Chemoluminescence immunoassay measuring kit and preparation method thereof for triiodothyronine magnetic particles
A triiodothyronine and chemiluminescence technology, applied in the field of immunoassay, can solve the problems of low sensitivity of enzyme-linked immunoassay, expensive instruments and reagents, large loss of activity, etc., and achieve good market application prospects, low price, and linear performance. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
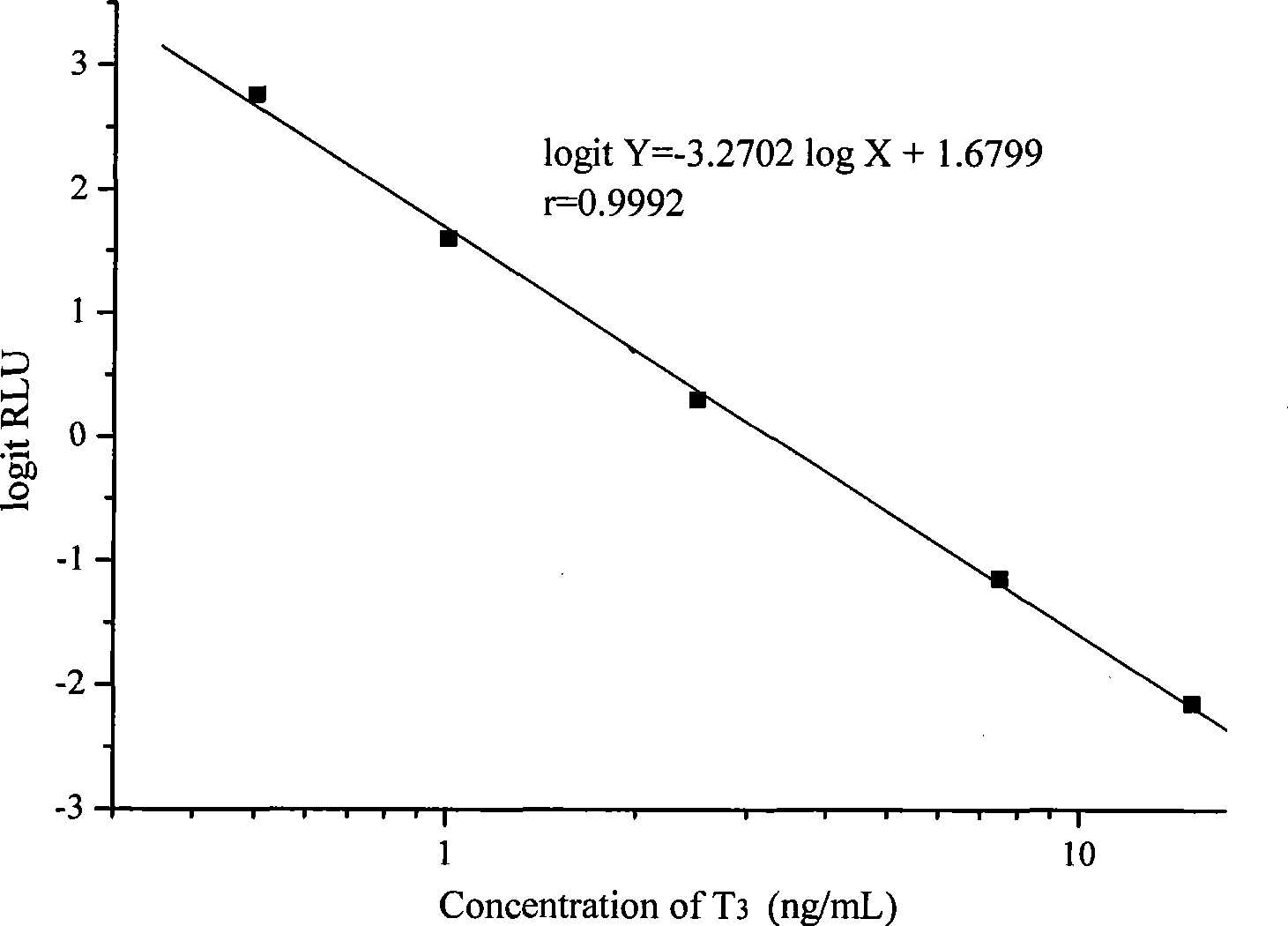
[0033] Embodiment 1 prepares T of the present invention 3 Magnetic particle chemiluminescence immunoassay assay kit
[0034] 1. T 3 Preparation of calibrator
[0035] T 3 The pure product was diluted into a calibrator, and divided into 6 bottles of 0, 0.5, 1.0, 2.5, 7.5, 15ng / mL.
[0036] 2. Preparation of anti-FITC monoclonal antibody coated magnetic particle solution
[0037] Activate the magnetic particles with a particle size of 2-3 μm with glutaraldehyde, stir at room temperature, mix for 2 hours, apply a magnetic field, let stand for 20-25 minutes, pour out the supernatant, and use 0.01mol / L with a pH value of 7.4 Wash with phosphate buffer three times, and suspend with this solution, the concentration is 50-100 mg / mL; add 60-100 μg of anti-FITC monoclonal antibody to each ml of suspension, stir overnight at 4°C, add a magnetic field, and let it stand for 10 ~ 15min, pour out the supernatant, and block with phosphate buffer (pH 7.2) containing 0.2% ~ 1.0% bovine ser...
Embodiment 2
[0064] Embodiment 2 The using method of kit of the present invention
[0065] 1. Sample pretreatment
[0066] The fasting morning serum samples were taken from people, centrifuged at 3000rpm for 5min, and the upper serum was taken for analysis.
[0067] 2. Detection method
[0068] Follow the steps below to use this kit:
[0069] 1) Preparation of washing buffer: dilute the washing buffer provided in the kit with distilled water 20 times;
[0070] 2) Take the calibrators, antibody-coated magnetic particles, biotin-labeled substances, FITC-labeled substances and enzyme-labeled substances out of the refrigerator at 4°C, place them for 15 minutes, and equilibrate to room temperature;
[0071] 3) Number the round-bottom polystyrene test tubes required for the experiment and fix them on the plate holder;
[0072] 4) Add 0, 0.5, 1.0, 2.5, 7.5, 15 ng / mL of 0, 0.5, 1.0, 2.5, 7.5, 15 ng / mL to the reaction wells respectively, and add 1 blank tube for each test, and then add 50 μL to...
Embodiment 3
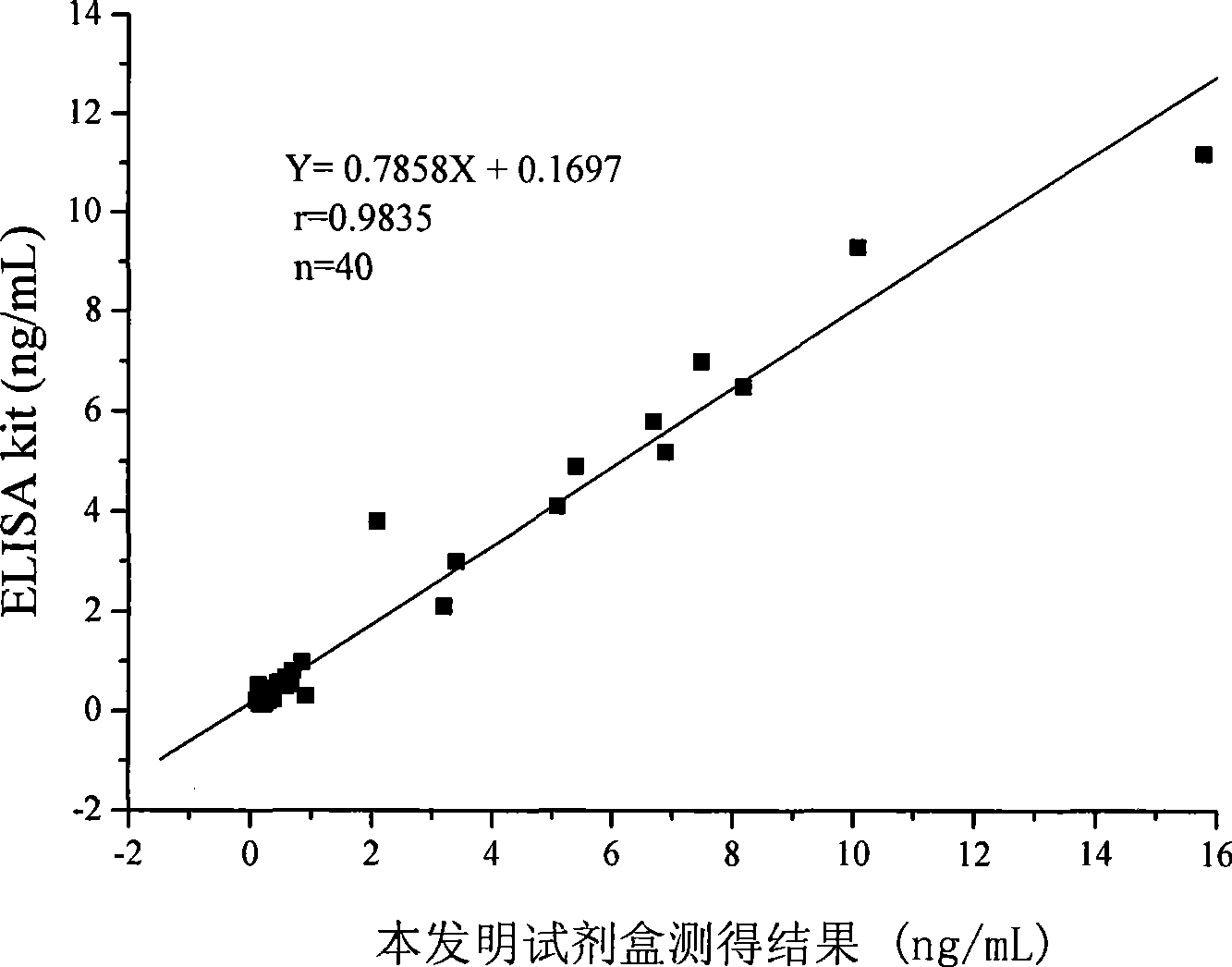
[0078] The methodological examination of the kit of the present invention of embodiment 3
[0079] The test kit prepared in Example 1 is tested according to the routine test procedures in the art. The present invention has carried out specific experimental operations on the precision, accuracy, sensitivity, specificity and stability of the test kit, and the results are as follows :
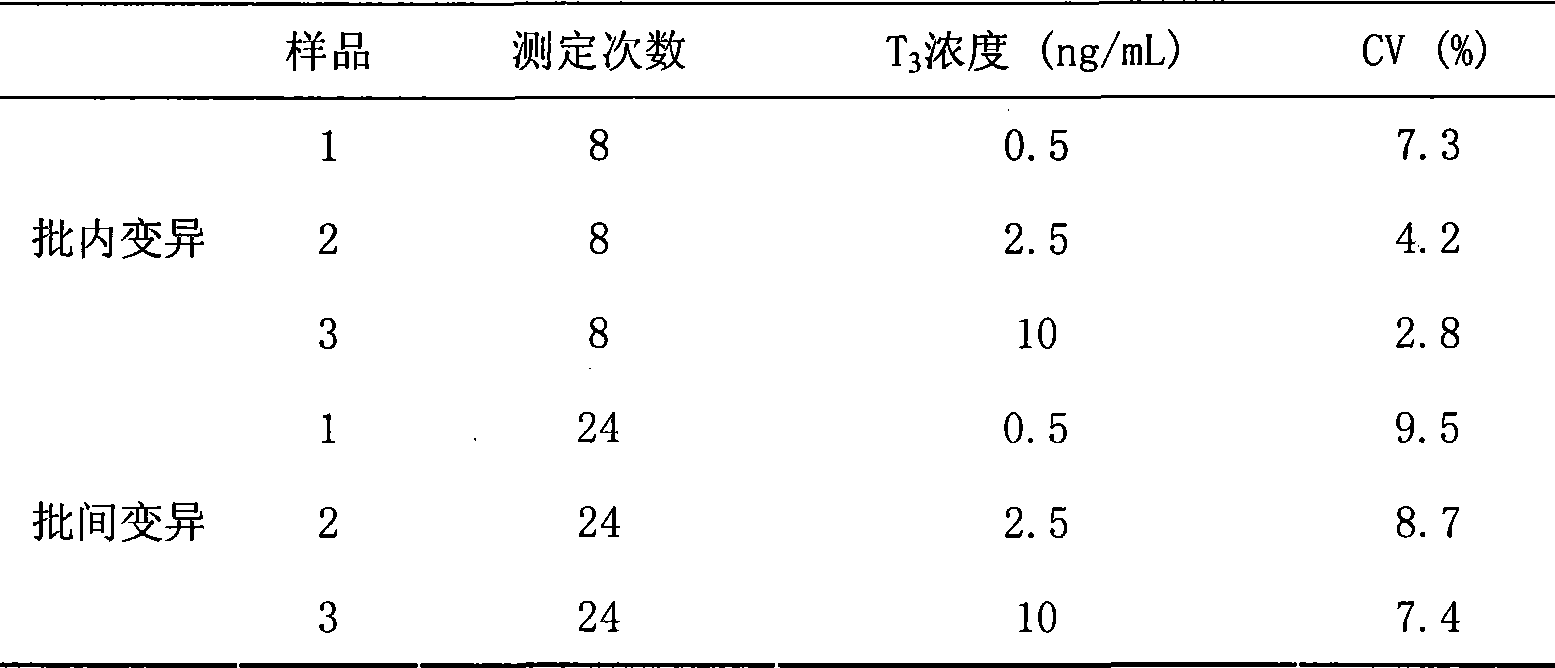
[0080] 1. Determination of kit precision
[0081] Three batches of the kits prepared in Example 1 were used for precision experiments, and three different concentrations of serum, namely low, medium, and high, were measured respectively. 8 wells were measured in parallel, and repeated 3 times to obtain the intra-assay and multiple of each analysis. Inter-assay variability (Table 1). The intra-assay and inter-assay coefficients of variation were both less than 10%.
[0082] Table 1 Inspection of method precision
[0083]
[0084] 2. Determination of kit accuracy
[0085] Accuracy refers to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com