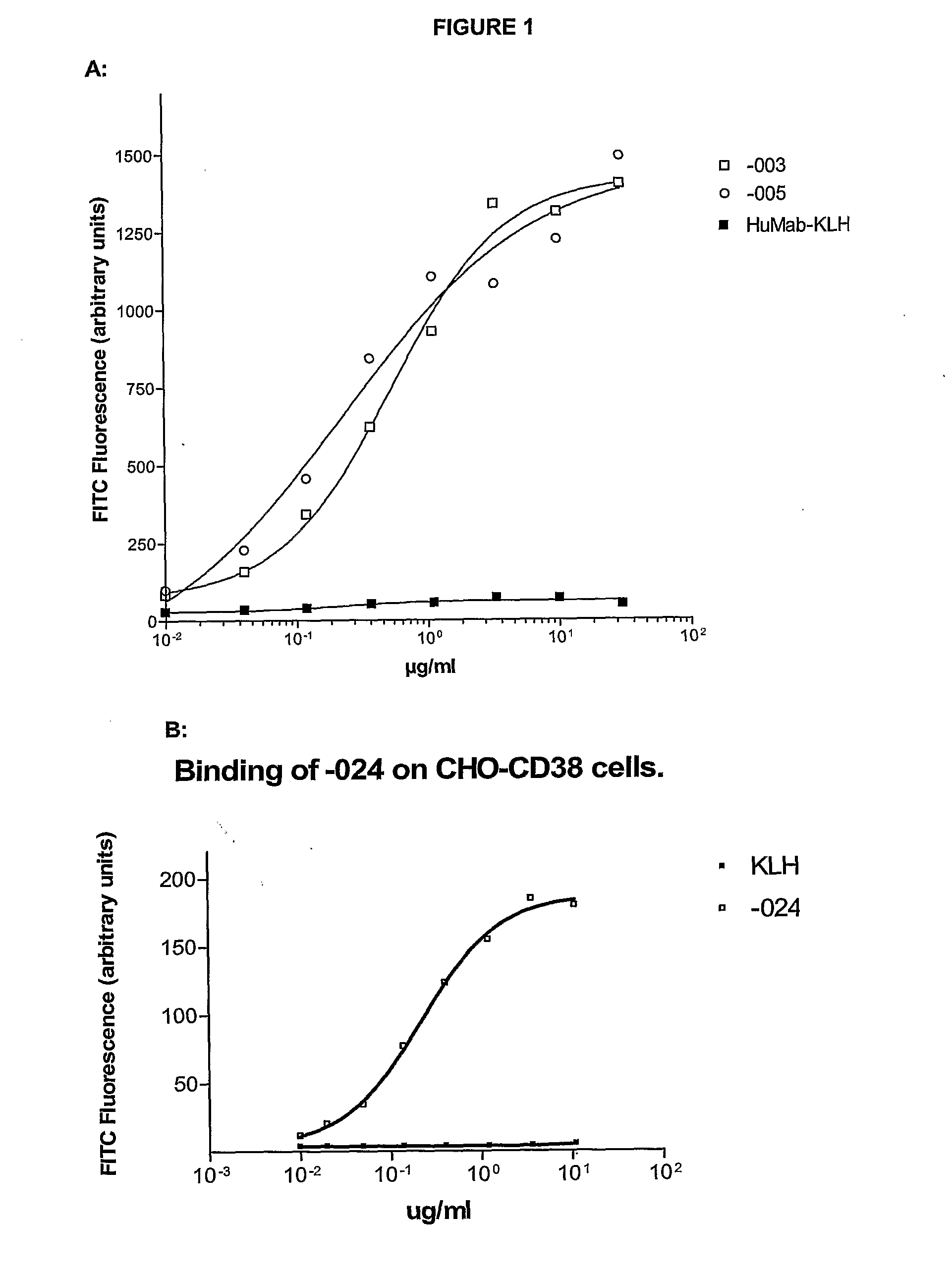
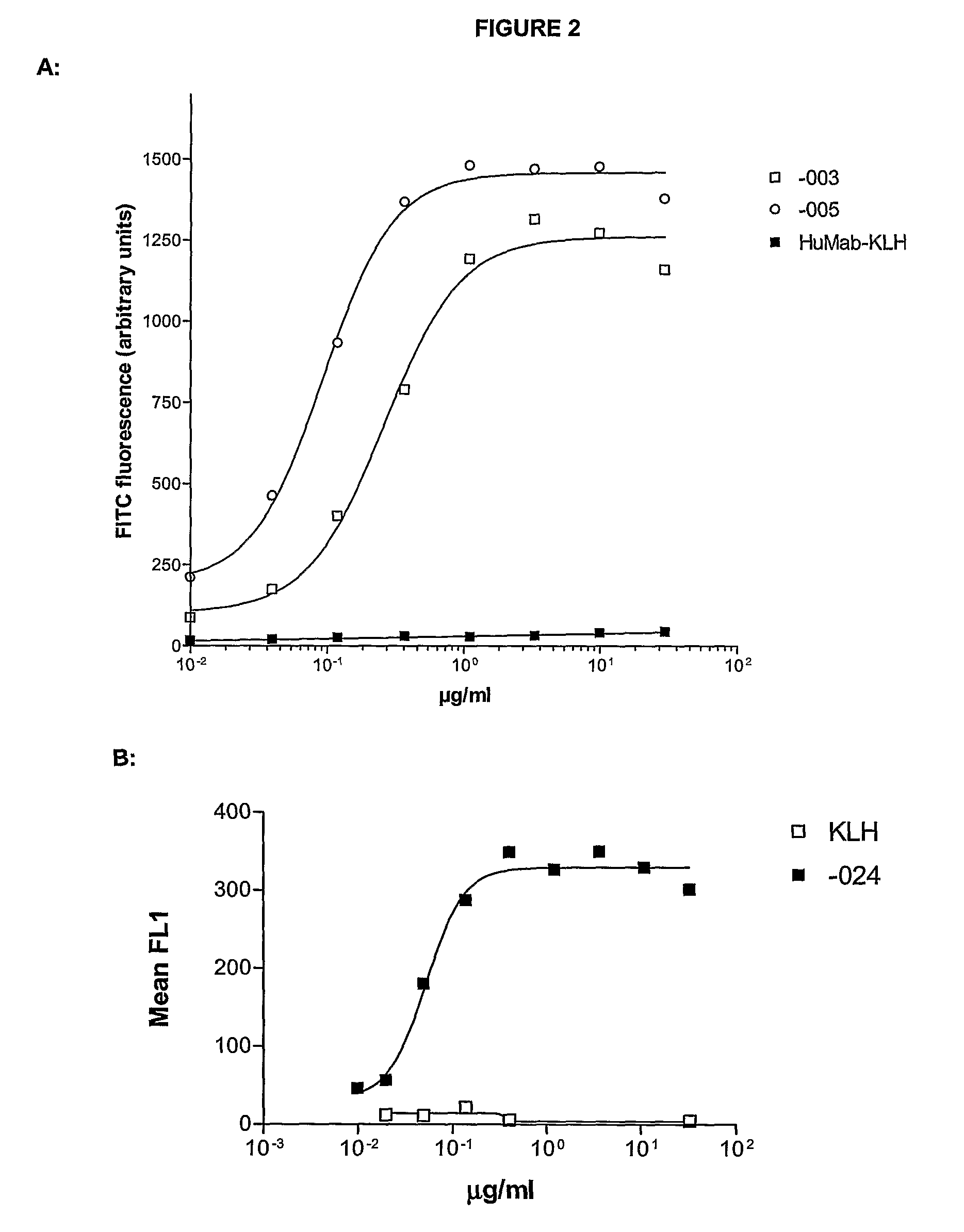
Antibodies against CD38 for treatment of multiple myeloma
ActiveUS7829673B2Compounds screening/testingSenses disorderAntiendomysial antibodiesPharmaceutical drug
Isolated human monoclonal antibodies which bind to human CD38, and related antibody-based compositions and molecules, are disclosed. Also disclosed are pharmaceutical compositions comprising the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB AS
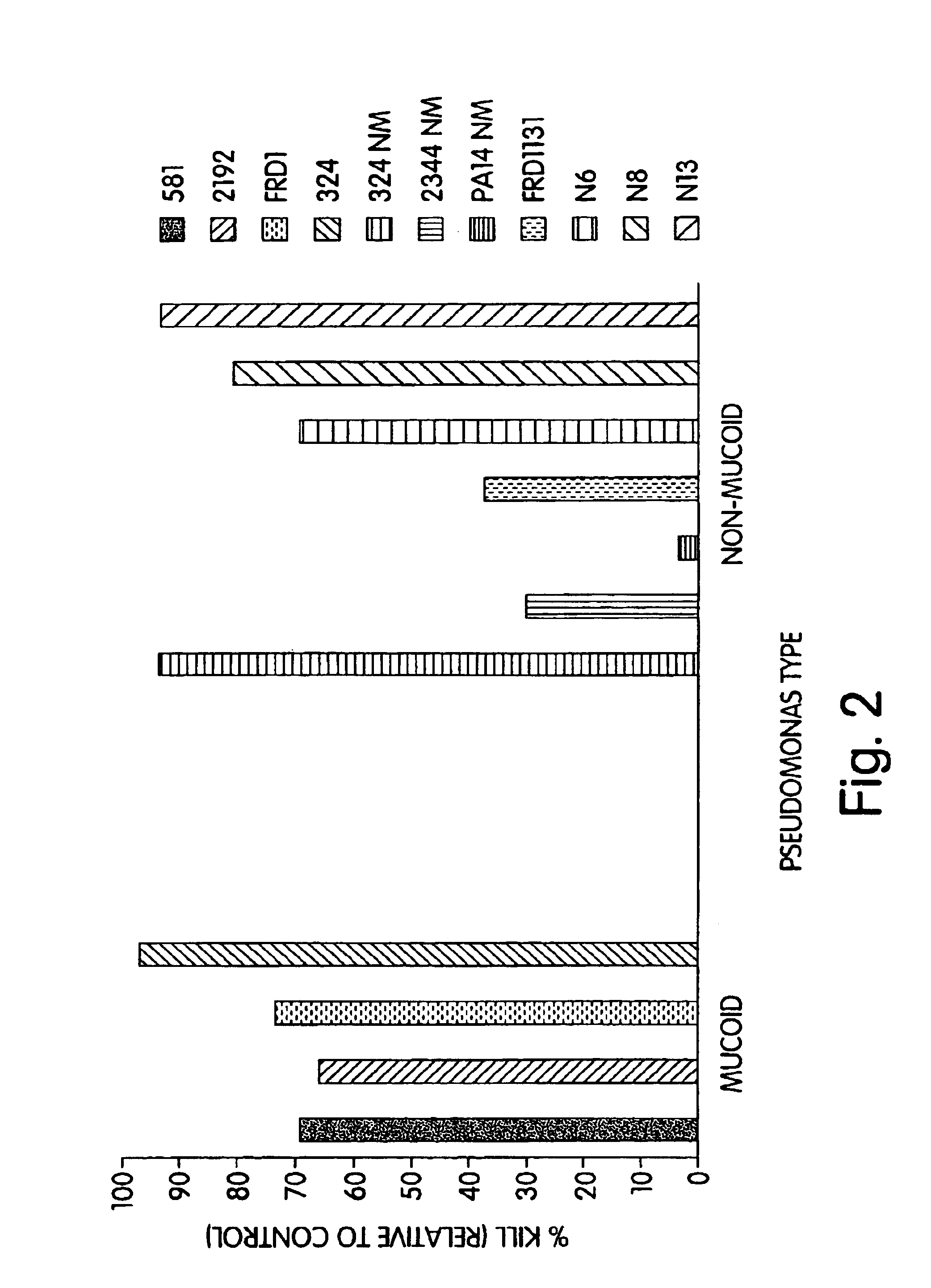
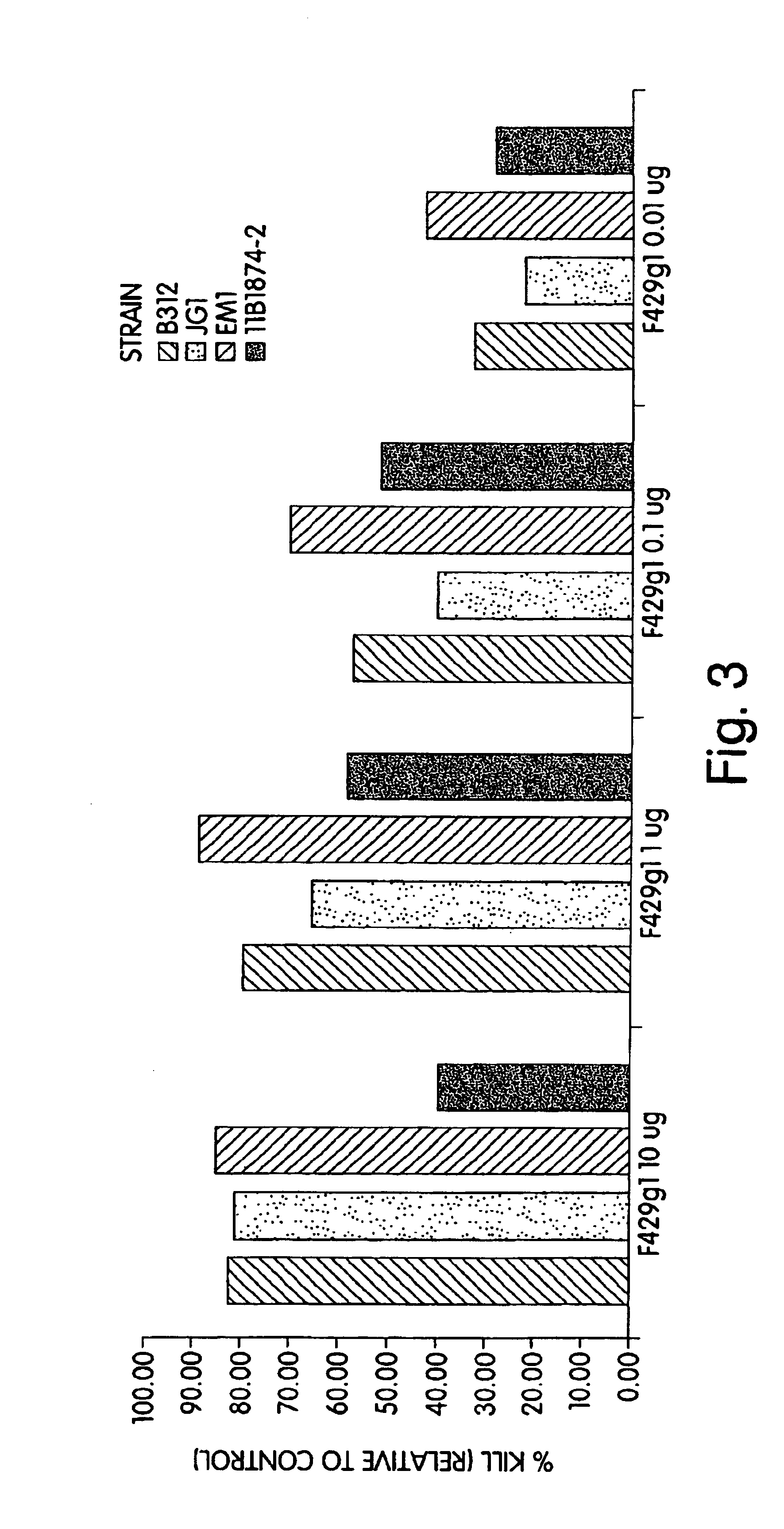
P. aeruginosa mucoid exopolysaccharide specific binding peptides
InactiveUS6962813B2Enhance opsonization and phagocytosisEnhance cytocidal effectAnimal cellsImmunoglobulins against bacteriaDiseaseMonoclonal antibody
The present invention relates to peptides, particularly human monoclonal antibodies, that bind specifically to P. aeruginosa mucoid exopolysaccharide. The invention further provides methods for using these peptides in the diagnosis, prophylaxis and therapy of P. aeruginosa infection and related disorders (e.g., cystic fibrosis). Some antibodies of the invention enhance opsonophagocytic killing of multiple mucoid strains of P. aeruginosa. Compositions of these peptides, including pharmaceutical compositions, are also provided, as are functionally equivalent variants of such peptides.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
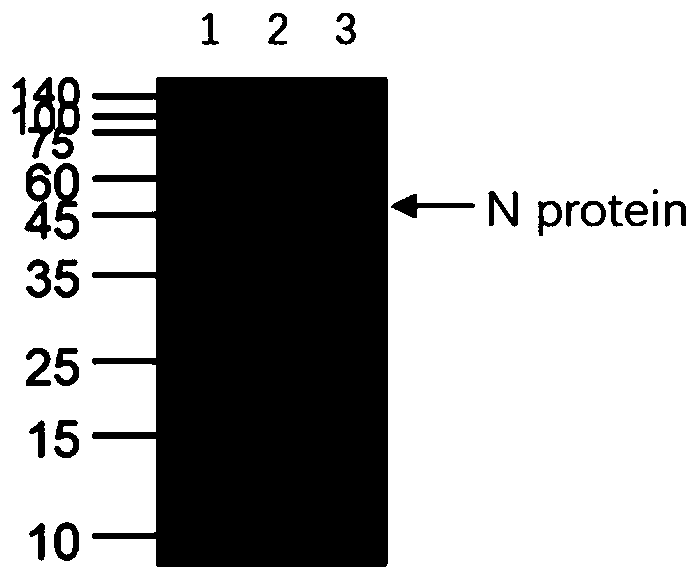
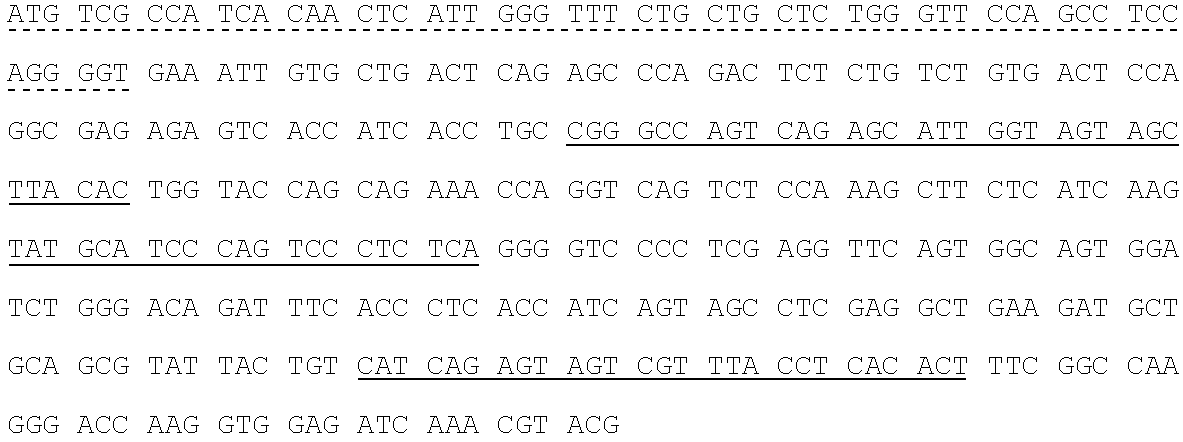
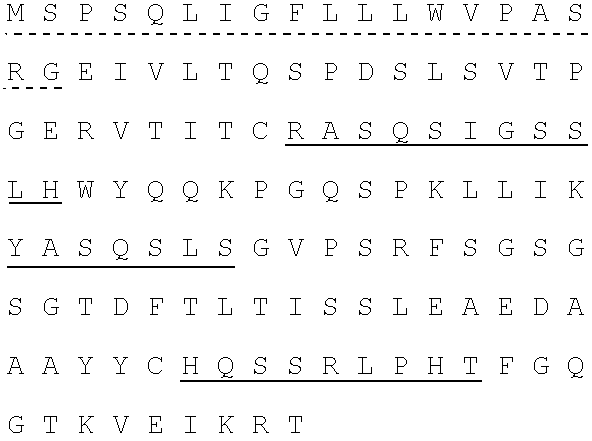
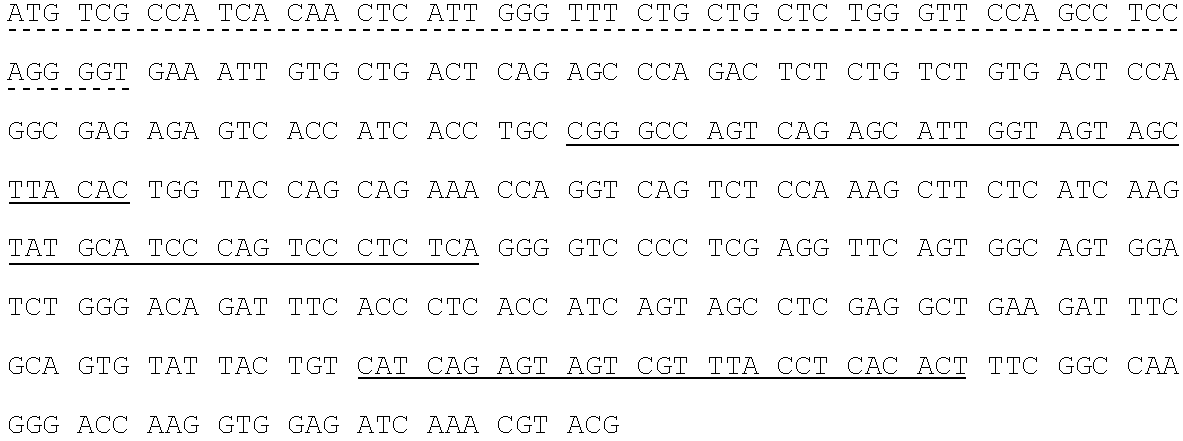
Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
PendingCN111153991AHigh affinityStrong specificitySsRNA viruses positive-senseVirus peptidesBALB/cMonoclonal antibody agent
The invention discloses a human SARS-CoV-2 monoclonal antibody. The preparation method of the human SARS-CoV-2 monoclonal antibody comprises the steps: adopting SARS-CoV Nucleocapsid recombinant protein as immunogen, immunizing BALB / c mice, performing fusion and subcloning on spleen cells and myeloma cells of mice, then performing a large amount of repeated screening and domestication of cell lines through commercialized products SARS-CoV-2 Nucleocapsid and MERS Nucleocapsid so as to obtain a hybridoma cell line capable of secreting the SARS-CoV-2-resistant N monoclonal antibody with high affinity and high specificity finally and successfully, and finally performing ascites preparation and purification so as to obtain the monoclonal antibody, wherein the amino acid sequence of the SARS-CoVNucleocapsid recombinant protein is shown in SEQ ID No. 1. The invention also discloses application of the monoclonal antibody in preparation of SARS-CoV-2 virus detection products and preparation ofdrugs for inhibiting the SARS-CoV-2 viruses. The monoclonal antibody can be used for detecting the SARS-CoV-2 in human throat swabs / pulmonary secretions and other samples by using a double-antibody sandwich method, and can be applied to diagnosis and prevention and control of SARS-CoV-2 virus infection and scientific researches of viruses and other study.
Owner:BEIJING BIOSYNTHESIS BIOTECH
Neutralizing human anti-IGFR antibody
The present invention includes fully human, neutralizing, monoclonal antibodies against human Insulin-like Growth Factor Receptor-I (IGFR1). The antibodies are useful for treating or preventing cancer in a subject. Also included are methods of using and producing the antibodies of the invention.
Owner:MERCK SHARP & DOHME CORP
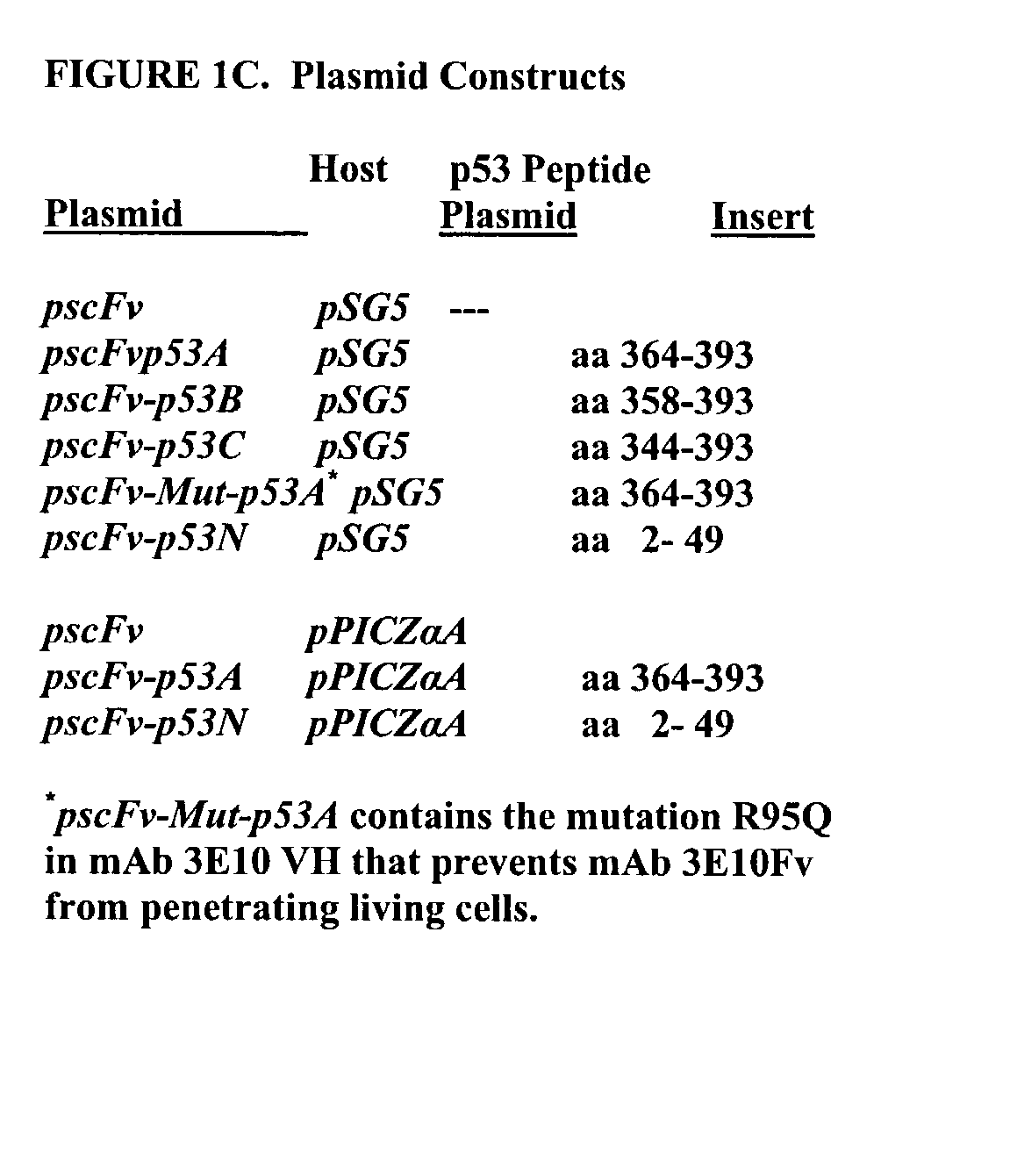
Delivery system using mAb 3E10 and mutants and/or functional fragments thereof
InactiveUS7189396B1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellCytotoxicity
A monoclonal antibody, 3E10, and active fragments thereof that selectively are transported in vivo to the nucleus of mammalian cells without cytotoxic effect are provided. The antibody and other molecules that bind to a variant of myosin IIb heavy chain found in the nucleus of skeletal muscle cells are useful as a non-viral delivery vector to target skeletal muscle in vivo. By contrast, in vitro the monoclonal antibody penetrates and is transported to the nucleus of multiple cell lines derived from different tissue types and can be used in screening tests to identify molecules that modulate growth of cells, such as cancer cells. Non-cytotoxic vectors for delivering a drug, polynucleotide or polypeptide selectively to skeletal muscle cells are also provided.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Antibodies against CD38 for treatment of multiple myeloma
InactiveUS20090076249A1Immunoglobulins against animals/humansFermentationAntiendomysial antibodiesPharmaceutical drug
Isolated human monoclonal antibodies which bind to human CD38, and related antibody-based compositions and molecules, are disclosed. Also disclosed are pharmaceutical compositions comprising the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:DE WEERS MICHEL +5
Antibodies against cd38 for treatment of multiple myeloma
ActiveUS20090148449A1Compounds screening/testingOrganic active ingredientsAntiendomysial antibodiesPharmaceutical drug
Isolated human monoclonal antibodies which bind to human CD38, and related antibody-based compositions and molecules, are disclosed. Also disclosed are pharmaceutical compositions comprising the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB AS
Fully human Anti-human nkg2d monoclonal antibodies
The invention relates to isolated fully human monoclonal antibodies having specificity for human NKG2D and compositions thereof. The invention further relates to methods for using such antibodies in treating diseases or conditions such as cancer, autoimmune disease, or infectious disease.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
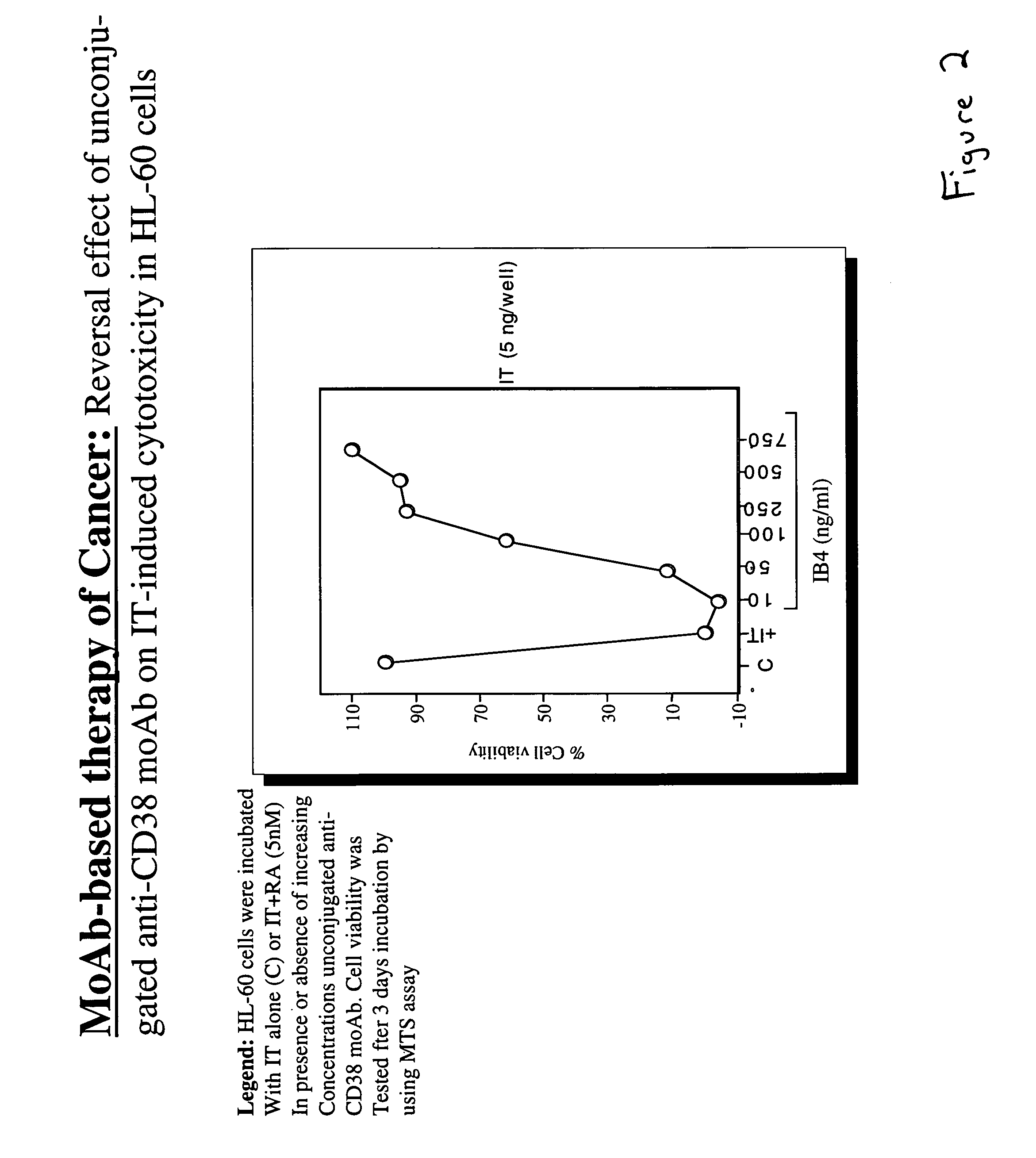
Potentiation of anti-CD38-Immunotoxin cytotoxicity
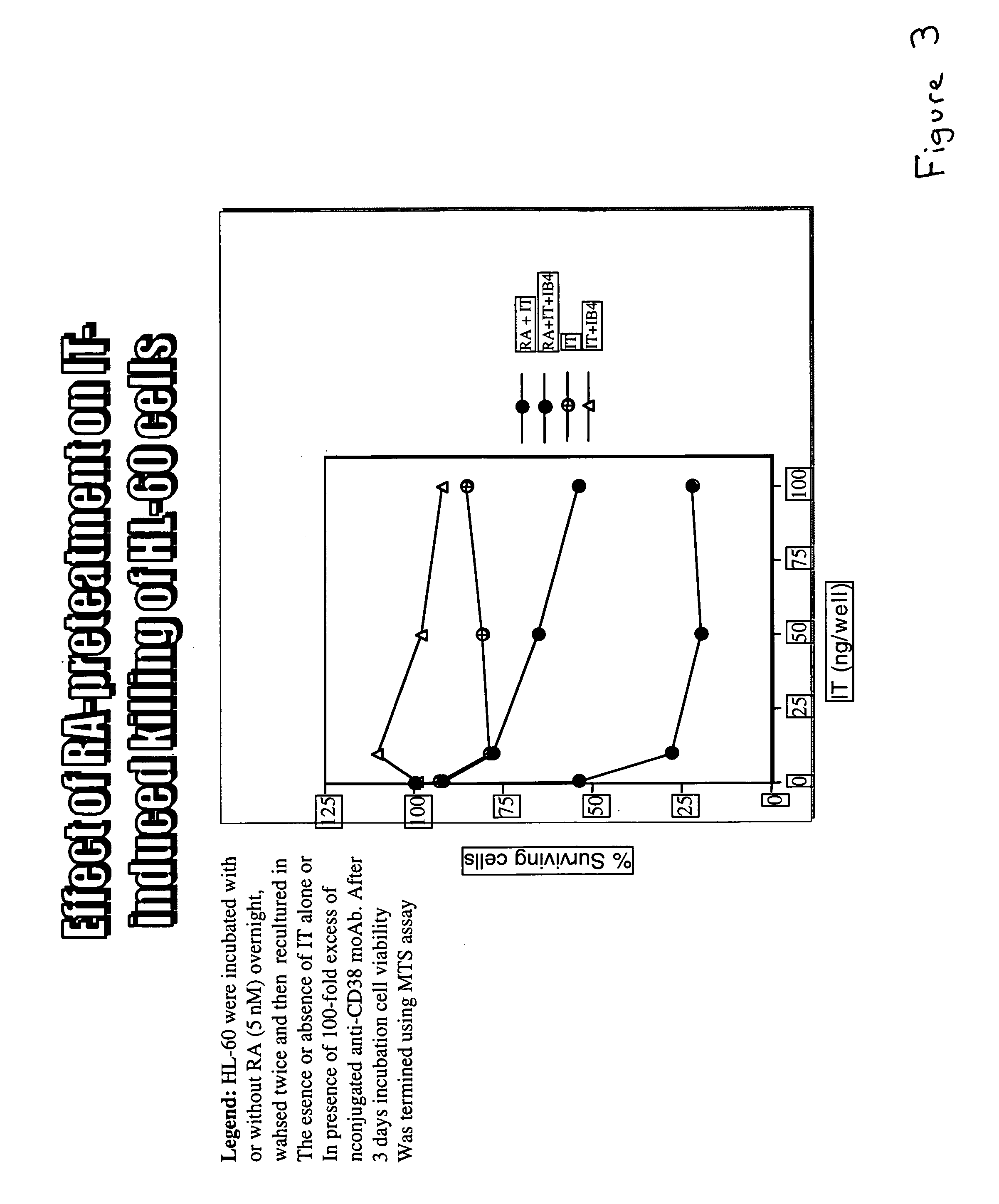
The present invention is directed to the use of agents that induce high levels of cell surface molecules to provide targets for immunotoxins directed against the same cell surface molecules. A specific example is given in which all-trans-retinoic acid (RA) is used to induce high levels of CD38 cell surface antigen expression in several myeloid and lymphoid leukemia cells. CD38 was then used as target for delivering plant toxin (gelonin) to leukemia cells. Treatment of leukemia cells with RA induced high levels of CD38 in those cells that otherwise had low CD38 expression. The RA-induced leukemia cells then became exquisitely sensitive to an immunotoxin constructed from an anti-CD38 monoclonal antibody conjugated to the plant toxin gelonin.
Owner:BOARD OF REGENTS
Solid forms of anti-EGFR antibodies
InactiveUS7960516B2Improve stabilityHigh purityPowder deliveryFrom normal temperature solutionsAdjuvantDissolution
The invention relates to solid forms of antibodies against the EGF receptor, in particular precipitates and crystals of monoclonal antibodies against the EGF receptor, particularly preferably of Mab C225 (cetuximab) and Mab h425 (EMD 72000), which result in biologically active antibody protein through dissolution or suspension in aqueous medium, obtainable by precipitation of the antibody and / or one of its variants and / or fragments dissolved or suspended in aqueous medium by means of a precipitation reagent. The invention furthermore relates to pharmaceutical preparations comprising at least one solid form of above-mentioned antibodies in precipitated non-crystalline, precipitated crystalline or in soluble or suspended form, and optionally excipients and / or adjuvants and / or further pharmaceutical active ingredients, and to a process for the preparation of solid forms of anti-EGFR antibodies according to the invention.
Owner:MERCK PATENT GMBH
Digital immunochromatographic test strip for semi-quantitative detection of aflatoxin B1 and preparation method thereof
InactiveUS20120034711A1Quick checkSimple procedureAnalysis using chemical indicatorsLamination ancillary operationsPaperboardBovine serum albumin
The present invention belongs to the field of biological detection. Multi-line immunochromatographic test strip for semi-quantitative detection of aflatoxin B1 comprises a paperboard, wherein a water-absorbing pad, a detection pad, a gold-labeled pad and a sample pad are adhered sequentially on one surface of the paperboard from top to bottom, wherein each adjacent pads is overlapped and connected, the detection pad uses a nitrocellulose film as a backing pad, the nitrocellulose film is provided with a transverse control line, a test line I, a test line II and a test line III, wherein the control line is coated with a rabbit anti-mouse polyclonal antibody, and the test line I, test line II and test line III are coated with aflatoxin B1-bovine serum albumin conjugate (AFB1-BSA), respectively: and the gold-labeled pad is transversely coated with a nanogold-labeled anti-aflatoxin B1 monoclonal antibody. Said test strip is used for semi-quantitative detection of aflatoxin B1, and is characterized by quick detection, simple procedure and high sensitivity.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Multi-test-line immunochromatographic test strip for semi-quantitatively detecting aflatoxin B1 and preparation method thereof
InactiveCN101900728AHigh practical application valueEasy to handleBiological material analysisAflatoxin BMonoclonal antibody
The invention belongs to the field of bioinstrumentation and relates to a multi-test-line immunochromatographic test strip for semi-quantitatively detecting aflatoxin B1, comprising a paperboard, wherein a water absorbent pad, a detection pad, a gold-labeled pad and a sample pad are adhered on one surface of the paperboard from top to bottom in sequence, wherein adjacent pads are overlapped and connected at the connection part, the detection pad takes a nitrocellulose film as a base pad, the nitrocellulose film is provided with a transverse quality control line, a detection line I, a detection line II and a detection line III from top to bottom, wherein the quality control line is wrapped with a rabbit anti-mouse polyclonal antibody, and the detection line I, the detection line II and the detection line III are respectively wrapped with an aflatoxin B1-bovine serum albumin (AFB1-BSA) conjugate; and the gold-labeled pad is transversely sprayed with a nanogold-labeled aflatoxin B1 monoclonal antibody. The immunochromatographic test strip is used for semi-quantitatively detecting the aflatoxin B1 and has the characteristics of fast detection, simple operation and high sensitivity.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
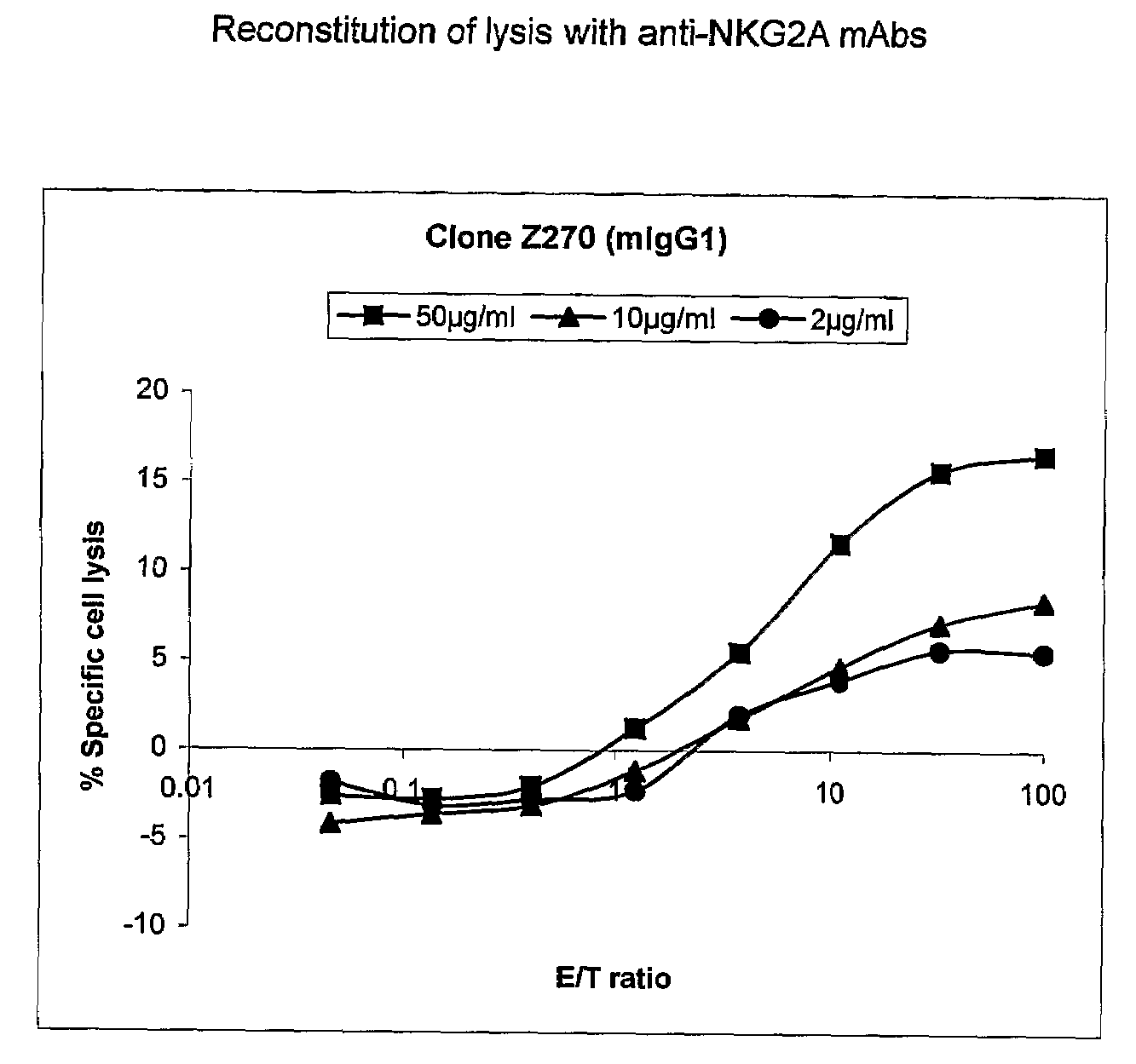
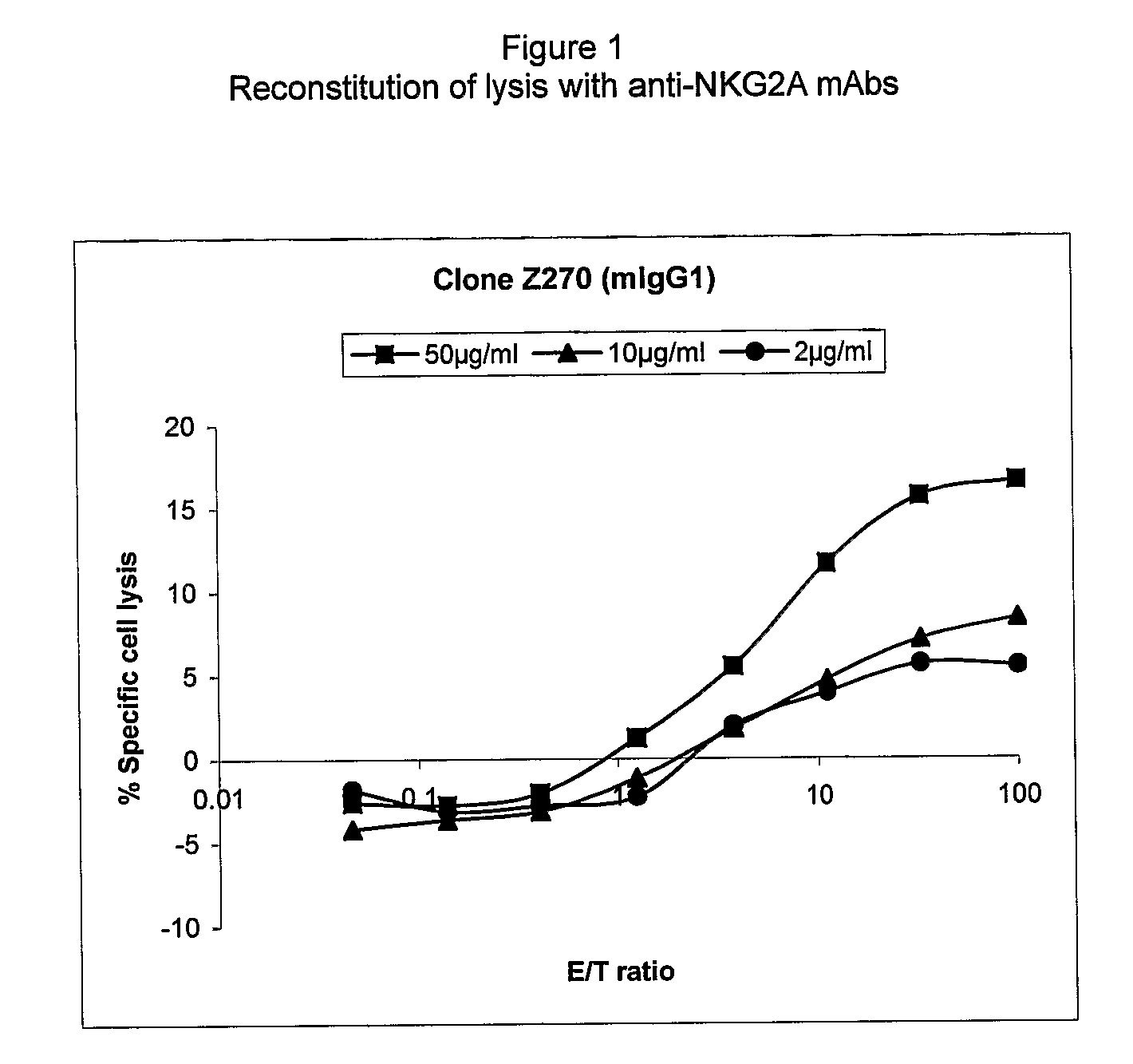
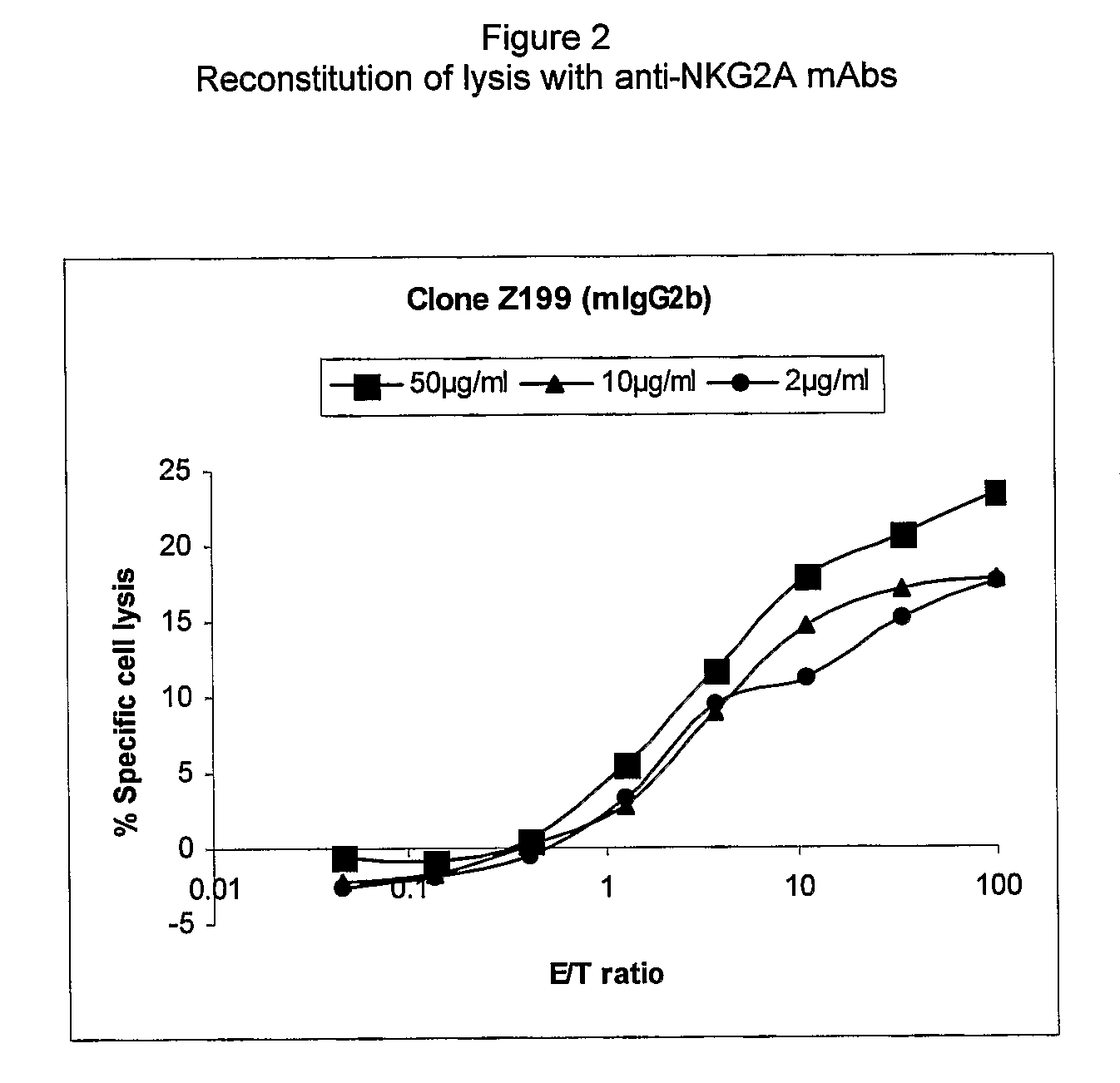
Monoclonal antibodies against nkg2a
ActiveUS20090208416A1Improve bioavailabilityImprove stabilitySenses disorderAntipyreticPresent methodDendritic cell
The present invention relates to methods of treating immune disorders, particularly autoimmune or inflammatory disorders, and methods of producing antibodies and other compounds for use in therapeutic strategies for treating such disorders. Generally, the present methods involve the use of antibodies or other compounds that prevent the stimulation of NKG2A receptors on NK cells, leading to the lysis of dendritic cells that contribute to the pathology of the disorders.
Owner:UNIV DEGLI STUDI DI GENOVA GENOVA IT +1
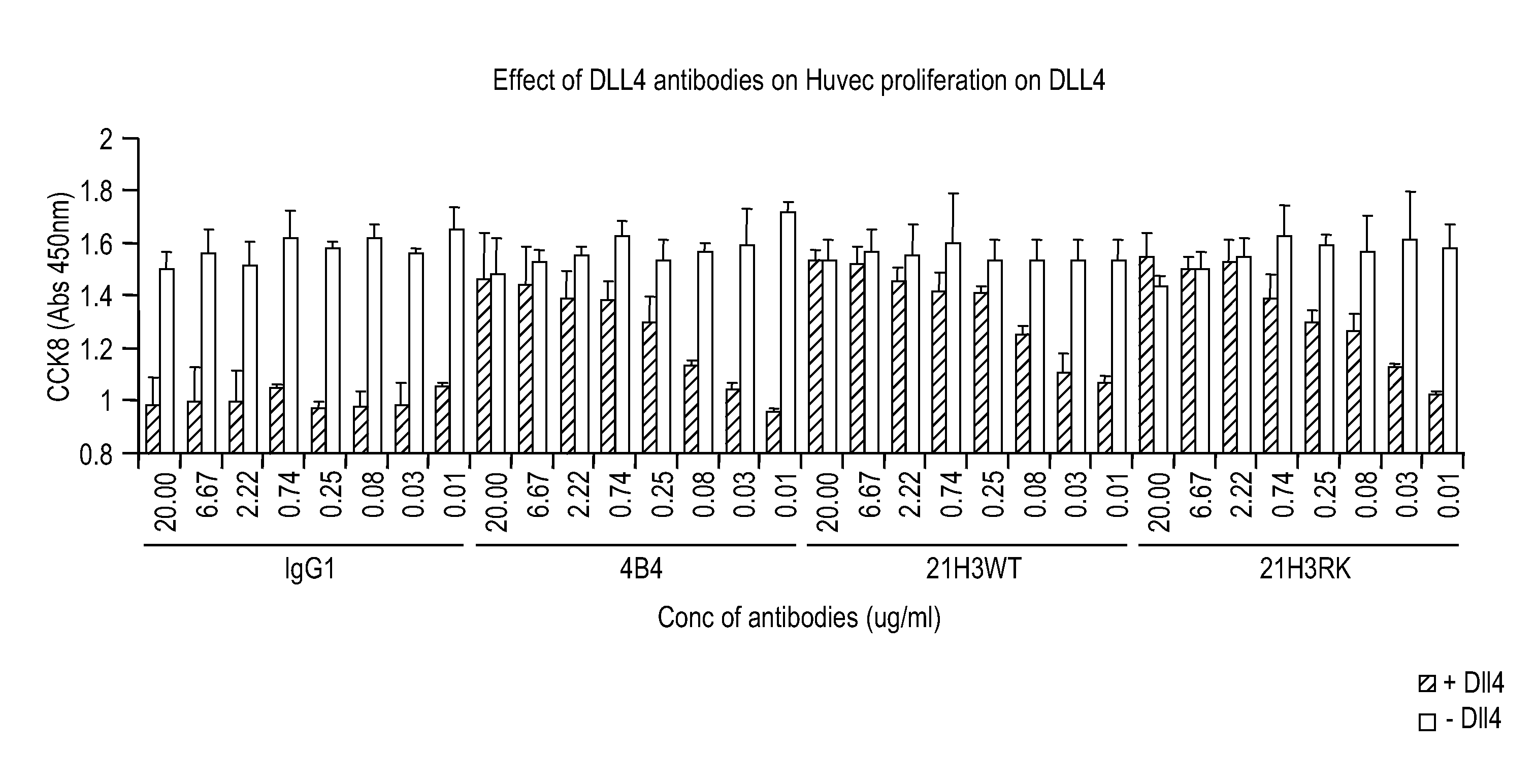
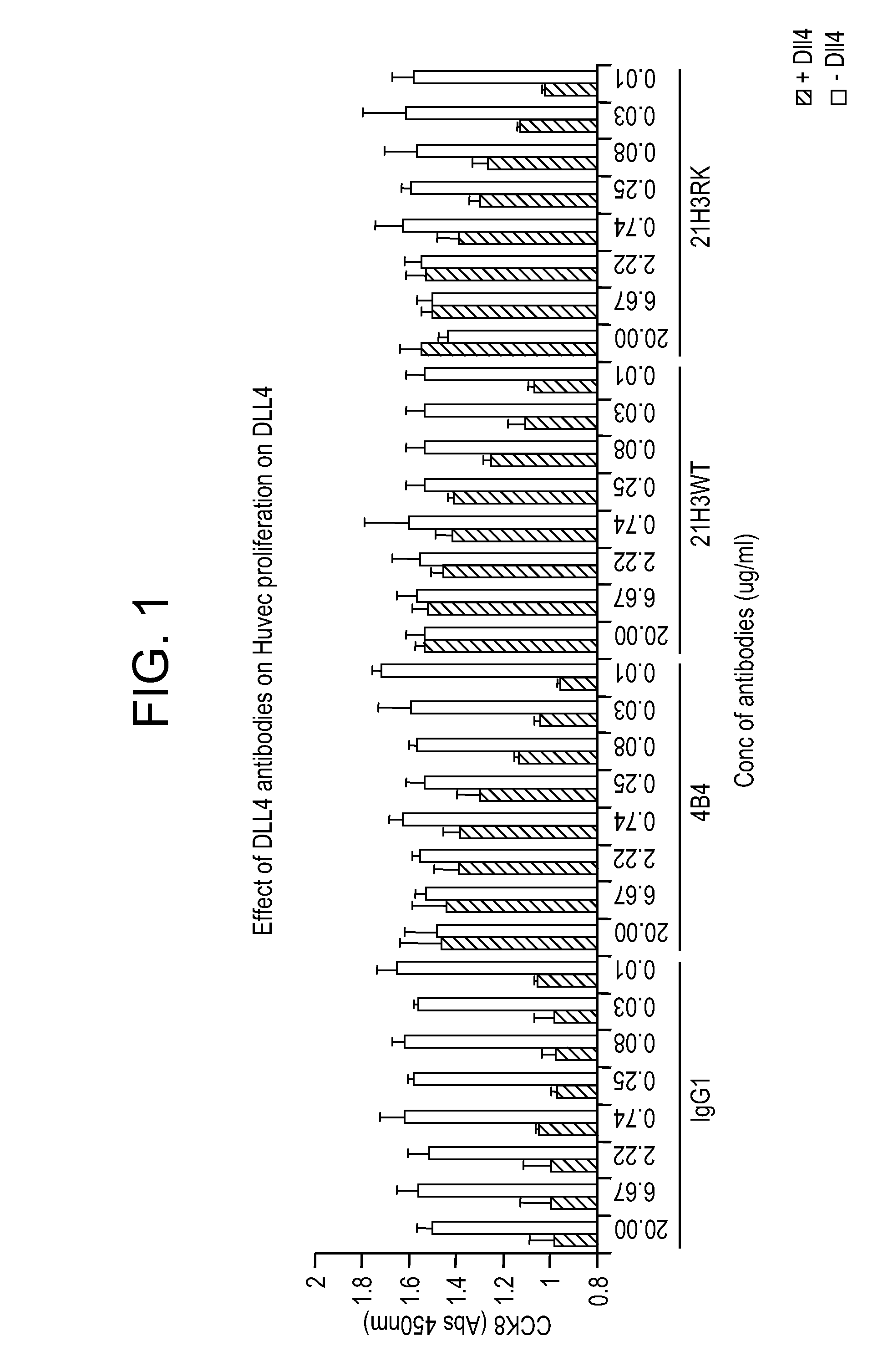
Targeted binding agents directed to dll4 and uses thereof 524
InactiveUS20100196385A1Improve abilitiesEasy to fixAntibacterial agentsSenses disorderDiseaseMonoclonal antibody
The invention relates to targeted binding agents against DLL4 and uses of such agents. More specifically, the invention relates to fully human monoclonal antibodies directed to DLL4. The described targeted binding agents are useful in the treatment of diseases associated with the activity and / or overproduction of DLL4 and as diagnostics.
Owner:MEDIMMUNE LLC
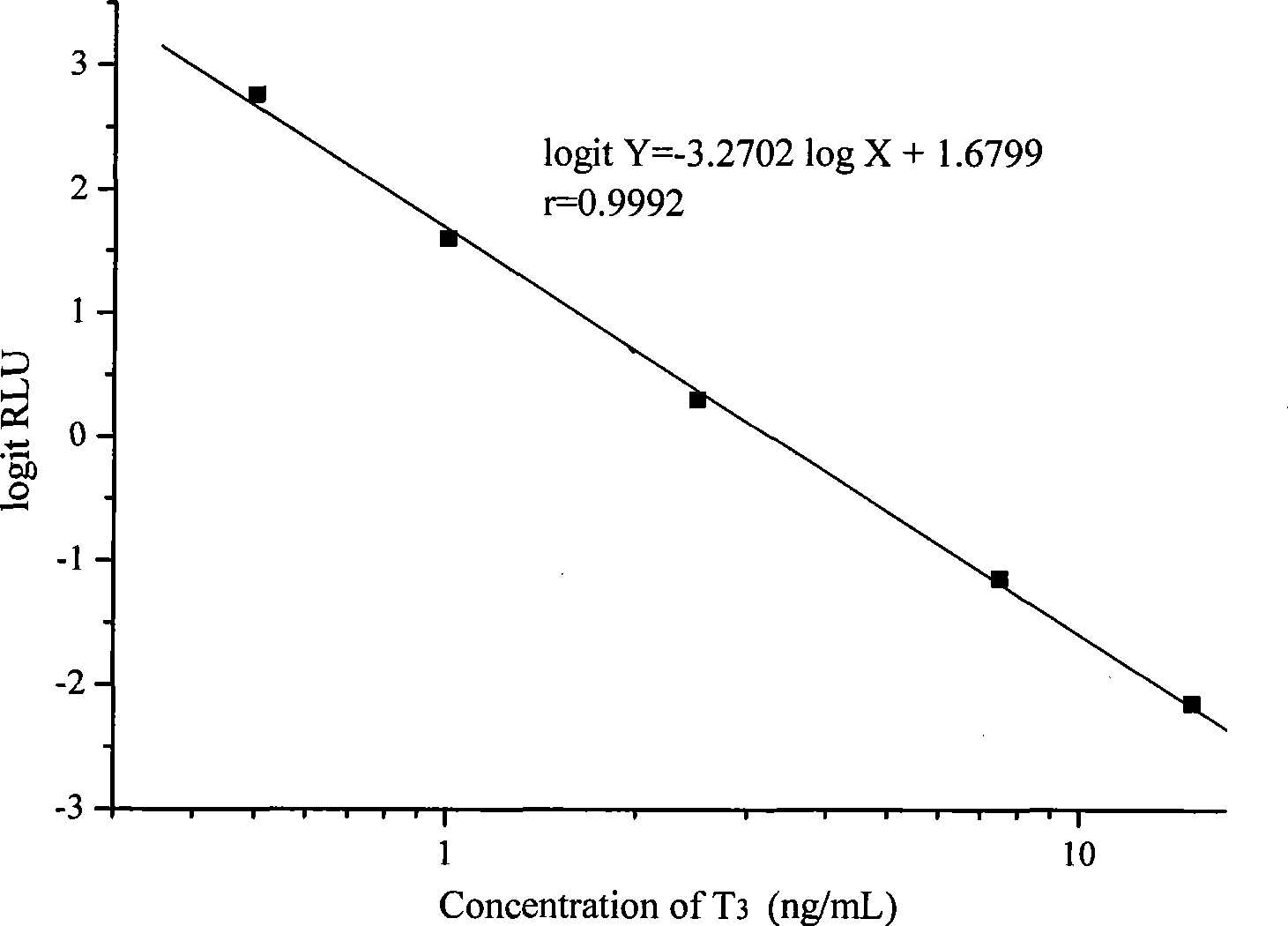
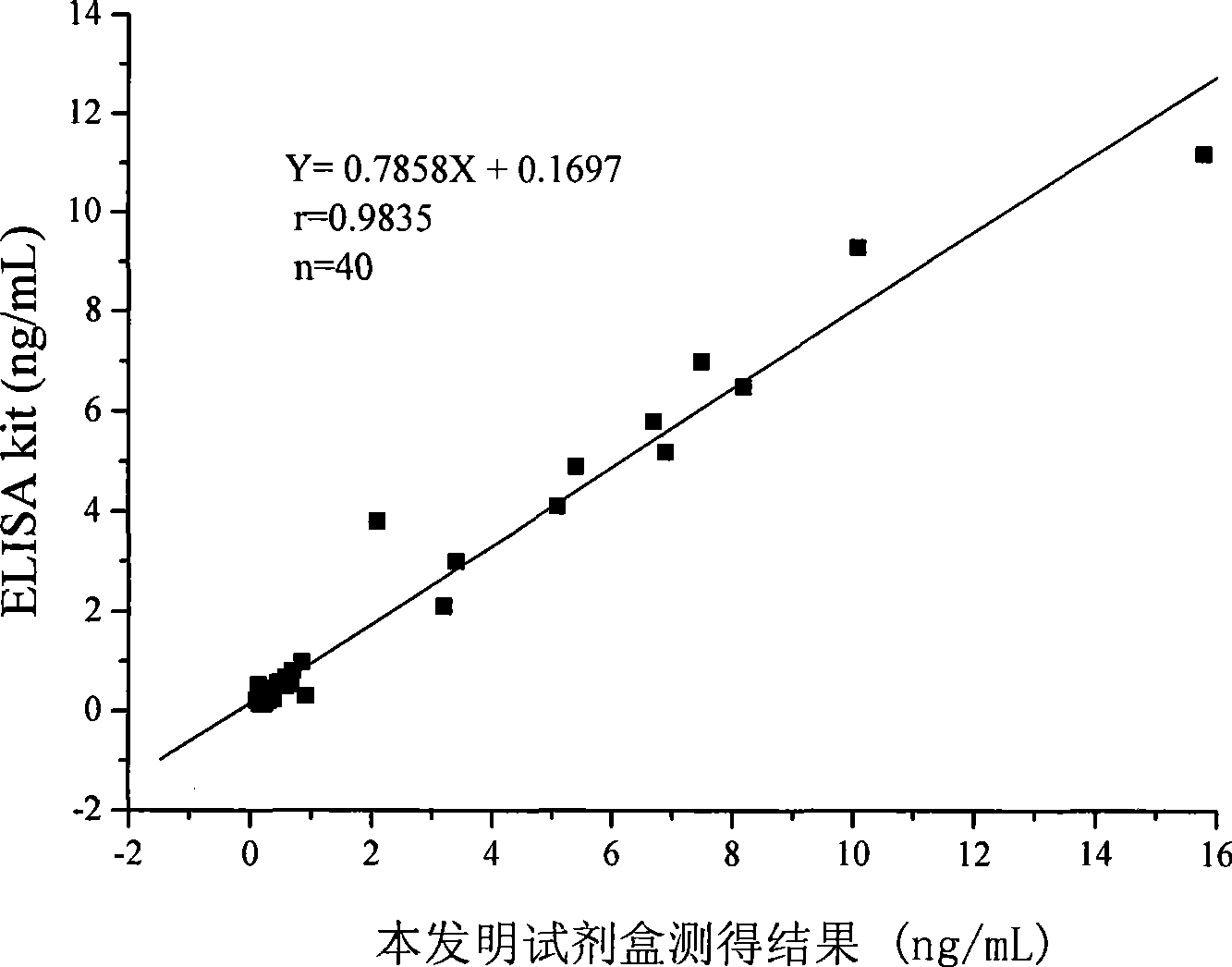
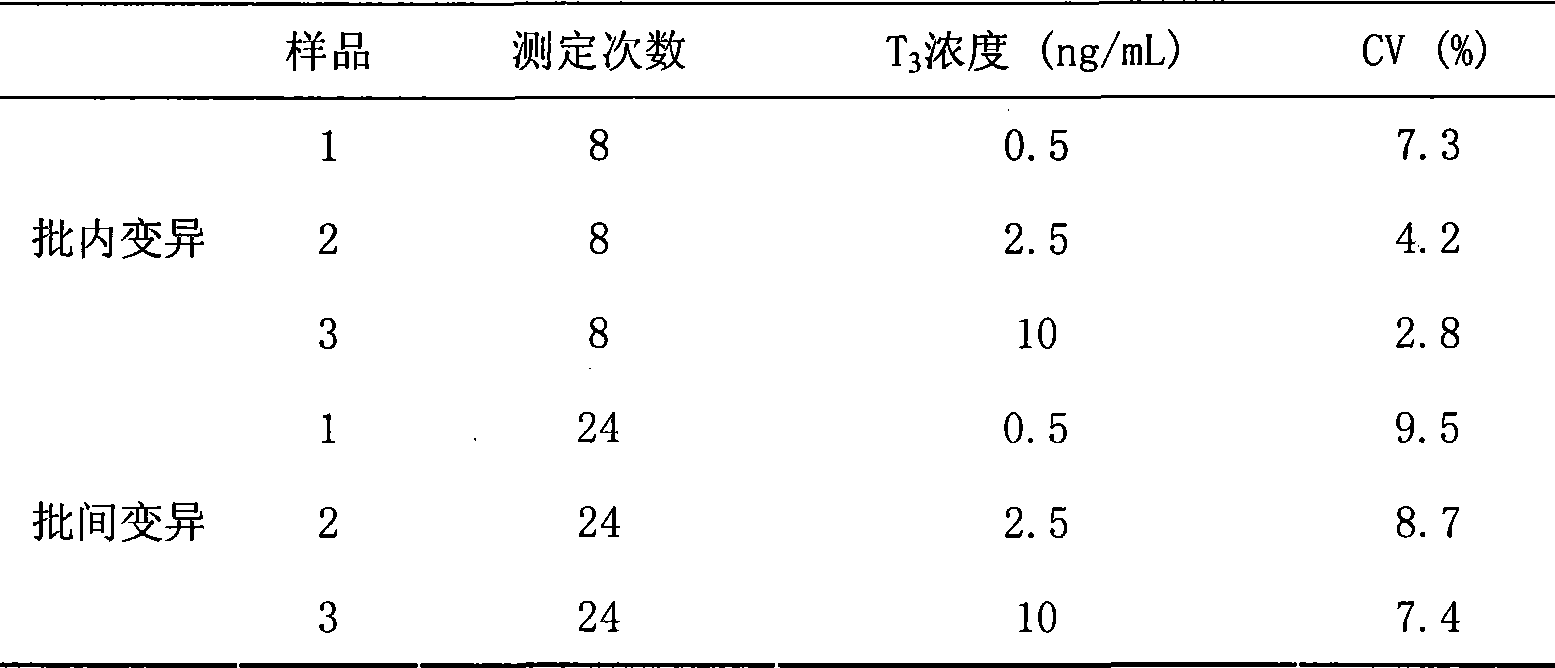
Chemoluminescence immunoassay measuring kit and preparation method thereof for triiodothyronine magnetic particles
InactiveCN101545913AEasy to measureStability determinationChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexAntigen
The invention provides a chemoluminescence immunoassay measuring kit and a preparation method thereof for quantificationally detect triiodothyronine (T3) magnetic particles. The kit mainly comprises a triiodothyronine serial calibration sample, magnetic particle solution coated by an anti-fluorescein isothiocyanate (FITC) monoclonal antibody, a T3 antigen marked by biotin, T3 monoclonal antibody marked by FITC, streptavidin marked by alkaline phosphatase, chemoluminescence substrate solution and 20-time concentrated washing solution. The invention adopts a competitive-method reaction mode, effectively utilizes the chemoluminescence technology combined with magnetic particles and biotin-avidin immunity magnifying technology principle to quantificationally detect the content of T3 in blood serum and blood plasma samples of human bodies and ensure the sensitivity of the detection. The kit is simple, convenient, fast, sensitive and stable to use, and provides a very valuable detection method for clinic diagnosis and scientific research works.
Owner:北京科美东雅生物技术有限公司
Preparation method of agarose immune magnetic microspheres and applications thereof
ActiveCN103212377ASeparation does not affectEliminate the pre-filtering stepOther chemical processesImmunoglobulins against cell receptors/antigens/surface-determinantsMicrosphereGenetic engineering
The invention discloses a preparation method of agarose immune magnetic microspheres and applications thereof, and relates to a preparation method of microspheres and applications thereof, which solves the technical problems that an existing purified monoclonal antibody is complex in process, is low in yield, and is high in cost, and coupling agents are hypertoxic. The preparation method comprises the following steps of: 1. preparing a ferric oleate precurser; 2. preparing a magnetic fluid; 3. preparing an oil phase and a water phase; 4. preparing agarose magnetic microspheres; 5. preparing activated and crosslinked agarose magnetic microspheres; 6. preparing agarose magnetic microspheres joined with spacer arms; and 7. preparing agarose immune magnetic microspheres. The agarose immune magnetic microspheres provided by the invention are used for purifying genetic engineering recombination trastuzumab monoclonal antibodies. The agarose immune magnetic microspheres prepared by the invention are used for the field of separating and purifying the monoclonal antibodies.
Owner:HARBIN YICAI NEW MATERIAL
Enzyme linked immunosorbent assay kit for combined diagnosis of gastrosis or evaluation of gastric cancer risks
InactiveCN102087279AIncreased sensitivityImprove featuresComponent separationTissue cultureAntigenPepsinogen I
The invention discloses an enzyme linked immunosorbent assay kit for combined diagnosis of the gastrosis or evaluation of gastric cancer risks and a preparation method thereof. The kit comprises a micropore plate coated with an antibody against a pepsin antigen I or an antibody against a pepsin antigen II, an enzyme labeled antibody, a color-developing agent, a stop solution and a concentrated cleaning solution, wherein the pepsin antigen I or the pepsin antigen II is a natural protein obtained from extraction of human gastric mucosa tissue. The kit disclosed by the invention adopts a mouse immunized with pepsinogen I and pepsinogen II which are separated from human gastric mucosa to prepare immunogen of a monoclonal antibody, the used standard sample also adopts the pepsin antigen I or the pepsin antigen II separated from the human gastric mucosa, thereby the defects caused by adopting different structures of animal pepsinogen and human pepsinogen are filled. The kit can be used for accurately diagnosing the gastrosis or early gastric cancer and has the advantages of high sensitivity, strong specificity, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Immune antibody for detecting pesticide organic phosphorus residus and its application
InactiveCN1598586AFast immunoassaySimple immunoassay techniqueMaterial analysisAcetic acidFiltration
The invention discloses a rudimental immune antibody which is used to detect organic phosphorus pesticide residue and its application, the character of the invention is using diethyl phosphonic acid acetic acid as hapten, produce artificial immunogen through coupling with bovine serum albumin(BSA), and produce several clone antibody through immune Zelanian giant blanc or produce mono clone antibody through chmice immune, cell amalgamation, filtration, clone planting and other steps, the several and single antibody can be used to indirect emulative ELISA, direct ELISA, or immune test paper to detect the rudimental phosphorus pesticide. The excellences of the invention including: the selected hapten has several organic phosphorus pesticides general structure, the structure reforming is not necessary, it can immune animal after coupling with protein, it can also be used to detect organic phosphorus pesticide in food and water source, this show the rapid and convenient of the immune detecting tehchnology.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
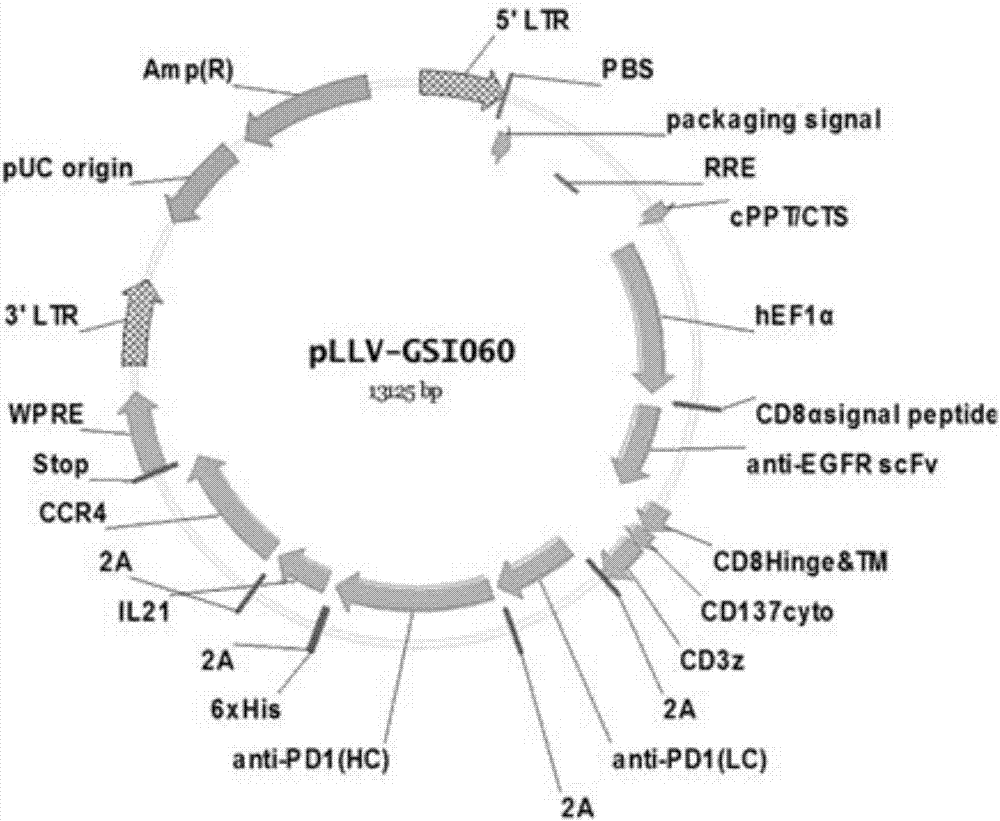
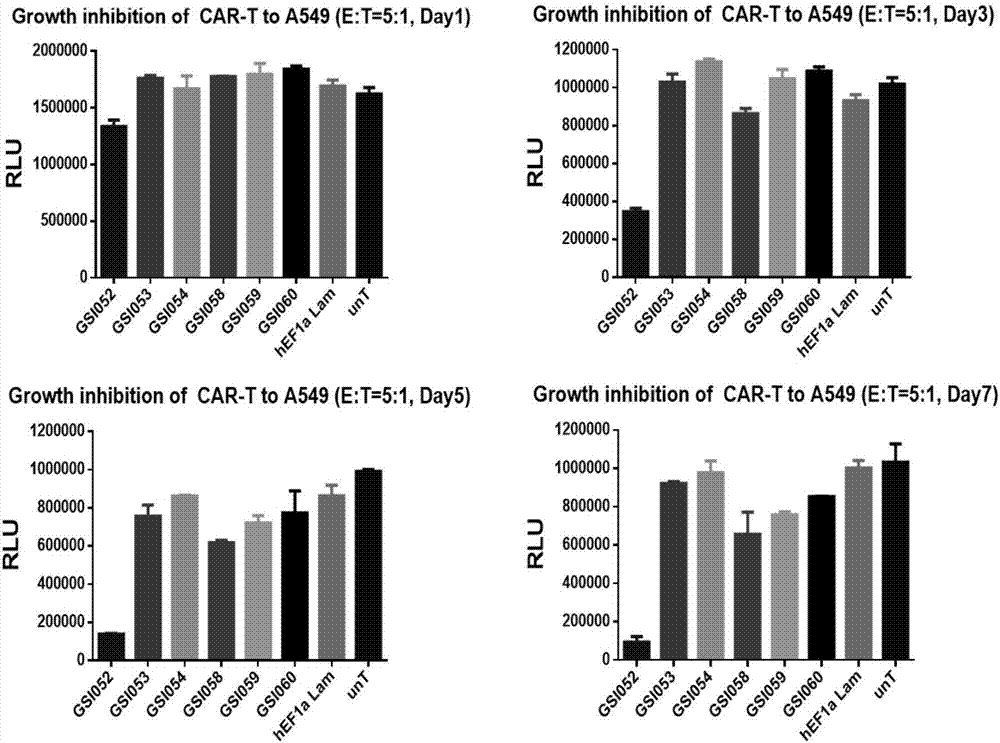
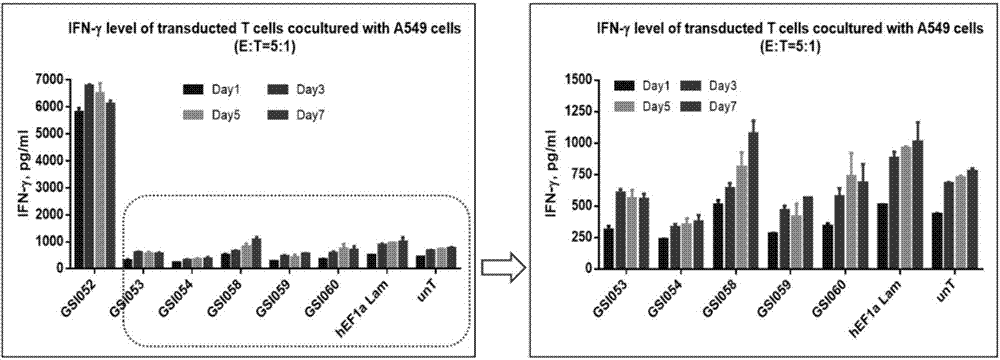
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
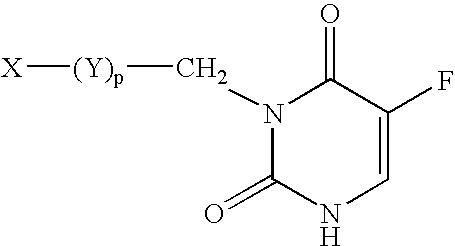
5-Fluoro-uracil immunoassay
ActiveUS7205116B2Accurate monitoringReaction can be selectiveEnzymologyDepsipeptidesBiological fluidsFluorouracil
Owner:SALADAX BIOMEDICAL INC
Detection for zearalenone
A detection method includes preparing single cloning antibody by steps of antigen synthesis, mouse immune, cell fusion, hybrid tumor selection and its cell line collection, extracting ZEN in corn sample by methanol / aqueous solution; filtering and deluting it; flowing liquid sample through affinity column and it by methanol; and using fluoresclence spectrophotometer to measure out content of zearlenone in set sample solution.
Owner:SHANGHAI UNIV
Method for a fully automated monoclonal antibody-based extended differential
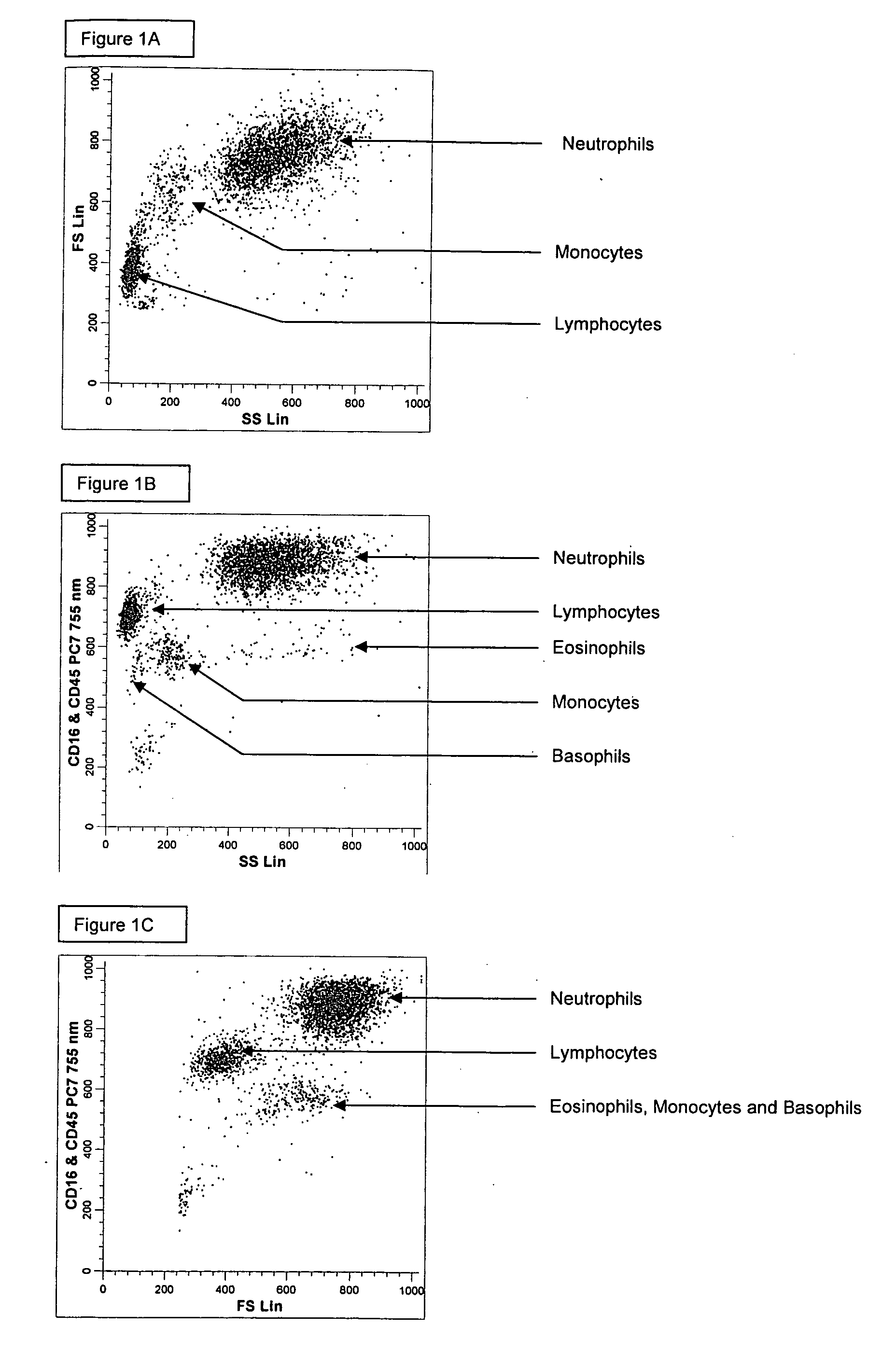
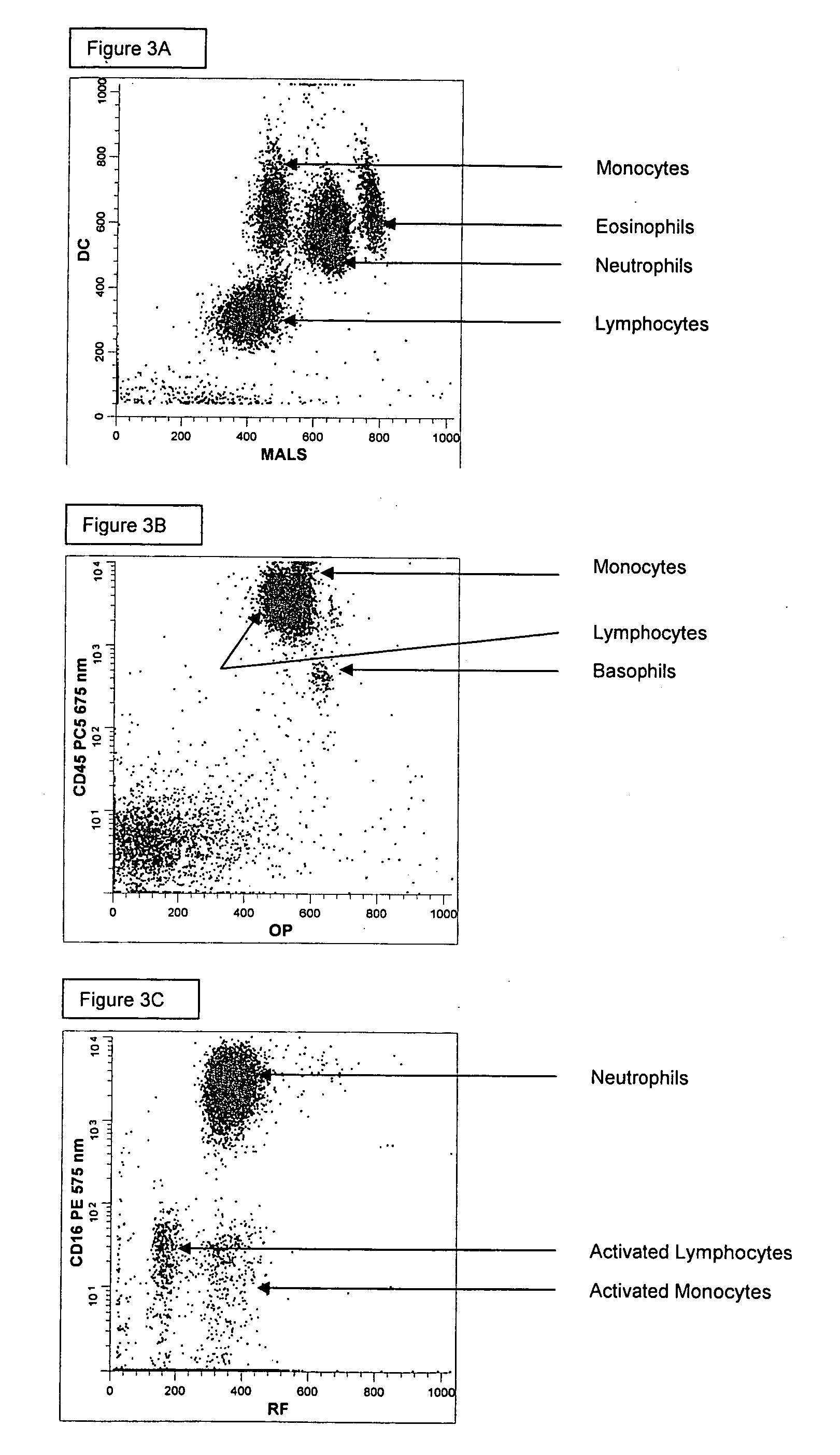
ActiveUS20060269970A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSpectral patternMonoclonal antibody agent
Methods for differentially identifying cells in an instrument employ compositions containing a combination of selected antibodies and fluorescent dyes having different cellular distribution patterns and specificities, as well as antibodies and fluorescent dyes characterized by overlapping emission spectra which form non-compensatable spectral patterns. When utilizing the compositions described herein consisting of fluorescent dyes and fluorochrome labeled antibodies with overlapping spectra that cannot be separated or distinguished based upon optical or electronic compensation means, a new fluorescent footprint is established. This new fluorescent footprint is a result of the overlapping spectra and the combined cellular staining patterns of the dyes and fluorochrome labeled antibodies chosen for the composition. The new fluorescent footprint results in histogram patterns that are useful for the identification of additional cell populations or subtypes in hematological disease.
Owner:BECKMAN COULTER INC
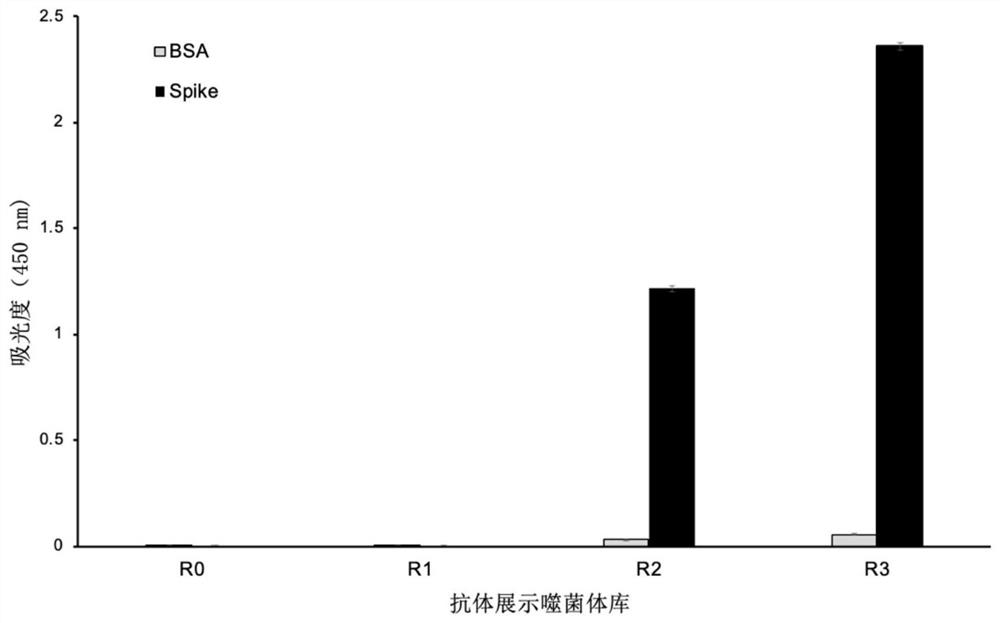
Phage display antibody library and monoclonal antibodies aiming at novel coronavirus SARS-CoV-2 and obtained by panning based on same
ActiveCN111778218ANeutralizes contagionMicroorganism based processesImmunoglobulins against virusesEscherichia coliMonoclonal antibody agent
The invention discloses a phage display antibody library and five strains of screened antibodies capable of being combined with S protein of novel coronavirus SARS-CoV-2. Mutation is introduced into an ultra-variable region of an antibody variable region based on synthetic biology and a phage display technology, and a gene is transferred into escherichia coli, so that a synthetic antibody librarycontaining 108 kinds of antibodies is constructed; the phage display antibody library provided by the invention can perform screening to obtain the antibody with the specificity and the detection function, so that powerful resources of biological research and medical diagnosis are expanded; and the five strains of antibodies capable of being combined with the S protein of the novel coronavirus arefurther screened out and can be used for detecting the virus, part of the antibodies can block combination of the virus and cells, and the antibodies have the capacity of neutralizing novel coronavirus infectivity, can be used for preparing a novel coronavirus detection product, preparing a drug for inhibiting the novel coronavirus and preparing a pharmaceutical preparation for preventing or treating diseases caused by the novel coronavirus, and have a wide application prospect.
Owner:山东宽和正生物医药有限公司
Ultra-sensitive superparamagnetic nano immunization microsphere and GP73 antigen detection method
ActiveCN105699653AThe detection process is fastStrong specificityBiological testingMicrosphereEnzyme immunoassays
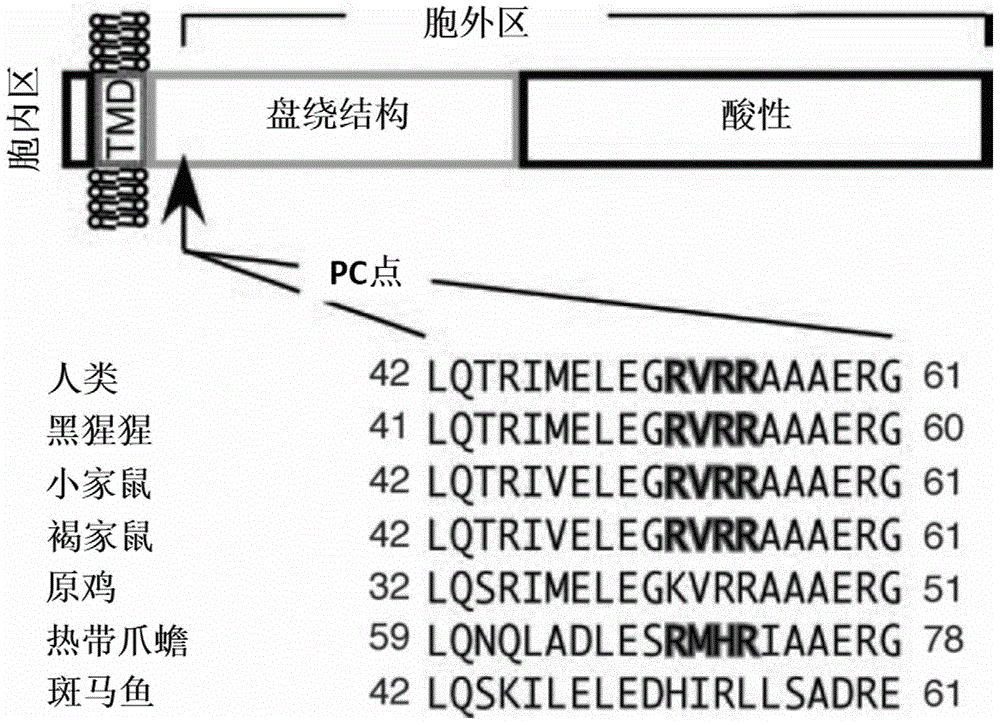
The present invention relates to the technical field of diagnostic reagents, and particularly relates to a magnetic immunization in-vitro diagnostic reagent. In order to solve the technical problems of smaller capacity of an immobilized antibody, long reaction time, relatively low sensitivity and low stability of the immobilized antibody of a conventional enzyme immunoassay, the present invention provides a GP73 monoclonal antibody and a superparamagnetic nano immunization microsphere with the GP73 monoclonal antibody coupled with the surface, and a human serum or plasma GP73 antigen detection method using the superparamagnetic nano immunization microsphere by double-antibody sandwich enzyme immunoassay or chemiluminescence method. An antigen for producing the GP73 monoclonal antibody has the following amino acid sequence AAAERGAVELKK. The superparamagnetic nano immunization microsphere has the characteristics of being capable of coupling more antibodies, fast in immunoreaction speed, high in specificity, good in repeatability, low in cost, and simple in experimental condition requirements and the like.
Owner:BEIJING INST OF HEPATOLOGY +1
Production and use of test paper for fast detecting deoxynivalenol in cereal
InactiveCN101482566AImprove the coupling ratioHigh sensitivityMaterial analysisSocial benefitsCellulose
The invention provides a preparation method of test paper scrip capable of quickly detecting deoxynivalenol test paper in grain and use thereof, comprising (1) DON monoclonal antibody-colloidal gold label; (2) coating of conjugate releasing mat; (3) coating of cellulose nitrate film; (4) production of test paper scrip. The preparation method has features of specificity, high sensitivity; visual detection result; simple operation and high speed, no need of professional operation, obtaining the detection result in 10min; easy large-scale popularization and application, widely application on foodstuff purchasing scene detection, food safety detection, customs quarantine control or the like, with wide market prospect, bigger economic, social benefits.
Owner:NANCHANG BOHENG BIOLOGICAL PROD
Colloidal gold immunity percolation sensitization method for detecting avian influenza virus and its reagent kit
The invention provides a colloidal gold immunity infiltration sensitivity raising method for checking avian influenza virus and a reagent kit therefore. The colloidal gold immuno infiltration sensitivity raising method comprises: (1) preparing an immunity infiltration test strip; (2) fixing influenza virus multi-clonal antibody on the immunity infiltration test strip; (3) preparing avian influenza virus gold marking probes; 4) preparing sensitivity raising agent; (5) detecting avian influenza virus. The invention utilizes the oxidation reduction reaction between chloric acid and ac=scorbic acid, to generate gold atoms which can be absorbed by colloidal gold, to position the deposition part of the colloidal gold to develop and enhance color, thereby improving the detection sensitivity on object antigen by 8 to 50 times. Based on colloidal gold spot immunity infiltration test method (double-antibody sandwich), the invention adopts nanometer gold particle sensitivity raising technique, to improve the detection sensivity on avian influenza virus, screen sample of high flux, and adapt avian influenza virus detection in basic layer or in-situ field.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Antibody Specific Binding to A Beta Oligomer and the Use
InactiveUS20100260783A1Harmful side-effectsSuppresses senile plaque amyloid formationNervous disorderHybrid immunoglobulinsDiseaseOligomer
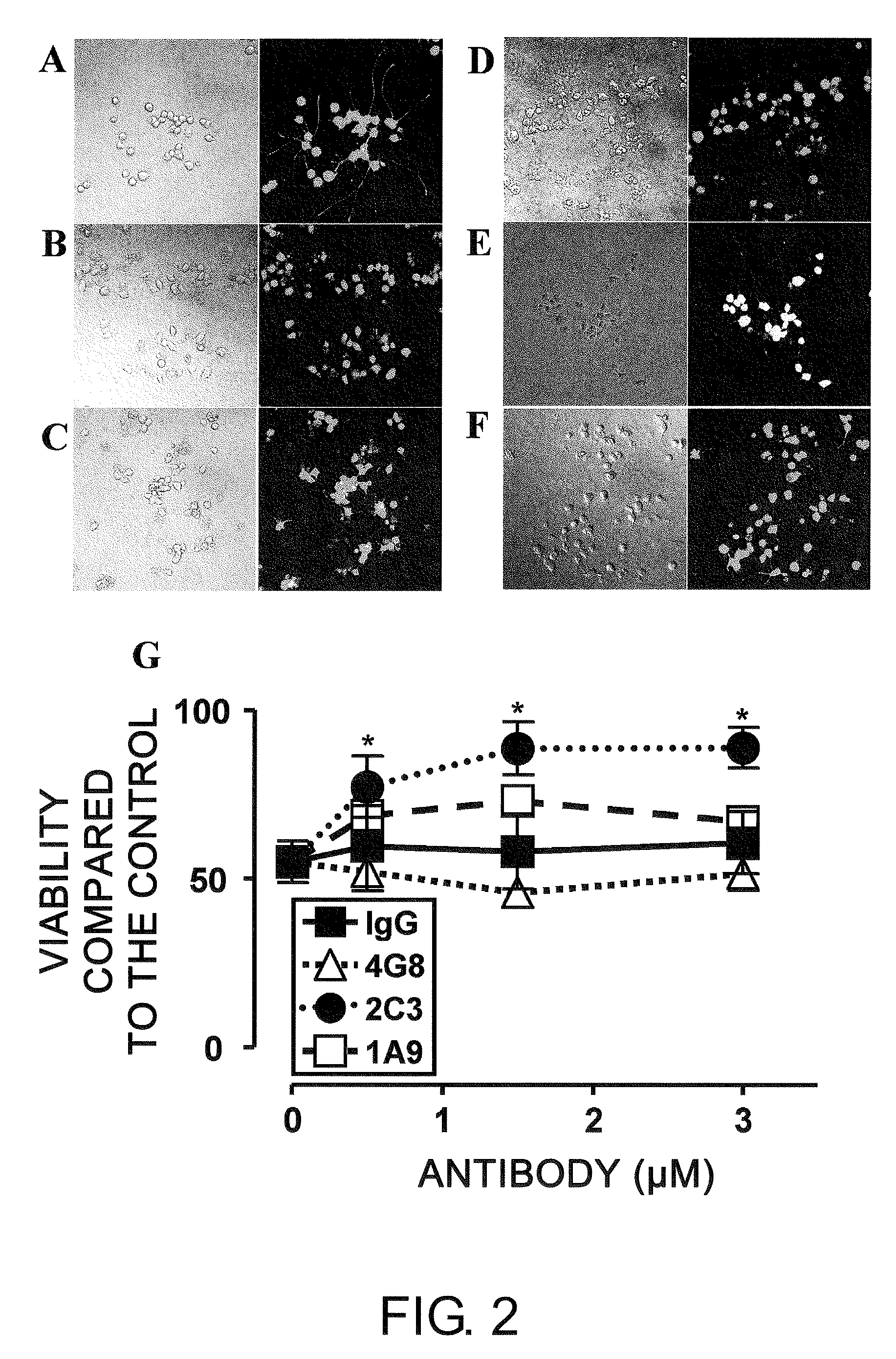
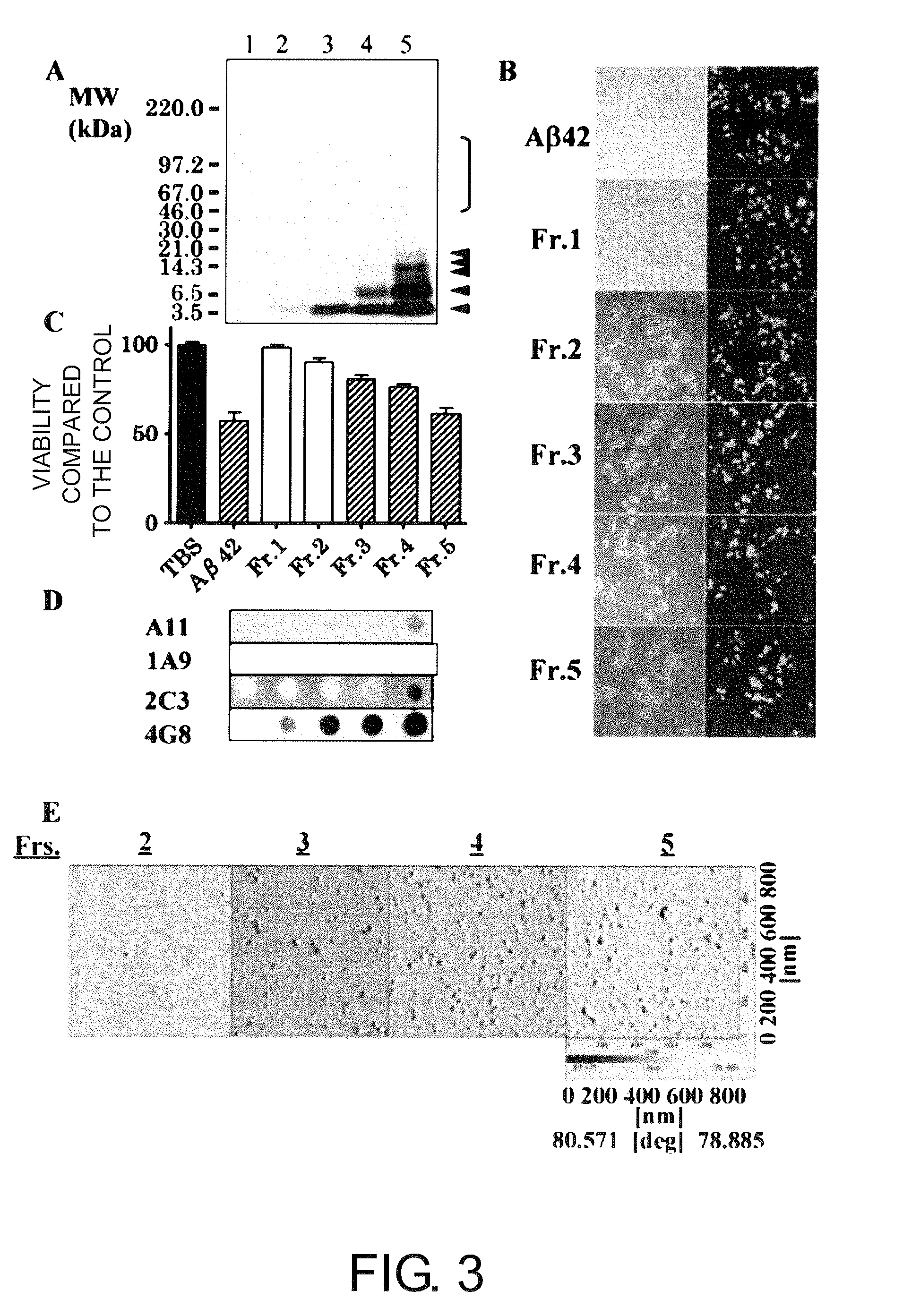
The present inventors successfully produced monoclonal antibodies that are specific to only soluble Aβ oligomers, but do not recognize soluble Aβ monomers, which are physiological molecules. It was demonstrated that the antibodies are useful as diagnostic / therapeutic monoclonal antibodies for Alzheimer's disease.
Owner:IMMUNAS PHARMA +1
In-vitro culture method for T lymphocytes
ActiveCN102433303AKeep aliveExtended shelf lifeBlood/immune system cellsAntiendomysial antibodiesWhite blood cell
The invention discloses an in-vitro culture method for T lymphocytes, and radically solves the problems of high cost, inconvenience of use and unfavorable medical research and clinical application existing in the conventional in-vitro culture method for the T lymphocytes. The method comprises the following steps of: directionally sorting the T lymphocytes, inducing, differentiating, culturing, amplifying and the like. A special cell factor combination is used for performing induction differentiation on the T lymphocytes; three factors are a CD3 monoclonal antibody, gamma-interferon and interleukin-1 alpha respectively; the optimal concentration ratio of the three factors is 10:1:1; the total final concentration of the three factors in a culture medium is 1,200ng / ml; and the experiment shows that the combination is an extremely effective way of inducing the T lymphocytes to differentiate.
Owner:LIAONING MEDI BIOTECH CO LTD
Qualitative semi-quantitative dual-purpose female ovulation hormone detection test paper and colorimetric card
InactiveCN102183663ABiological testingColor measuring using colour chartsSpot urineLH - Luteinizing hormone
The invention discloses a test paper for qualitative semi-quantitative dual-purpose detection of female ovulation, which is formed mainly by overlapping and connecting a bottom plate, a nitrocellulose membrane, an absorption pad, a polyester film and a glass fiber membrane mutually, wherein the middle of the bottom plate is adhered with the nitrocellulose membrane on which two antibody lines are engraved, one line is a detection line coated by a first anti-LH monoclonal antibody, and the other line is a quality control line coated by goat anti mouse IgG monoclonal antibody; and the upper edge position of the nitrocellulose membrane is adhered with the water absorption pad, the lower edge position of the nitrocellulose membrane is adhered with a combined pad which combines another anti-LH monoclonal antibody of marking colloidal gold or color rubber latex, and the lower edge position of the combined pad is adhered with a sample pad. A woman can use the test paper of the invention at home to qualitatively and semi-quantitatively detect the content of LH luteinizing hormone of the urine or blood of the woman, and knows the ovulation date, or knows whether hormonal infertility exists or not.
Owner:WUHAN J H BIO TECH
Immune regulation based on the targeting of early activation molecules
InactiveUS20050002929A1Increase concentrationIncrease proliferative activitySenses disorderNervous disorderDiseaseAgonist
Disclosed are methods of treating subjects having disorders or conditions characterized by an unwanted immune response including administering an effective amount of an early activation molecule agonist, antagonist or depletor, to the subject. Human monoclonal antibodies specific to the early activation molecules, and methods of use, are also disclosed.
Owner:GOMEZ PILAR LAUZURICA +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com