Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
200 results about "B-cell lymphoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The B-cell lymphomas are types of lymphoma affecting B cells. Lymphomas are "blood cancers" in the lymph nodes. They develop more frequently in older adults and in immunocompromised individuals. B-cell lymphomas include both Hodgkin's lymphomas and most non-Hodgkin lymphomas. They are typically divided into low and high grade, typically corresponding to indolent (slow-growing) lymphomas and aggressive lymphomas, respectively. As a generalisation, indolent lymphomas respond to treatment and are kept under control (in remission) with long-term survival of many years, but are not cured. Aggressive lymphomas usually require intensive treatments, with some having a good prospect for a permanent cure.
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
Radiolabeling kit and binding assay
InactiveUS20020102208A1No reduction in immunoreactivityNegligible lossIn-vivo radioactive preparationsDepsipeptidesTherapeutic antibodyAssay
Antibody binding assays and radiolabeling kits are disclosed for radiolabeling and testing therapeutic antibodies in the commercial setting. In particular, the kits are designed for making and evaluating radiolabeled anti-CD20 conjugates to be used for the treatment and imaging of B cell lymphoma tumors. All kit reagents are sterile and are designed to achieve a high level of antibody radiolabeling and product stability with results which are highly reproducible.
Owner:BIOGEN INC
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS6896885B2Avoiding and decreasing and resistanceIncrease ratingsOrganic active ingredientsIn-vivo radioactive preparationsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies.The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN® (rituximab).
Owner:BIOGEN INC
Heterocyclic compounds and their uses
ActiveUS20090137581A1Low inhibitory potencyInhibitory activityBiocideSenses disorderDiseaseMyeloid leukemia
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
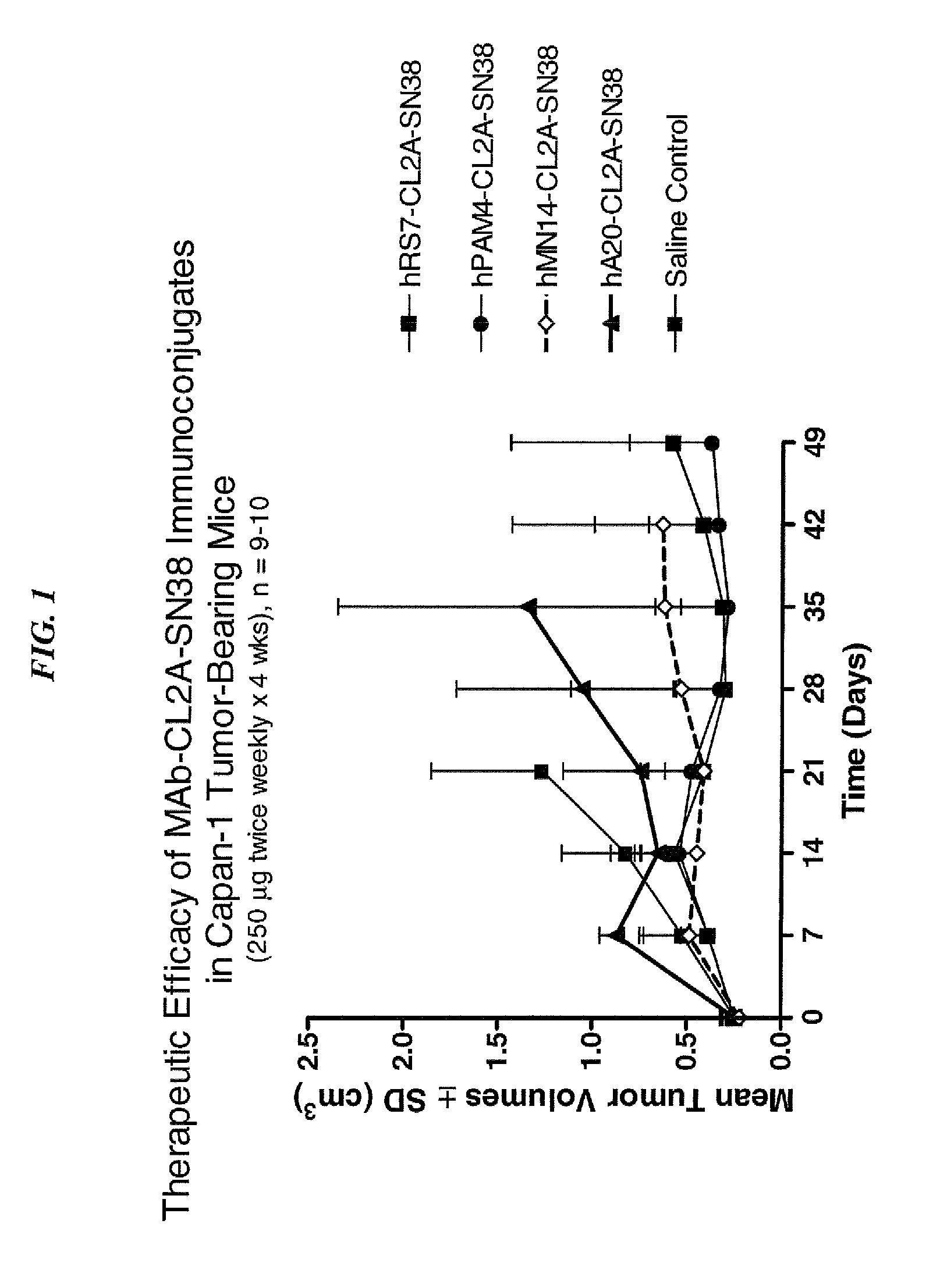
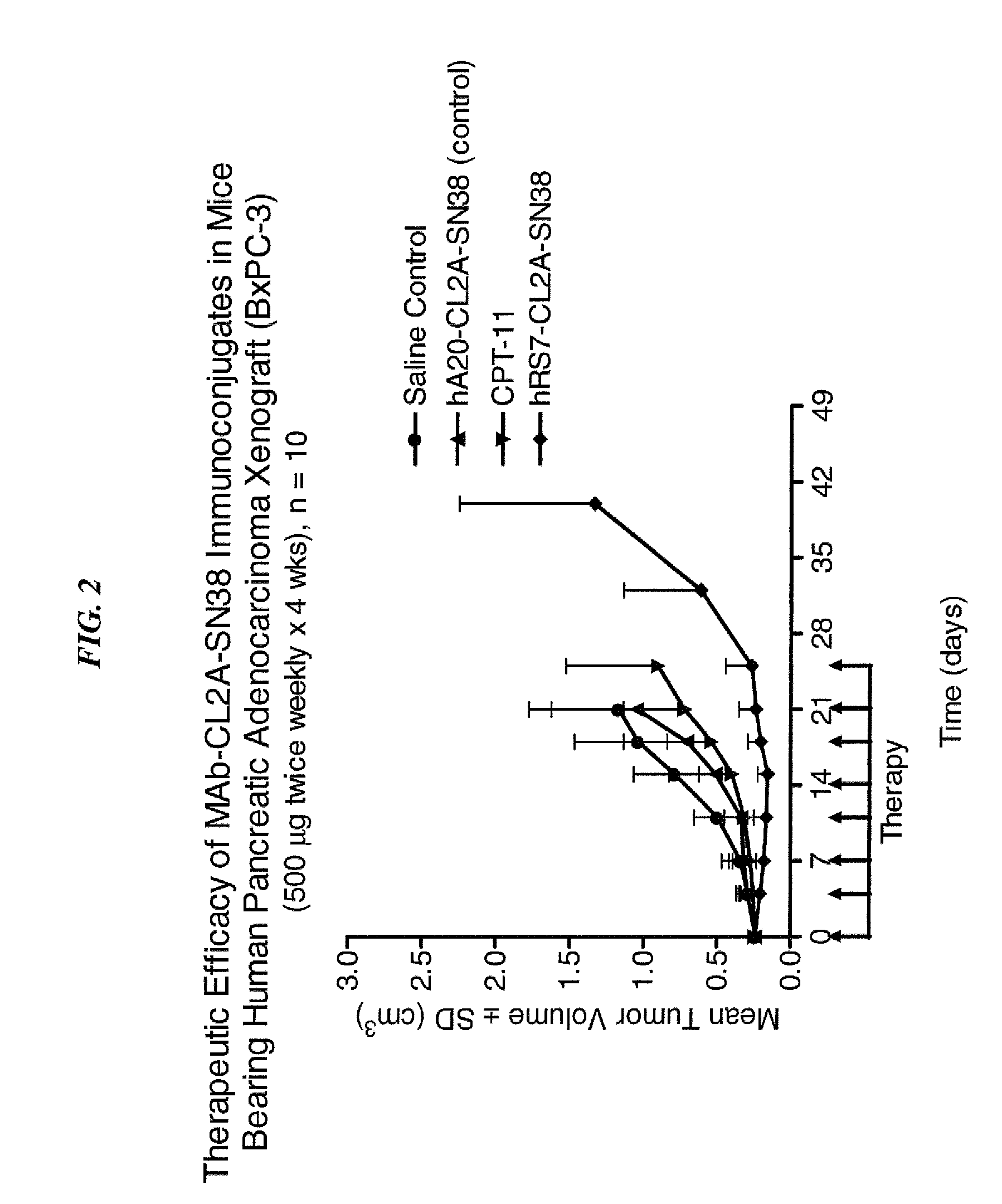
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
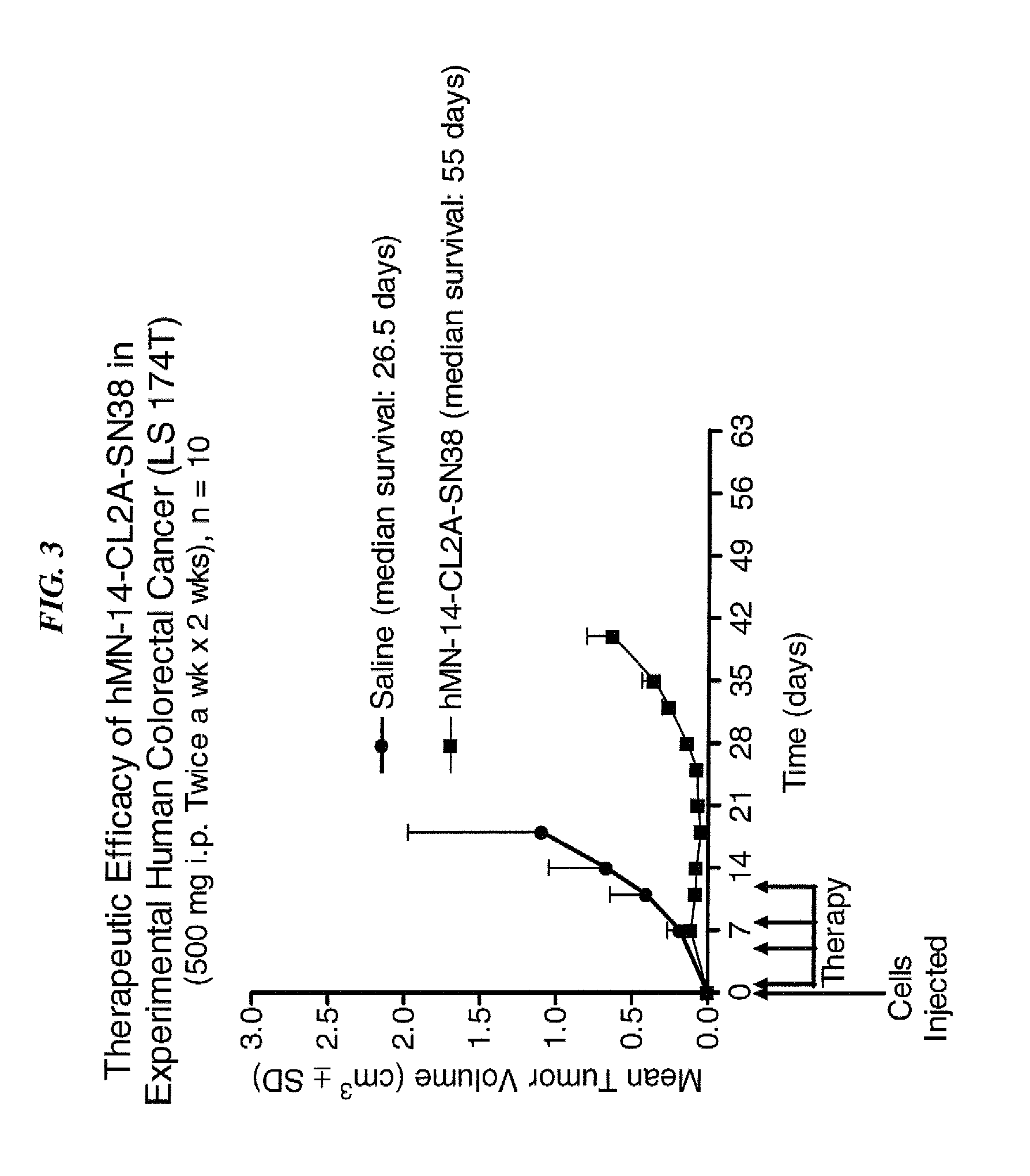
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
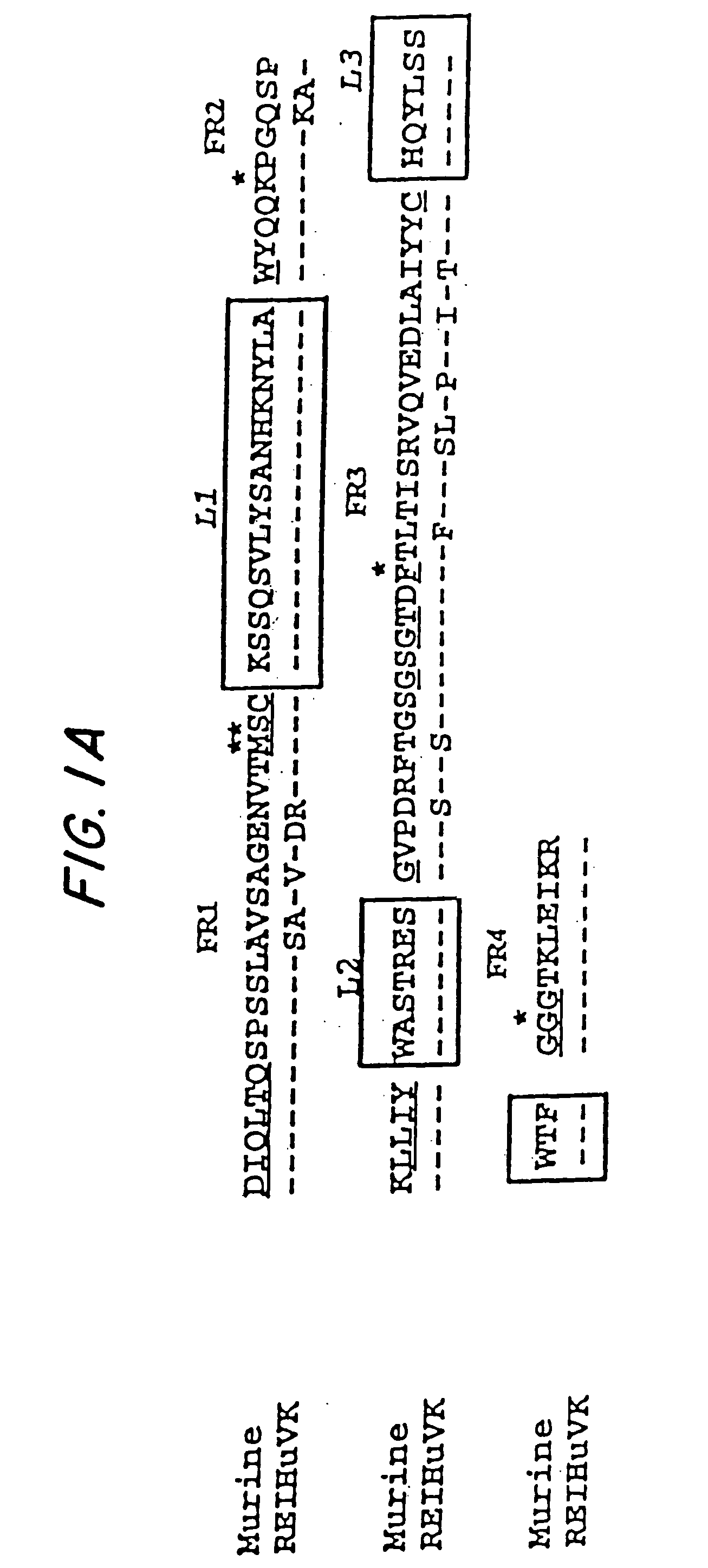
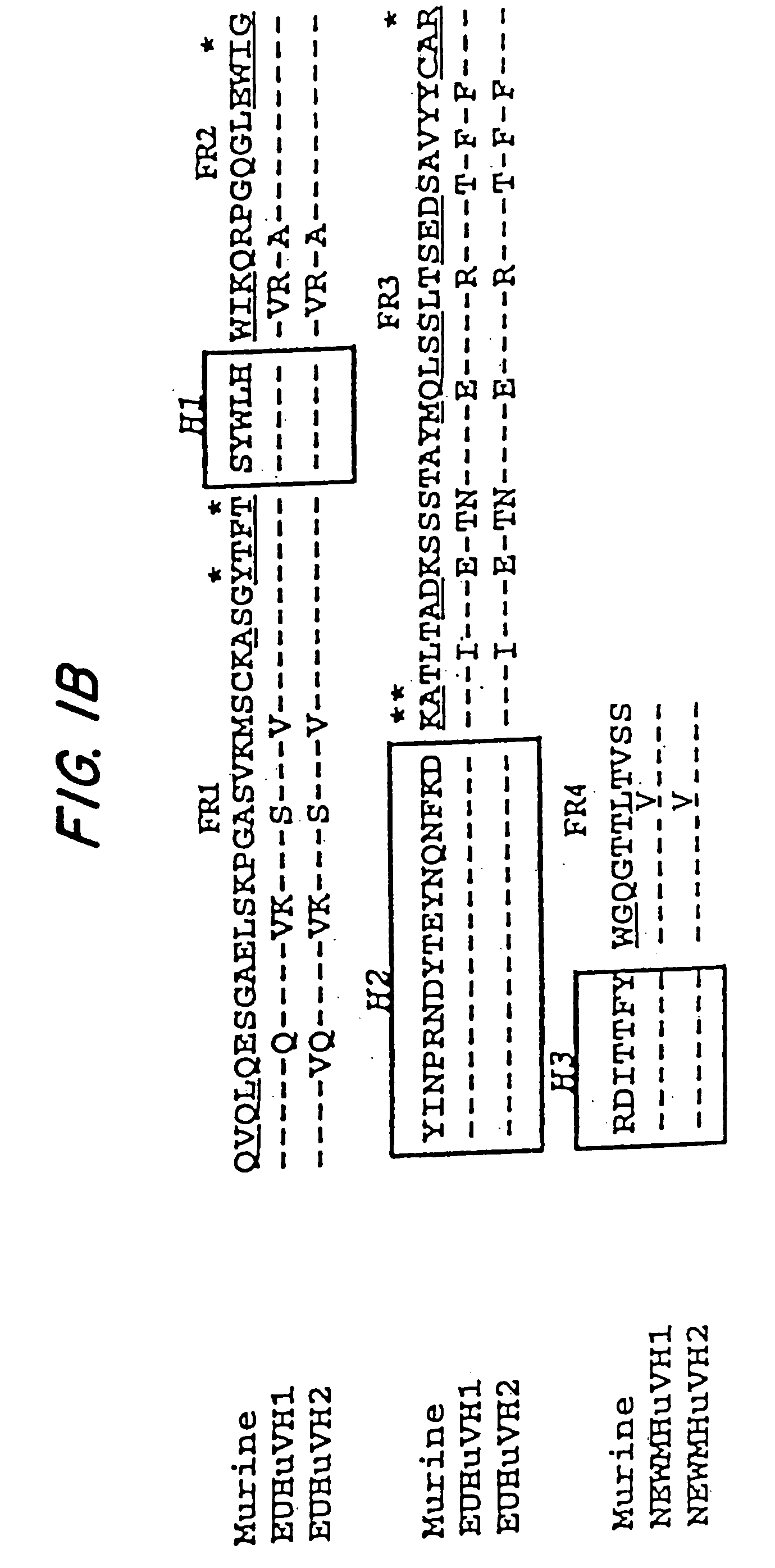
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Heterocyclic compounds and their uses
InactiveUS20100331306A1Inhibit biological activityLow inhibitory potencyBiocideSenses disorderDiseaseB-cell acute lymphoblastic leukaemia
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity. The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Use of chimeric Anti-cd20 antibody as in vitro or in vivo purging agent in patients receiving bmt or pbsc transplant
InactiveUS20110165159A1Reduce morbidityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsIn vivoDISEASE RELAPSE
Owner:BIOGEN INC
Compositions and methods useful for treating diseases
ActiveUS20120225851A1Blocking unwanted cell survival activityBiocideOrganic chemistryDiseaseAutoimmune disease
The present invention relates to a chemotherapeutic cancer treatment in which compounds of Formula Ia′, Ib′, Ic′, or II′ (referred to as a group as BH3Is) are administered to a mammal for the treatment of B-cell Lymphoma or other hematopoietic cancers, including diseases associated with MCL-1. In another aspect, the invention provides a method for treating particular types of hematopoietic cancers, such as B-cell lymphoma, using a combination of one or more compounds selected from the group consisting of compounds or Formula Ia, Ib, Ic, or II in combination with other therapies, for example, a class of therapeutics known as 26S proteosome inhibitors, such as, for example, Bortezomib. In another aspect the present invention relates to autoimmune treatment with pharmaceutical compositions comprising one or more compounds of Formula Ia′, Ib′, Ic′, or II′. In another aspect, this invention relates to methods for identifying compounds, for example, compounds of the BH3 mimic class, that have unique in vitro properties that predict in vivo efficacy against B-cell lymphoma tumors and other cancers as well as autoimmune disease.
Owner:EUTROPICS PHARMA
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
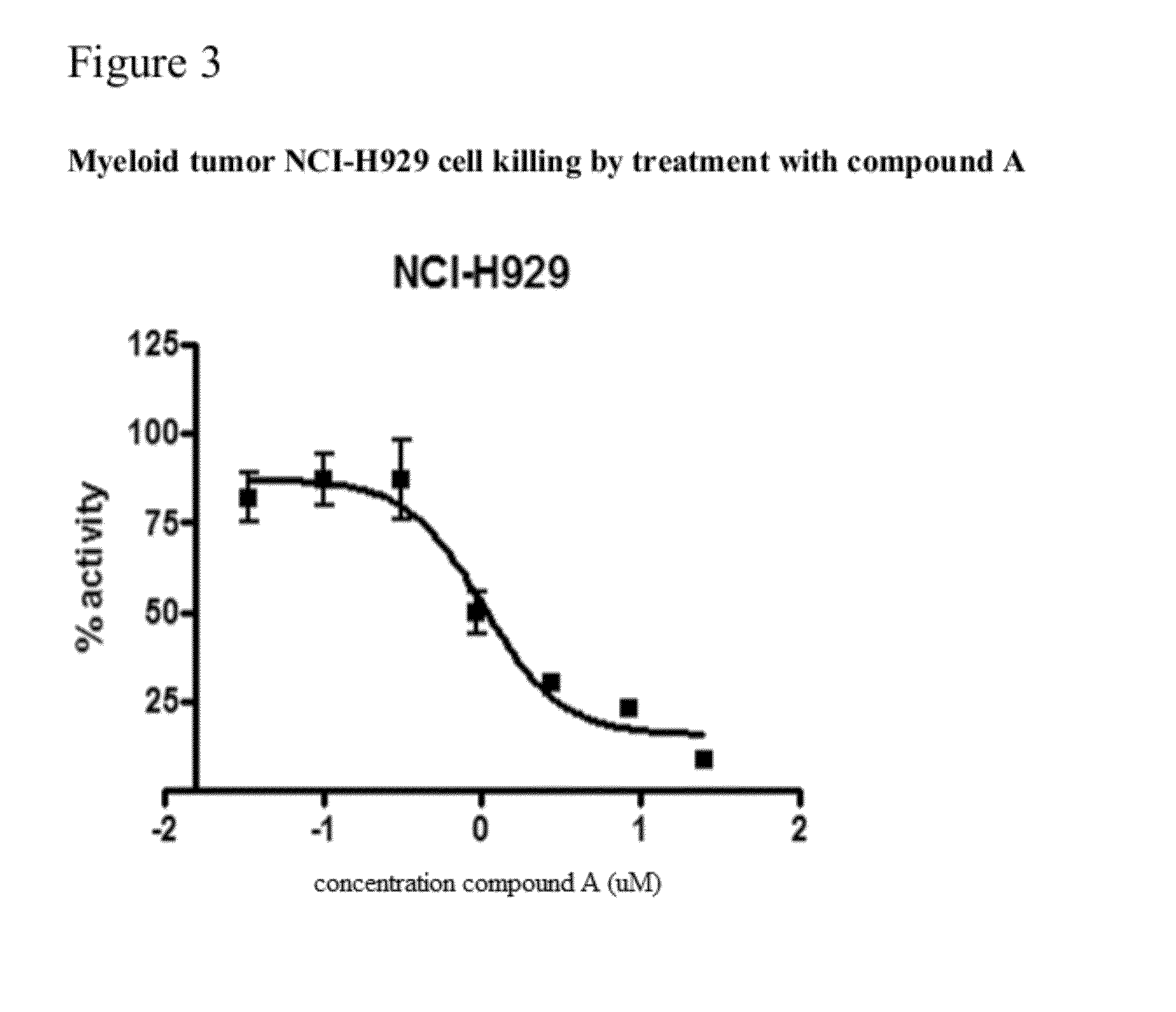
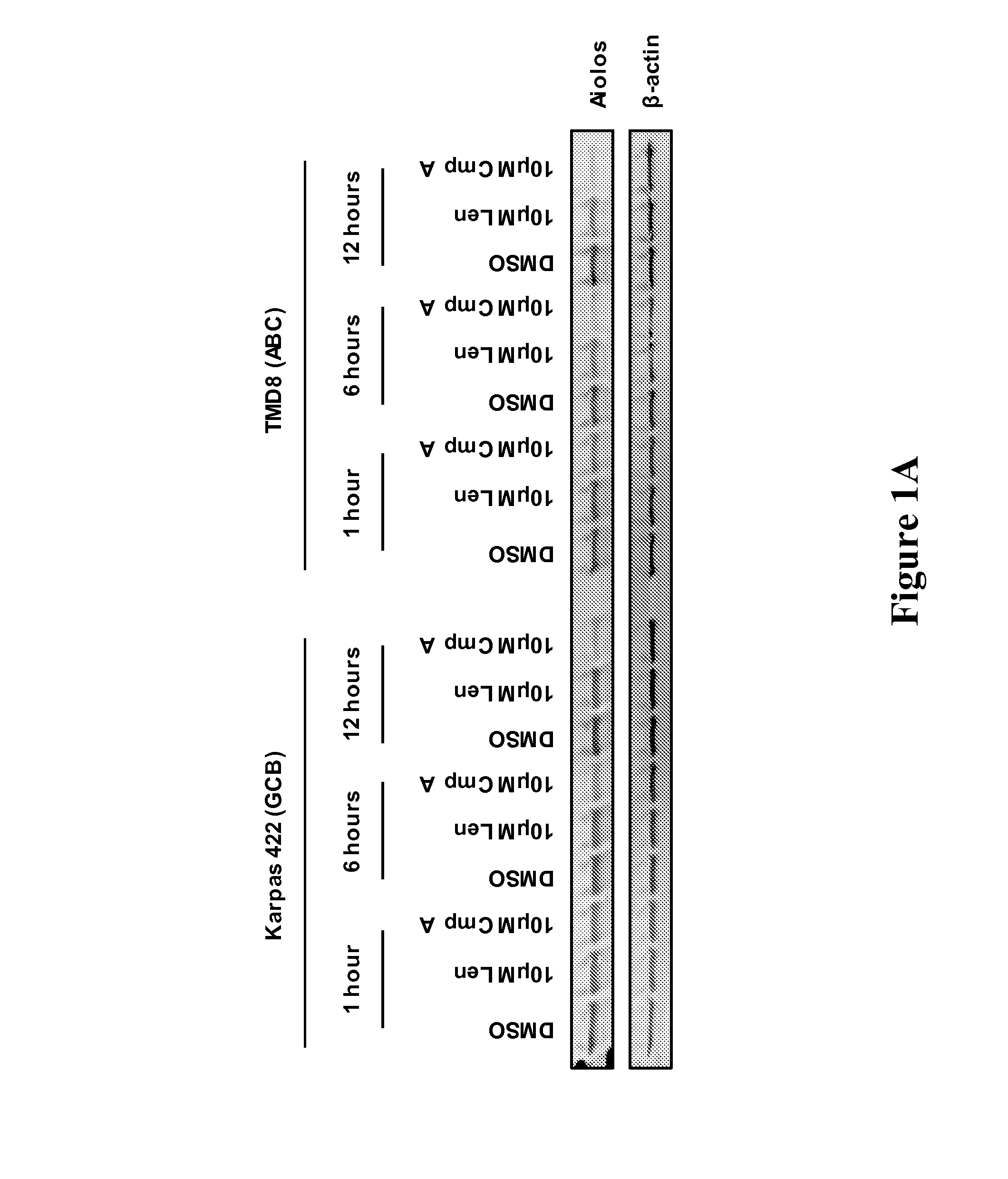
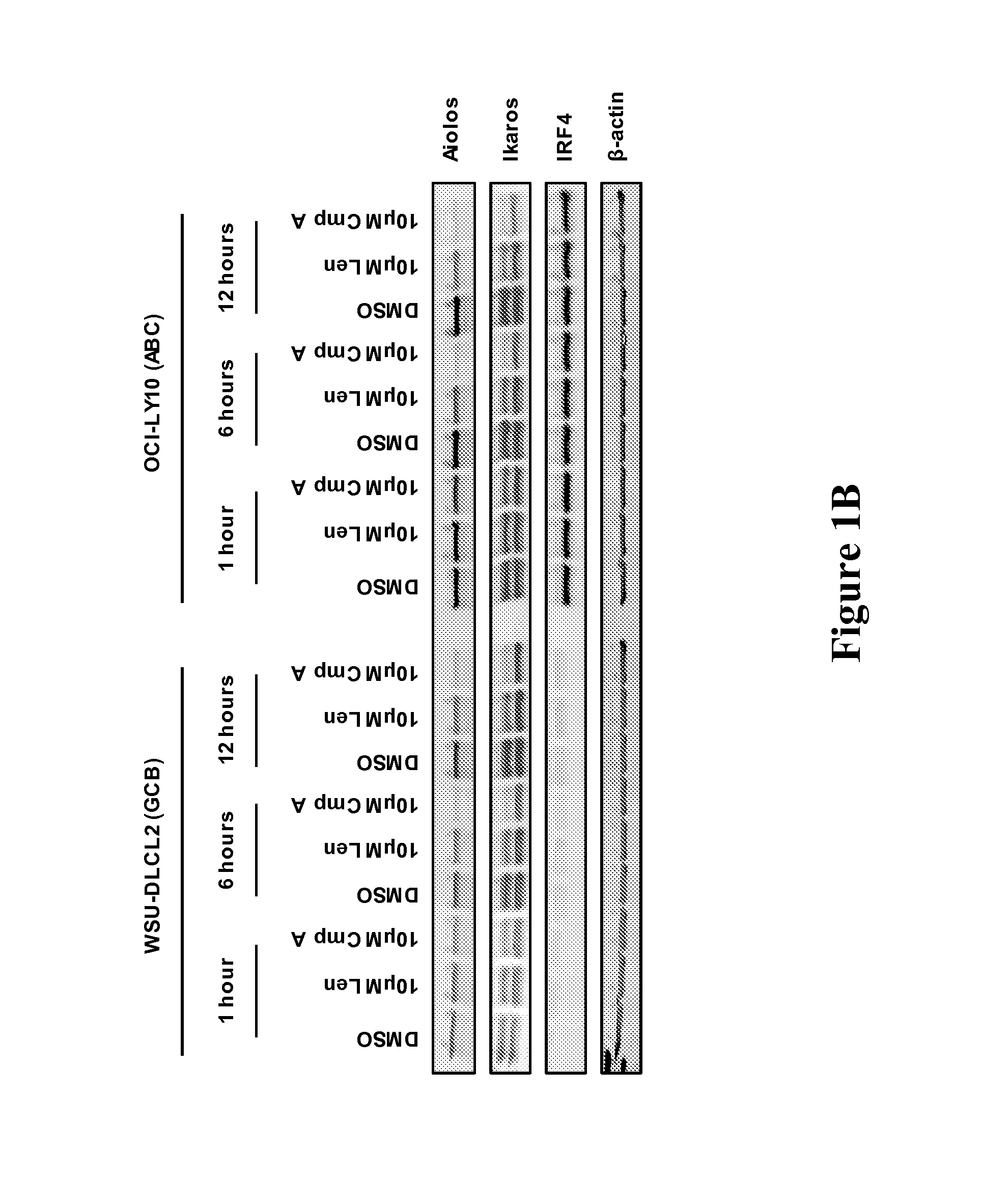
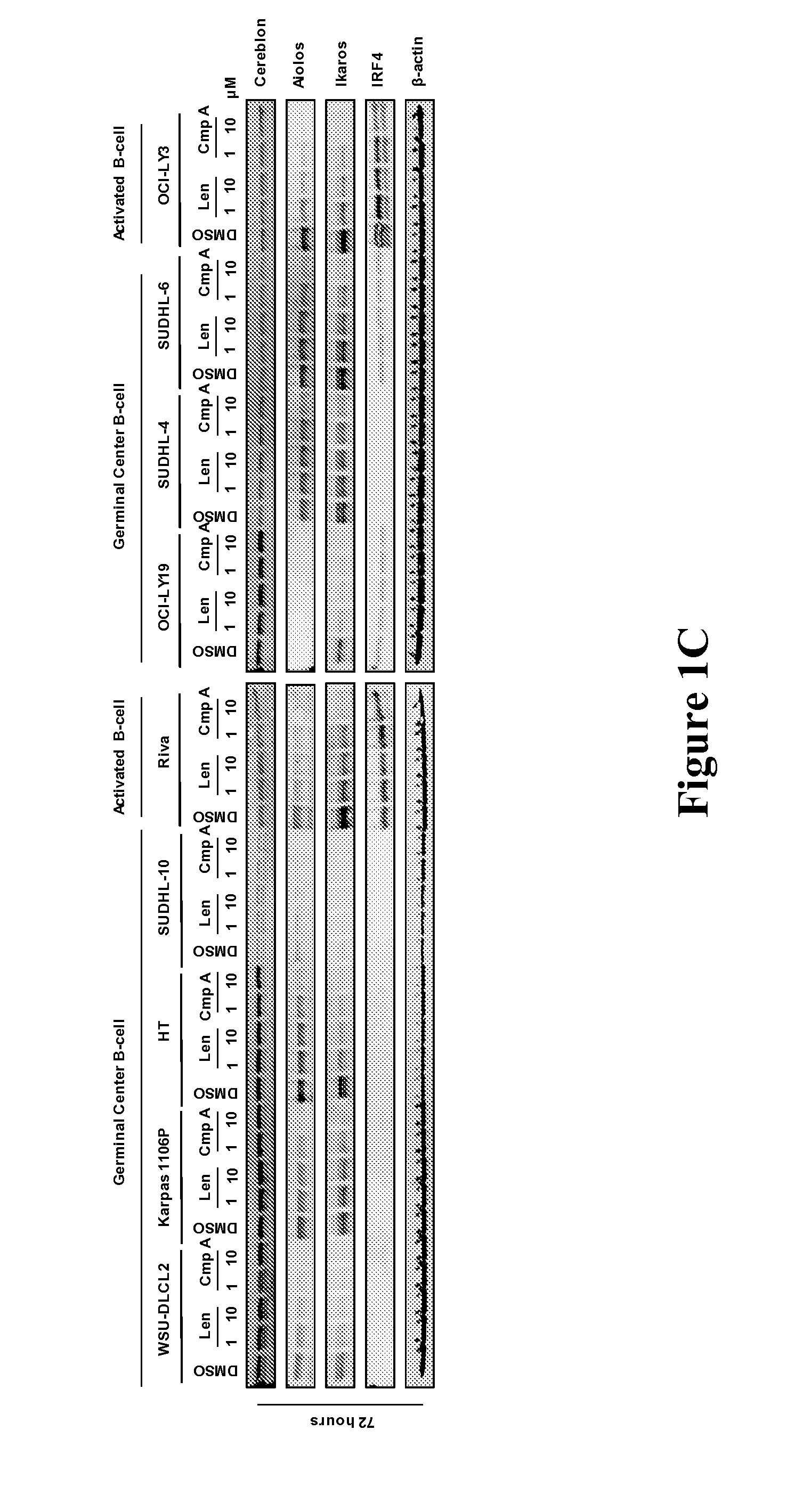
Methods for determining drug efficacy for the treatment of diffuse large b-cell lymphoma, multiple myeloma, and myeloid cancers
InactiveUS20160313300A1Organic active ingredientsNervous disorderMyeloid leukemiaBiomarker (petroleum)
Provided herein, in some embodiments, are methods of using certain cereblon-associated proteins, such as Aiolos, Ikaros, interferon (IFN), and IFN pathway proteins, casein kinase 1, alpha 1 (CSNK1A1), and ZFP9, as biomarkers for use in predicting and monitoring clinical sensitivity and therapeutic response to certain compounds in patients having various diseases and disorders, such as cancers (e.g., diffuse large B-cell lymphoma (DLBCL), multiple myeloma (MM), myelodysplasia syndromes (MDS) and acute myeloid leukemia (AML)) and IFN-associated disorders. Also provided herein, in certain embodiments, are methods of determining the efficacy of an immunomodulatory compound.
Owner:CELGENE CORP
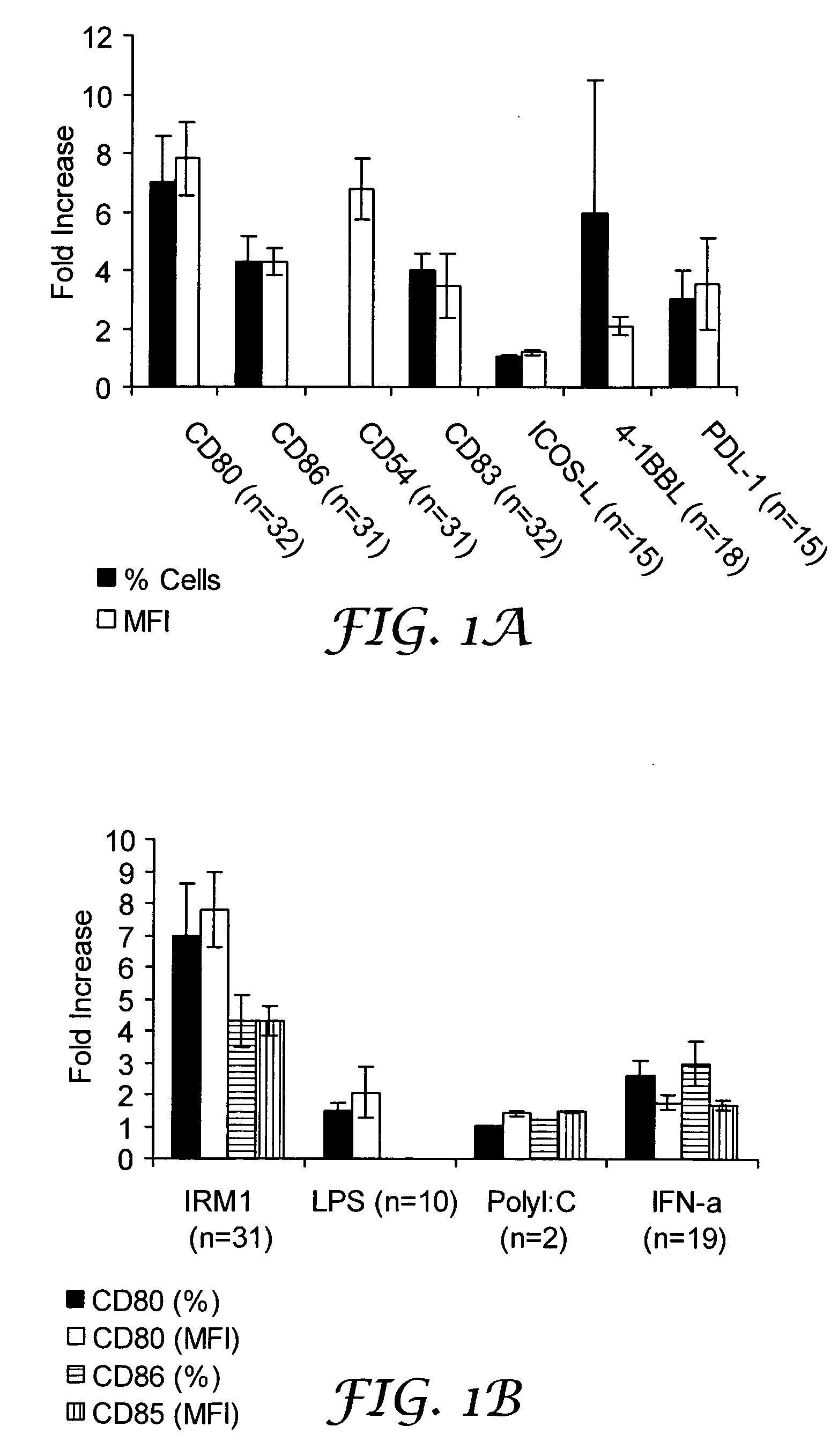
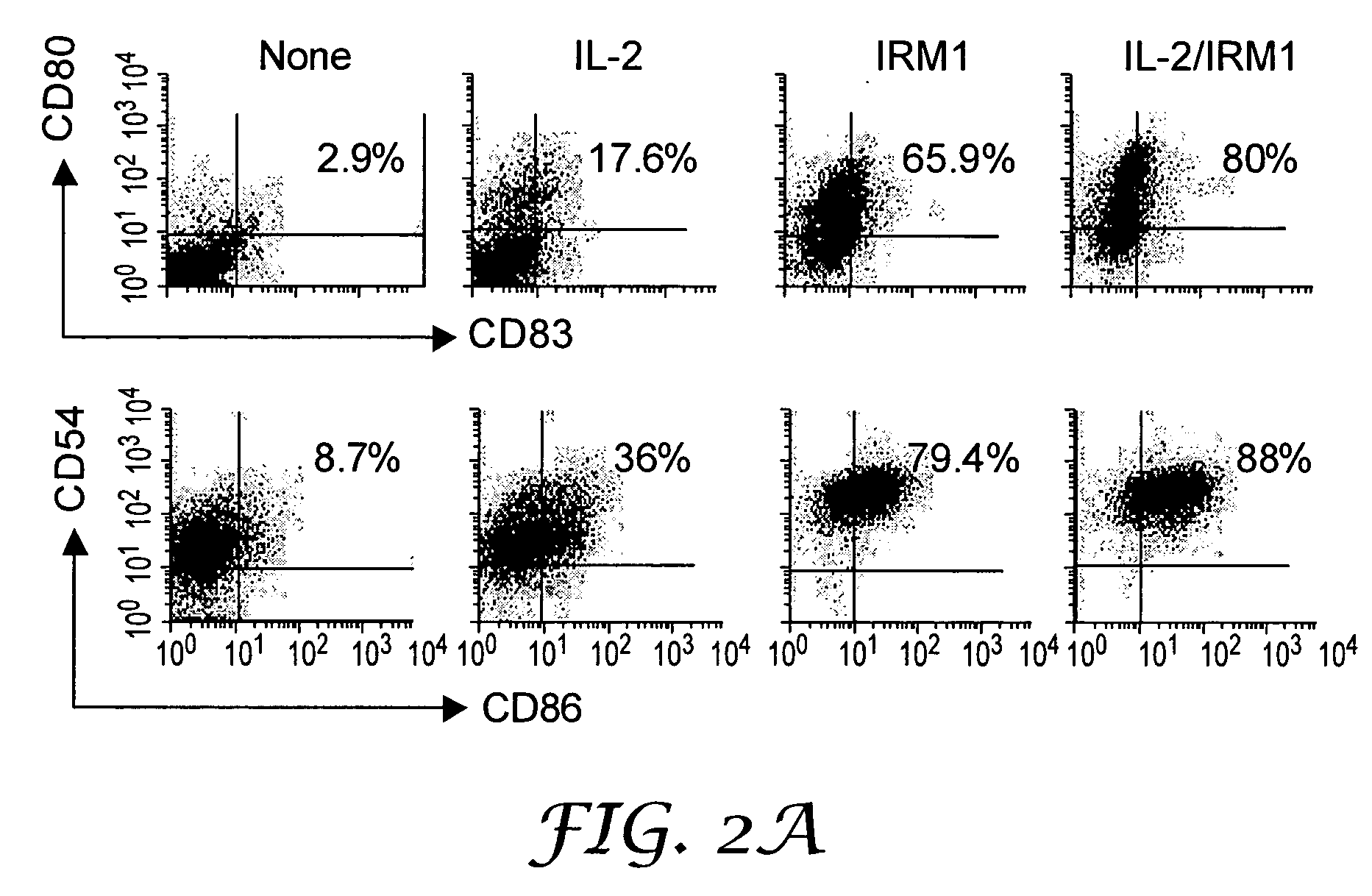
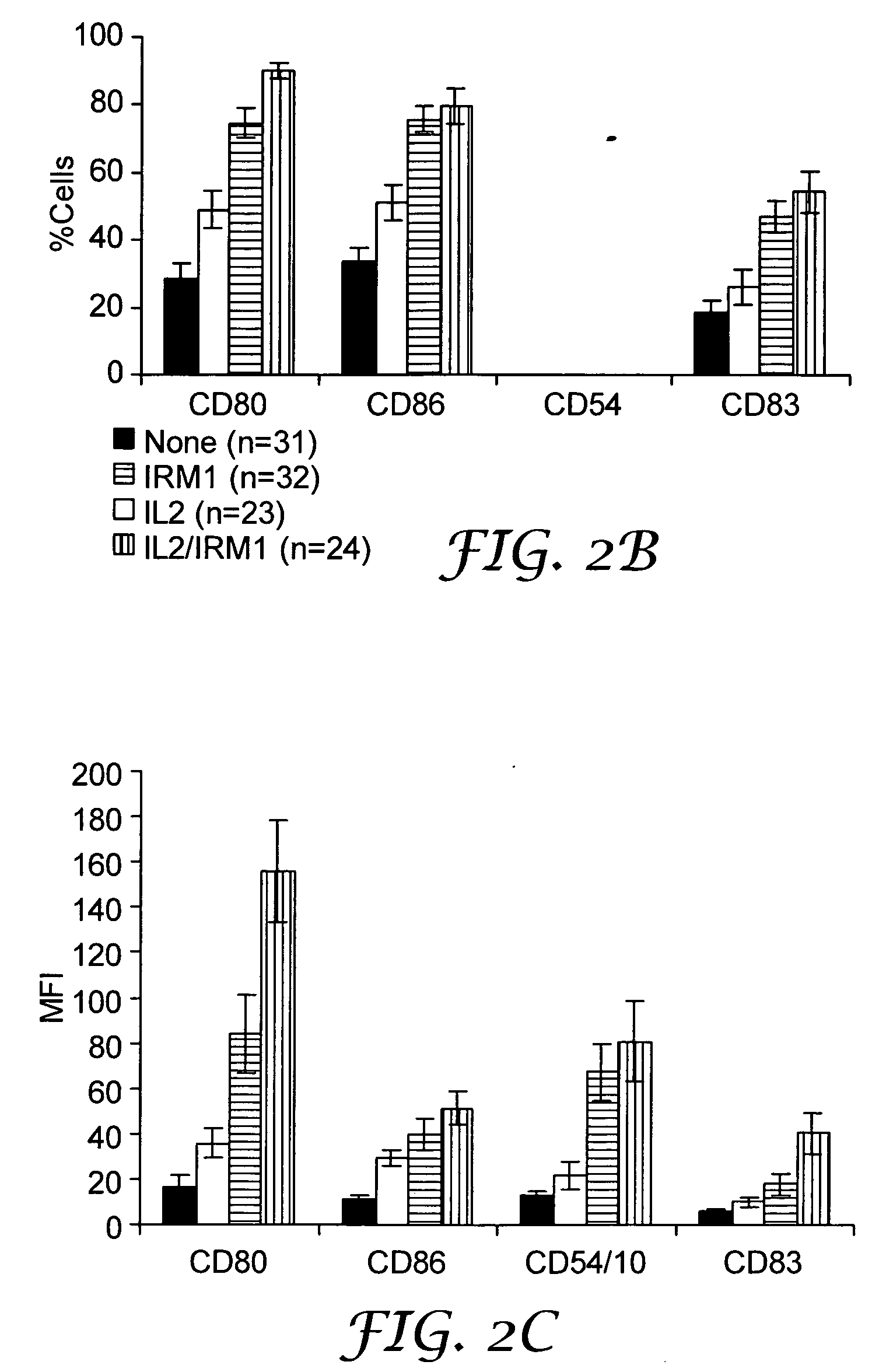
Treatment for CD5+ B cell lymphoma
InactiveUS20050054665A1Ameliorate at least one symptomDecrease in peripheral blood lymphocytes, lymphadenopathy, or splenomegalyBiocidePeptide/protein ingredientsTLR8Agonist
The present invention provides methods for increasing expression of cell surface molecules of CD5+ B cell lymphoma cells by contacting cells with immune response modifiers. The invention also provides methods for the treatment of CD5+ B cell lymphomas, including chronic lymphocytic leukemia and small lymphocytic lymphoma, by administering immune response modifier compounds to a subject in need of such treatment. Suitable immune response modifier compounds include agonists of TLR7 and / or TLR8.
Owner:COLEY PHARMA GRP INC
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelodysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
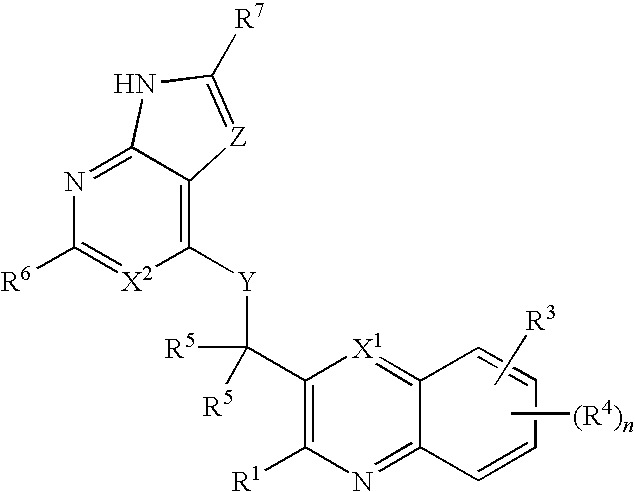
Substituted quinolines and their uses in treatment of inflammatory and related conditions
Substituted bicyclic heteroaryls having the general formulaand compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
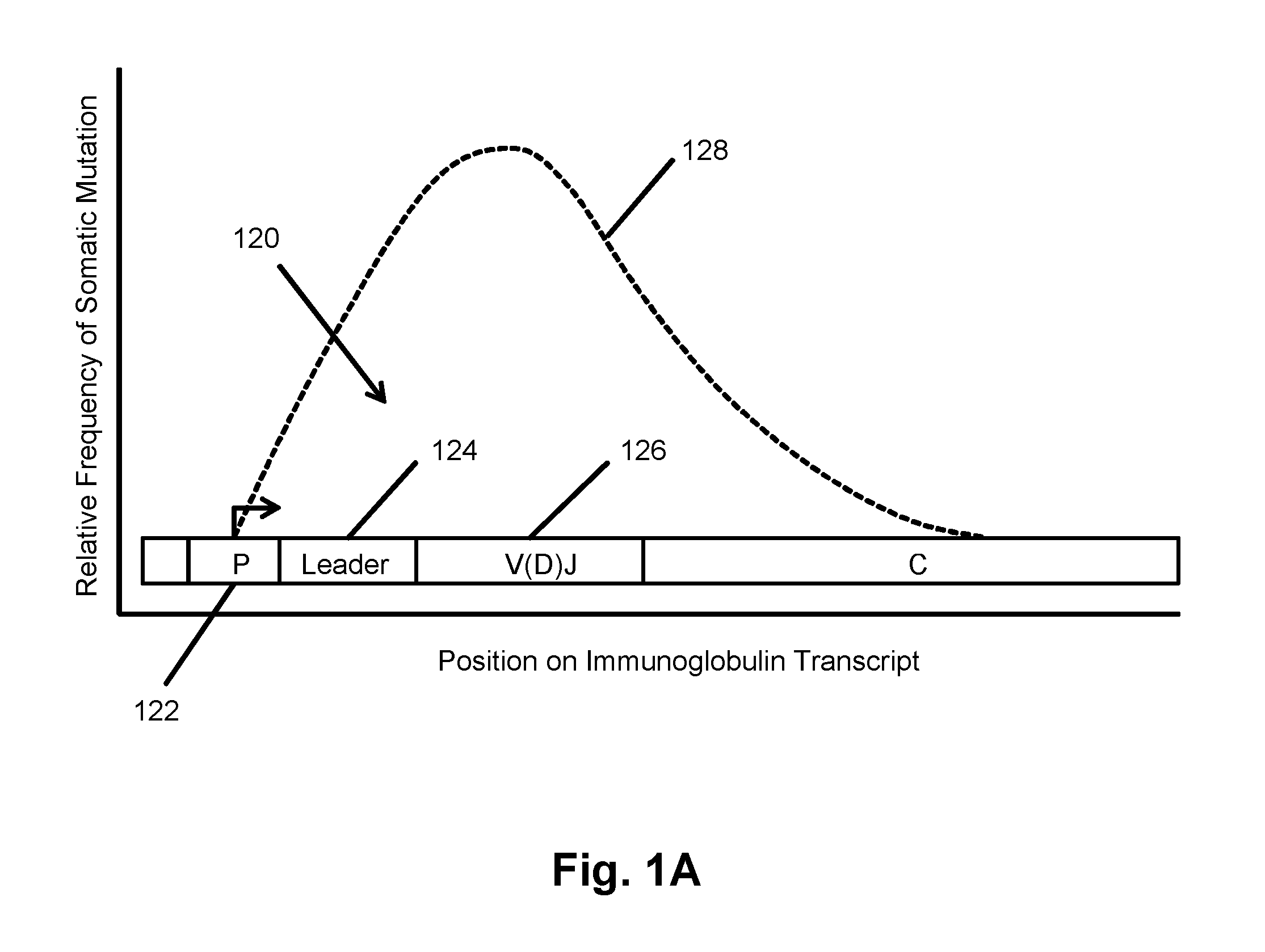
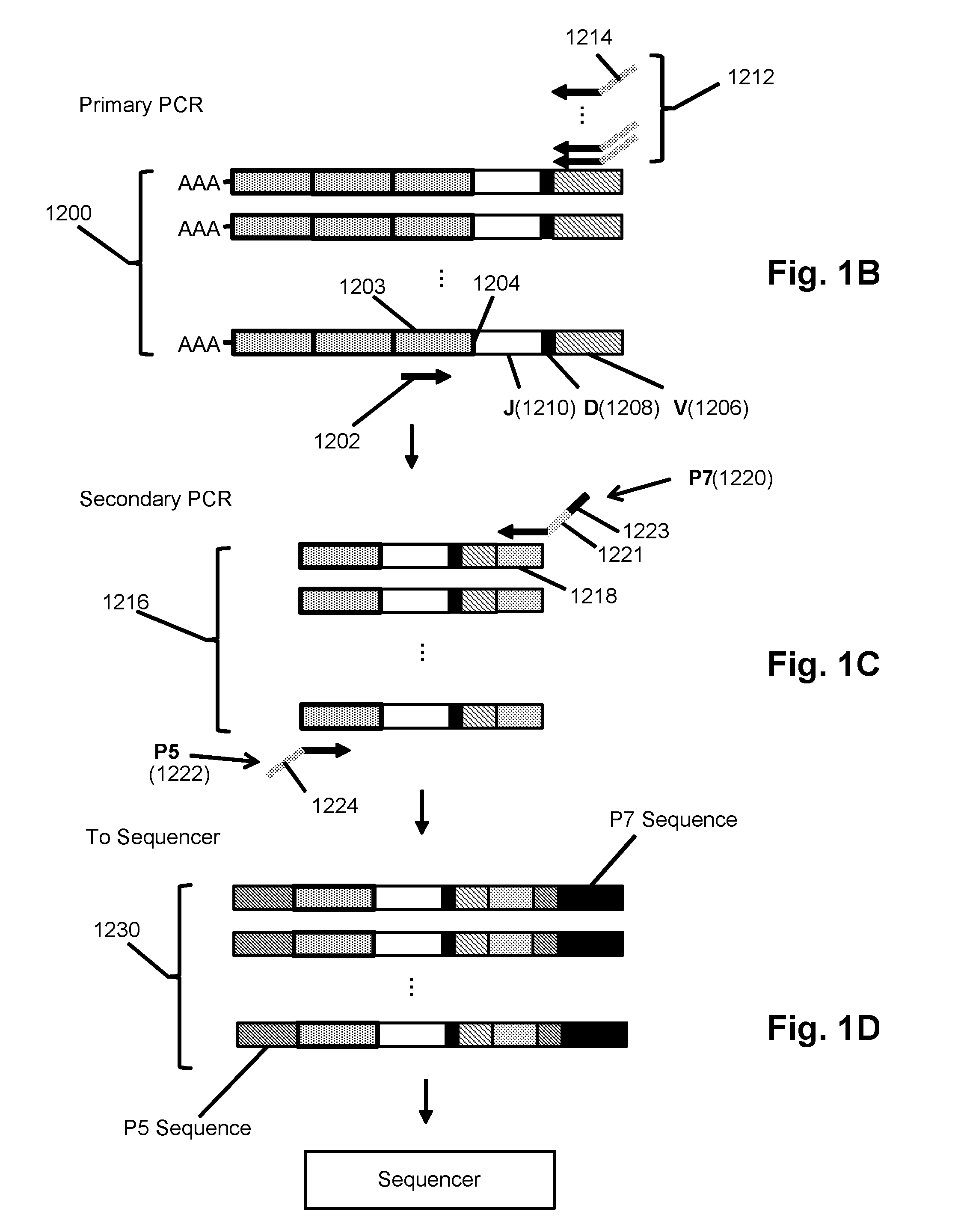
Monitoring transformation of follicular lymphoma to diffuse large b-cell lymphoma by immune repertoire analysis
InactiveUS20140349883A1Raise the possibilityMicrobiological testing/measurementLibrary screeningProgenitorSomatic cell
The invention is directed to a method of prognosing in an individual a transformation from follicular lymphoma to diffuse large B-cell lymphoma (DLBCL) by measuring changes and / or lack of changes in certain groups of related clonotypes, referred to herein as “clans,” in successive clonotype profiles of the individual. A clan may arise from a single lymphocyte progenitor that gives rise to many related lymphocyte progeny, each possessing and / or expressing a slightly different immunoglobulin receptor due to somatic mutation(s), such as base substitutions, inversions, related rearrangements resulting in common V(D)J gene segment usage, or the like. A higher likelihood of transformation from follicular lymphoma to DLBCL is correlated with the persistence of clans in successive clonotype profiles whose clonotype membership fails to undergo diversification over time.
Owner:ADAPTIVE BIOTECH
Method for the Production of a Monoclonal Antibody to CD20 for the Treatment of B-Cell Lymphoma
InactiveUS20090285795A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDNA constructMammalian expression
The present invention relates to the recombinant method used for the production of soluble form of an antibody that binds to CD20 for treatment of patients with relapsed or refractory, low-grade or follicular, CD20-positive, B-cell non-Hodgkin's lymphoma (NHL). The treatment will comprise the use of immunologically active anti-CD20 antibodies; or radiolabeled anti-CD20 antibodies and or cooperative strategies where both labeled and non-labeled antibodies will be used for treatment of NHL. The procedure describes the de novo synthesis of the nucleic acid sequence encoding anti-CD20, transformation of the constructed nucleic acid sequences into competent bacteria and the sub-cloning of the same into mammalian expression vectors for expression of the desired protein. DNA constructs comprising the control elements associated with the gene of interest has been disclosed. The nucleic acid sequence of interest has been codon optimized to permit expression in the suitable mammalian host cells.
Owner:AVESTHAGEN
Anti-cancer antibodies with reduced complement fixation
ActiveUS20050202021A1Reduce complement fixationReduce pain levelsPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthBlastoma
The invention provides modified antibodies directed against GD2 that have diminished complement fixation relative to antibody-dependent, cell-mediated cytotoxicity, which is maintained. The modified antibodies of the invention may be used in the treatment of tumors such as neuroblastoma, glioblastoma, melanoma, small-cell lung carcinoma, B-cell lymphoma, renal carcinoma, retinoblastoma, and other cancers of neuroectodermal origin.
Owner:MERCK PATENT GMBH
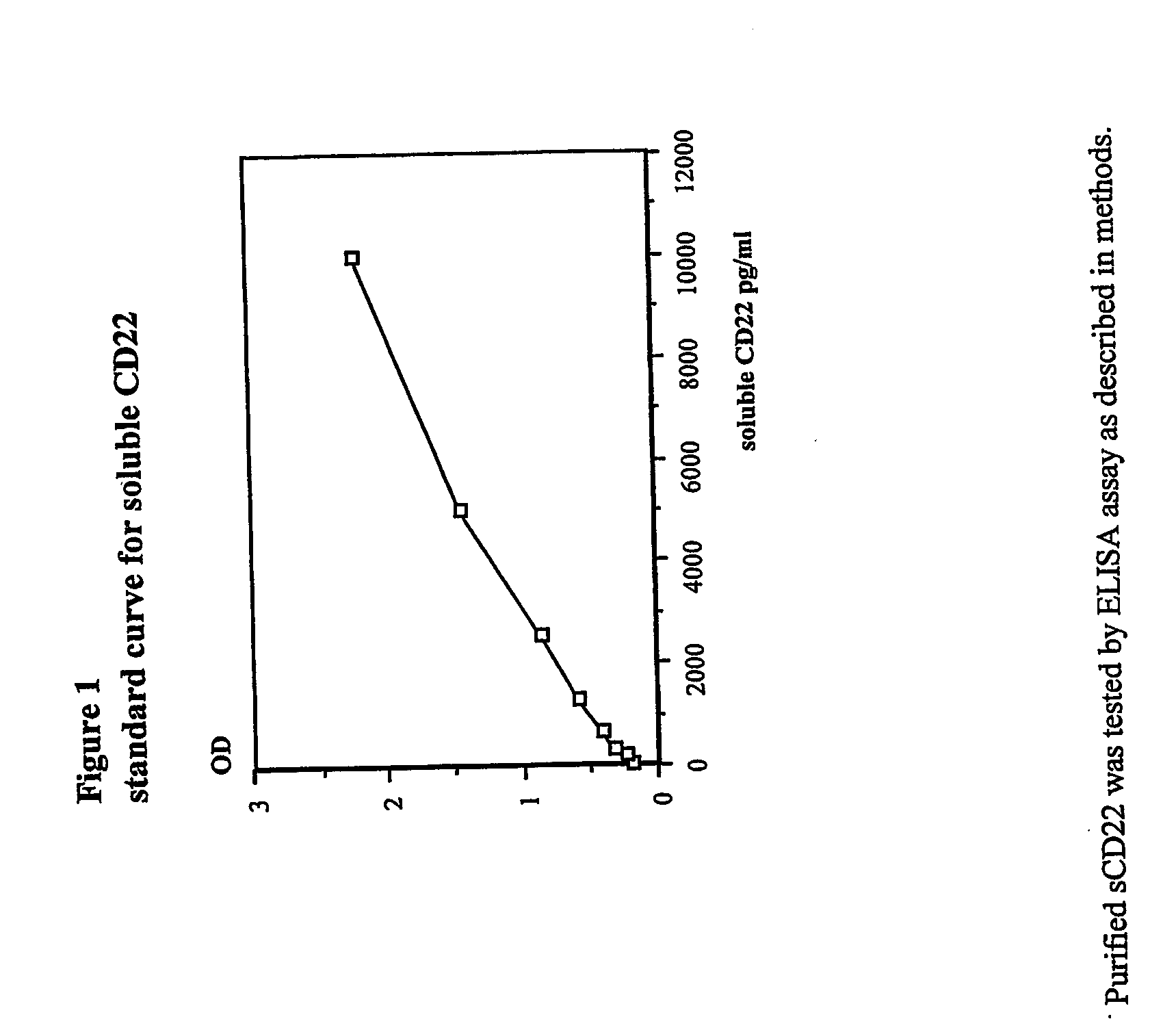
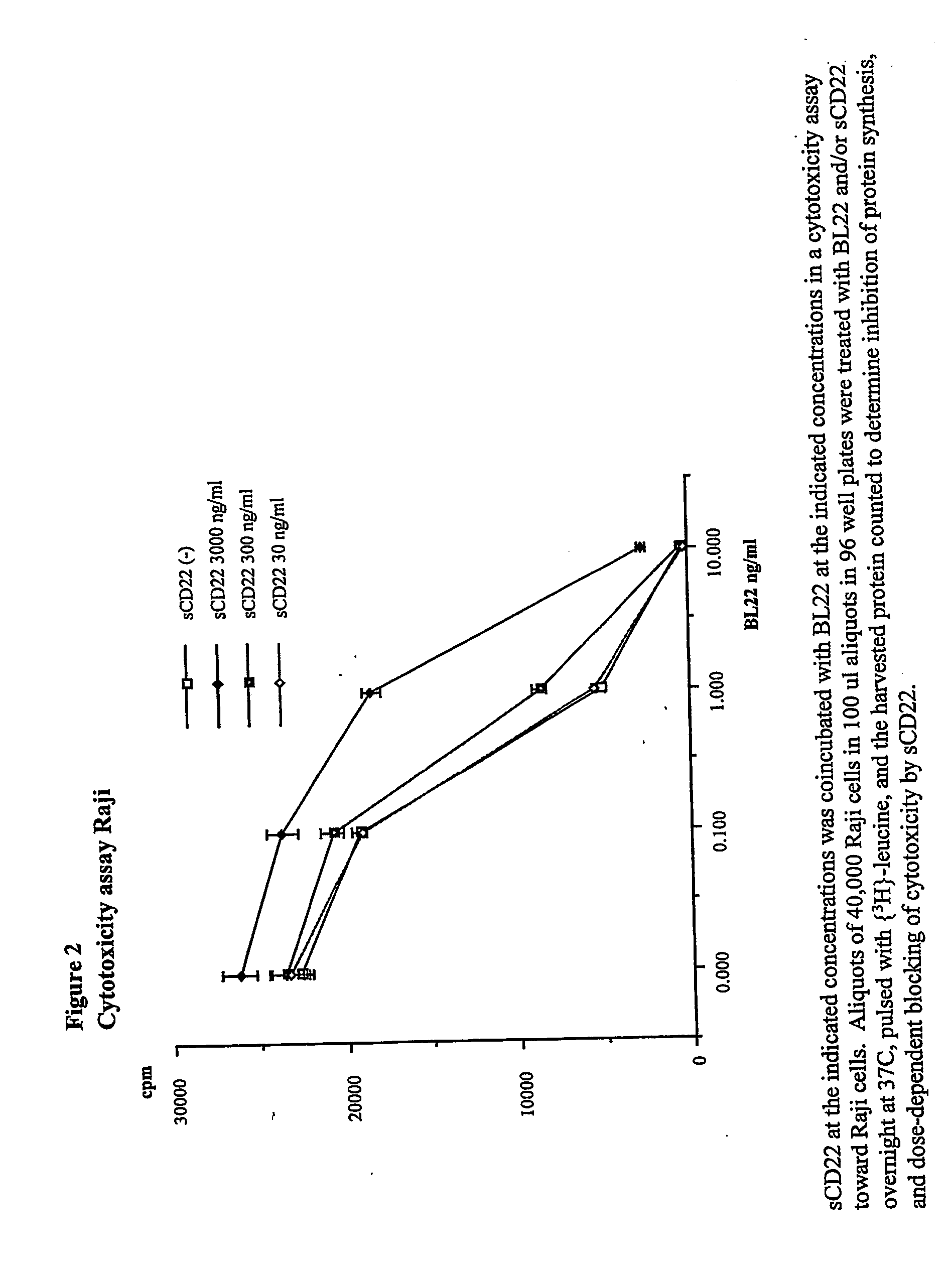
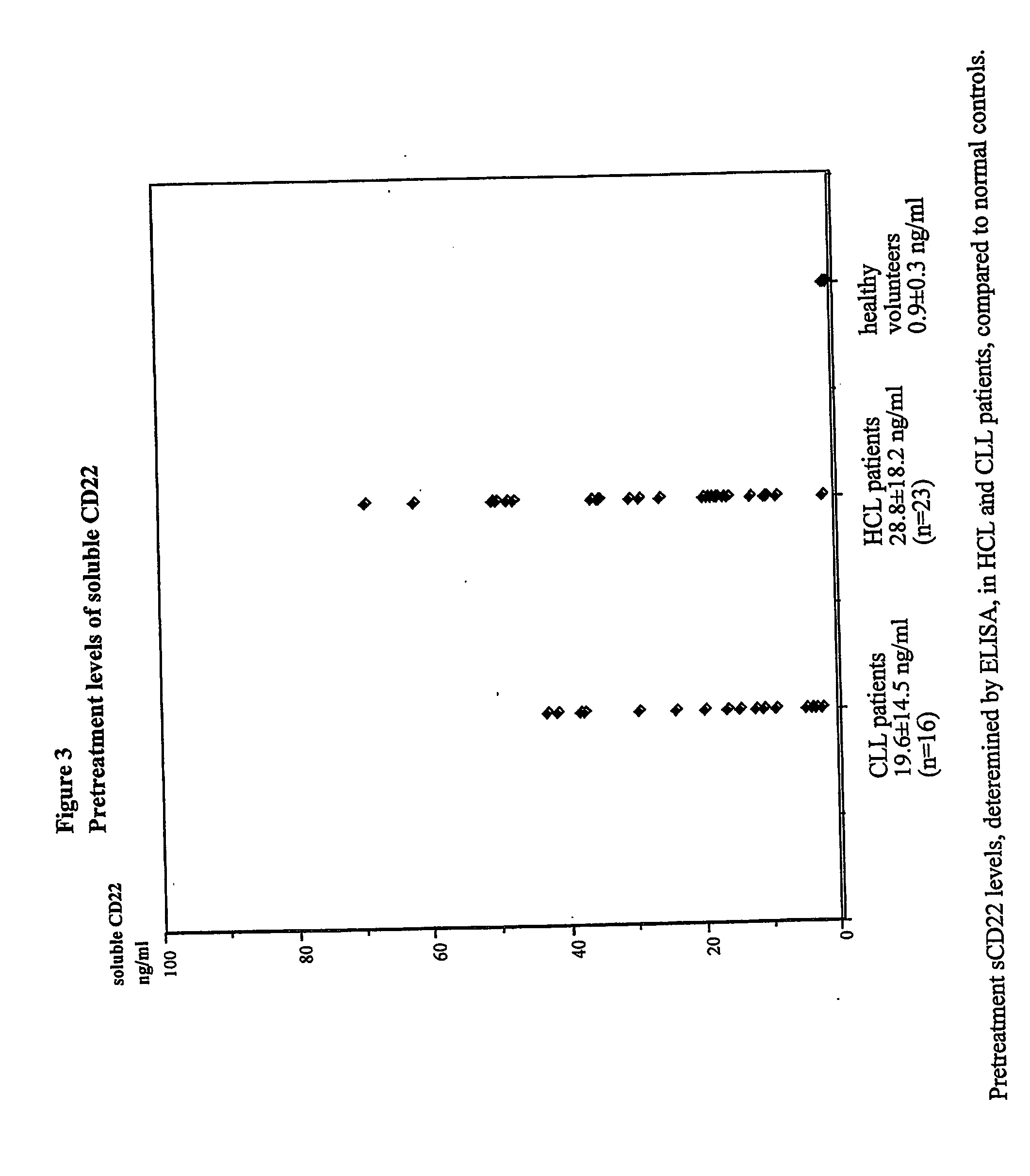
Elisa assay of serum soluble cd22 to assess tumor burnden/relapse in subjects with leukemia and lymphoma
InactiveUS20050244828A1Microbiological testing/measurementBiological material analysisAbnormal tissue growthTumor Load
Disclosed herein are methods of using previously unknown soluble forms of CD22 (sCD22) present in the serum of subjects with B-cell leukemias and lymphomas to assess tumor burden in the subjects. Also disclosed are methods of diagnosing or prognosing development or progression of a B-cell lymphoma or leukemia in a subject, including detecting sCD22 in a body fluid sample taken or derived from the subject, for instance serum.
Owner:GOVERNMENT OF THE UNITED STATES OF AMERICAS AS REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES THE
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20050180975A1Avoiding and decreasing and resistanceIncrease ratingsBiocidePeptide/protein ingredientsBiological activationHematologic malignancy
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN®.
Owner:BIOGEN INC
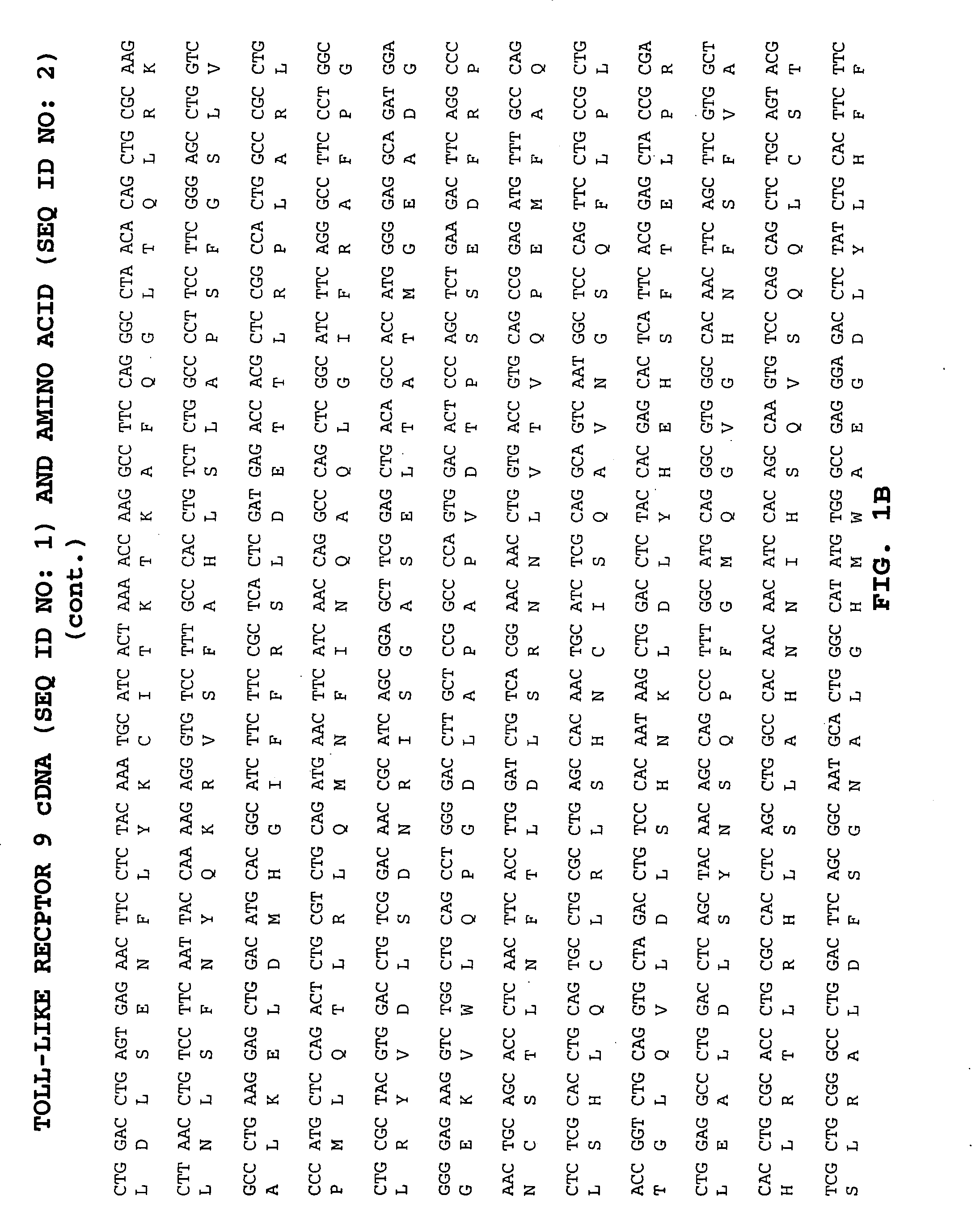
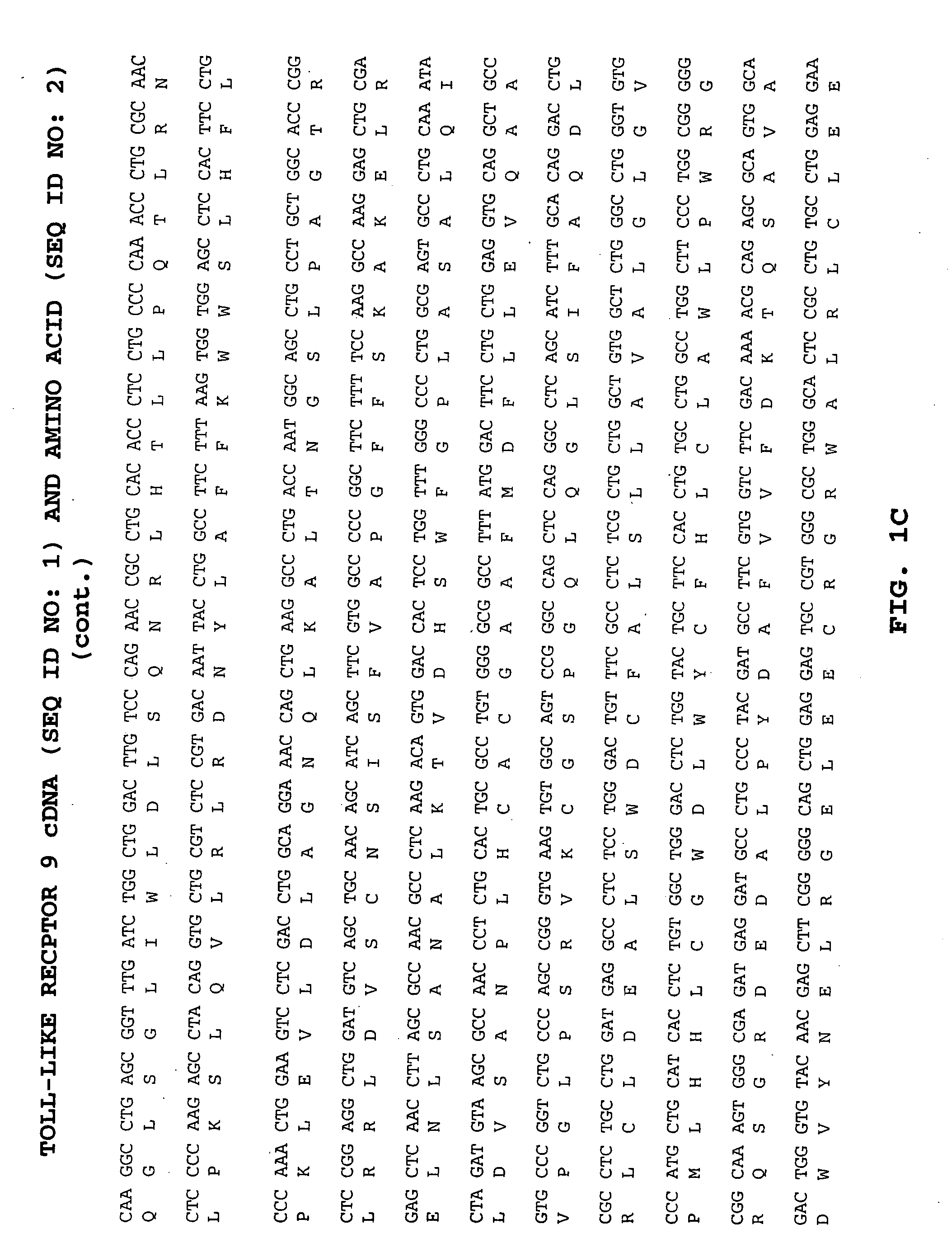
Methods of therapy and diagnosis using targeting of cells that express toll-like receptor proteins
InactiveUS20050281813A1Good effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellT cell
Certain cells, including types of cancer cells such as B-cell lymphomas, T cell lymphomas, Hodgkin's disease and myeloid leukemias, are capable of expressing Toll-like Receptor 9 (TLR9) or Toll-like Receptor 10 (TLR10) mRNA. Immunotargeting using TLR9 or TLR10 polypeptides, nucleic acids encoding for TLR9 or TLR10 polypeptides and anti-TLR9 or anti-TLR10 antibodies provides a method of killing or inhibiting that growth of cancer cells that express the TLR9 or TLR10 protein. Methods of immunotherapy and diagnosis of disorders associated with TLR9 or TLR10 protein-expressing cells, such as B-cell lymphoma, T cell lymphoma, acute myeloid leukemia, Hodgkin's disease, B cell leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia and myelodysplastic syndromes, are described.
Owner:NUVELO INC
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity. The present invention also enables methods for treating cancers that are mediated, dependent on or associated with pi 105 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Combination therapies for b-cell lymphomas comprising administration of Anti-cd20 antibody
InactiveUS20080038261A1Good synergyHigh response rateOrganic active ingredientsPeptide/protein ingredientsRegimenHodgkins lymphomas
New combined therapeutic regimens for treatment of B-cell lymphomas are disclosed which comprise in particular administration of anti-CD20 antibodies to patients having low-, intermediate- or high-grade non-Hodgkins lymphomas.
Owner:BIOGEN INC
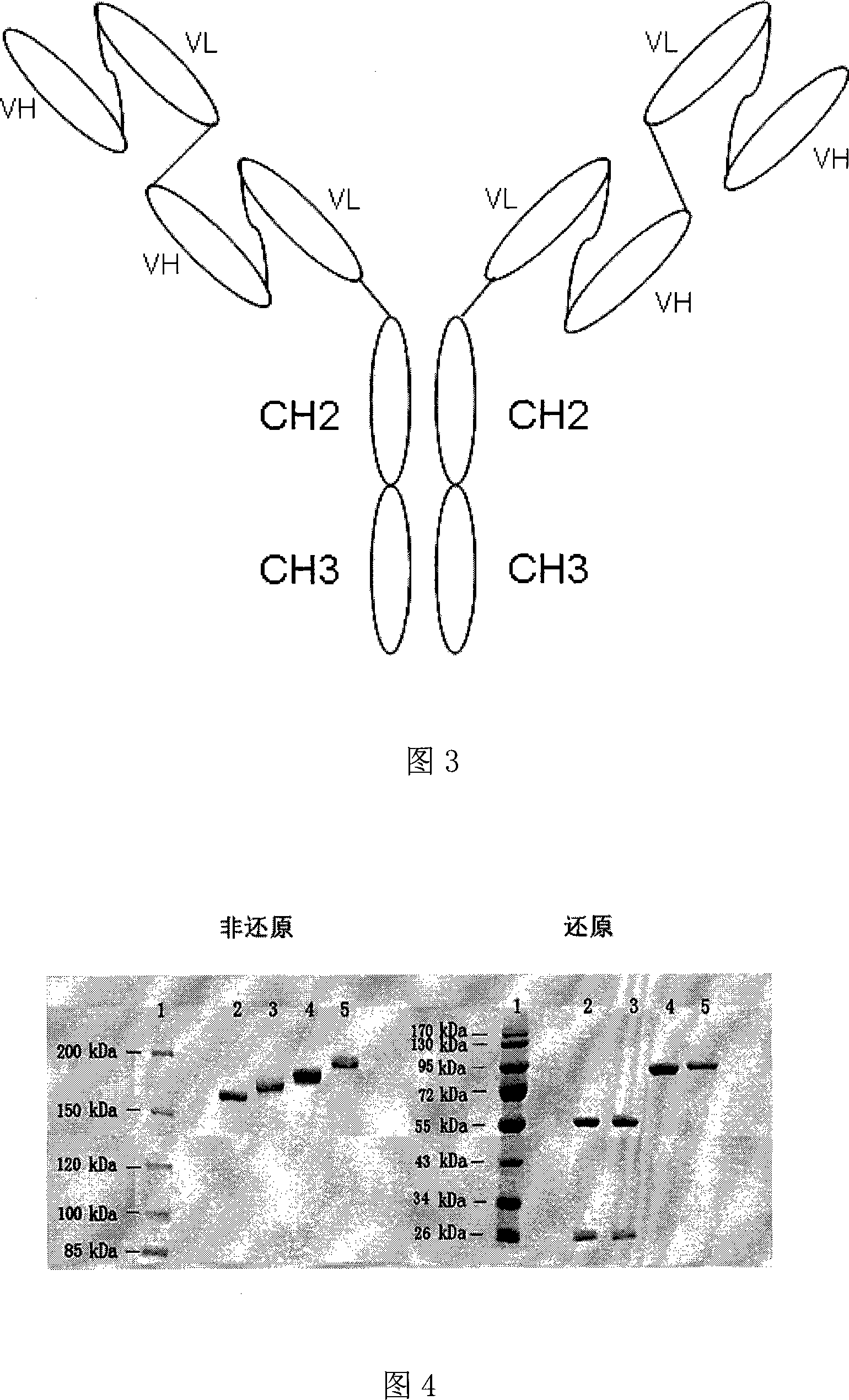
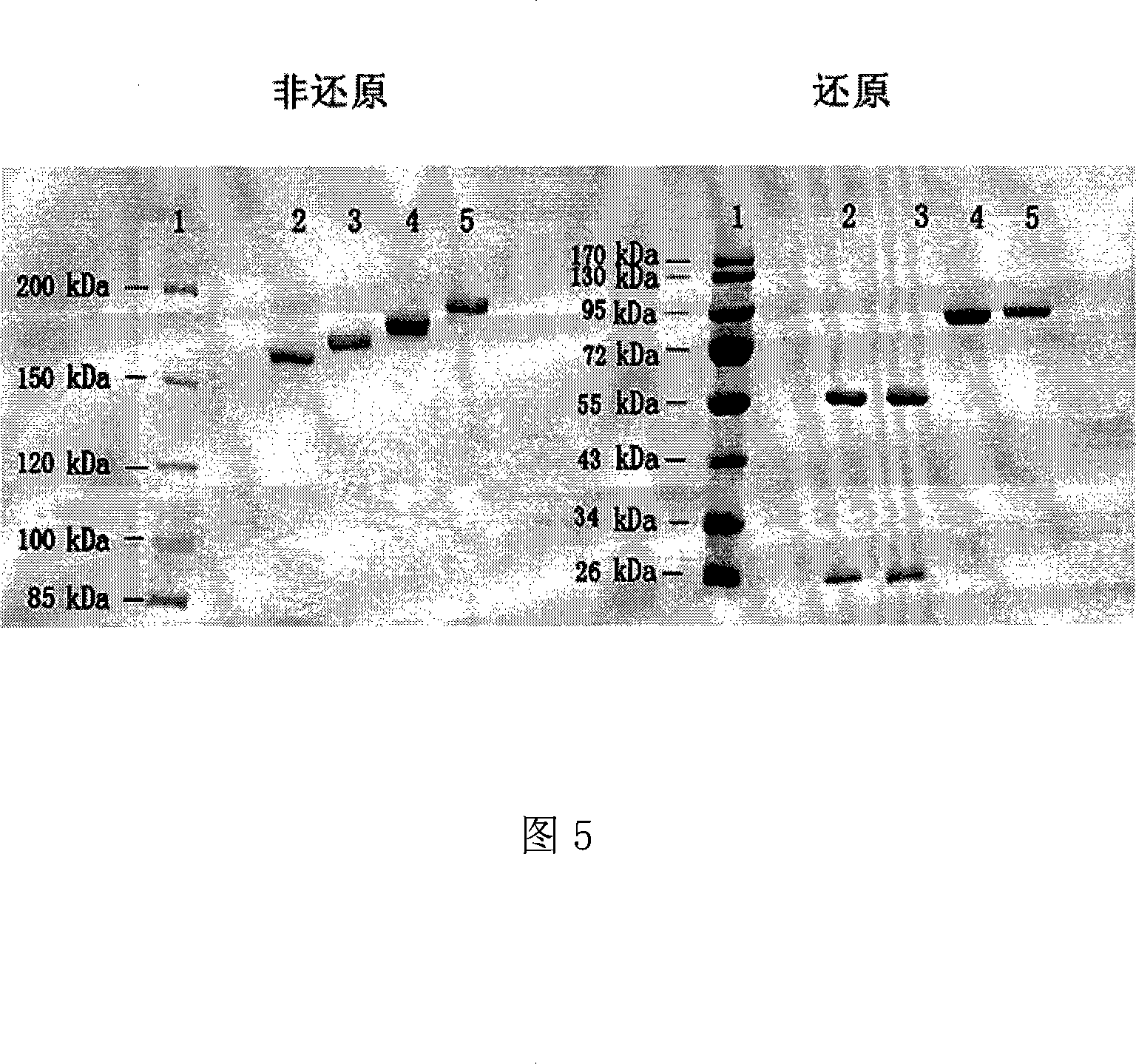
Anti CD20 tetravalent antibody, preparation method and uses thereof
InactiveCN101205255AReduce severityPrevent other symptomsHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCD20Medicine
The invention discloses an anti- CD20 tetravalent antibody and a preparation method as well as application thereof, in particular the invention disclsoes an anti- CD20 tetravalent antibody C2B8(ScFvHL)4-Fc and 2F2(ScFvHL)4-Fc, and the preparation method as well as the use thereof in the preparation of drugs for inhibiting B-cell lymphoma.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
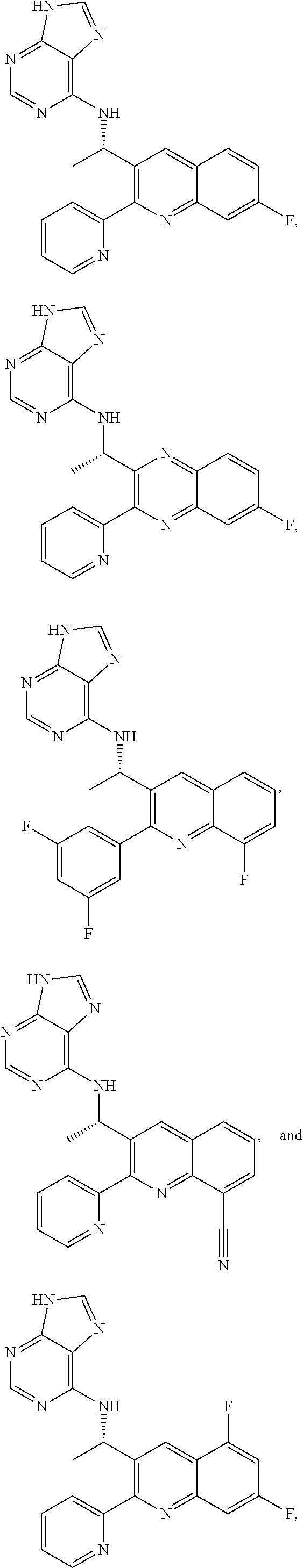
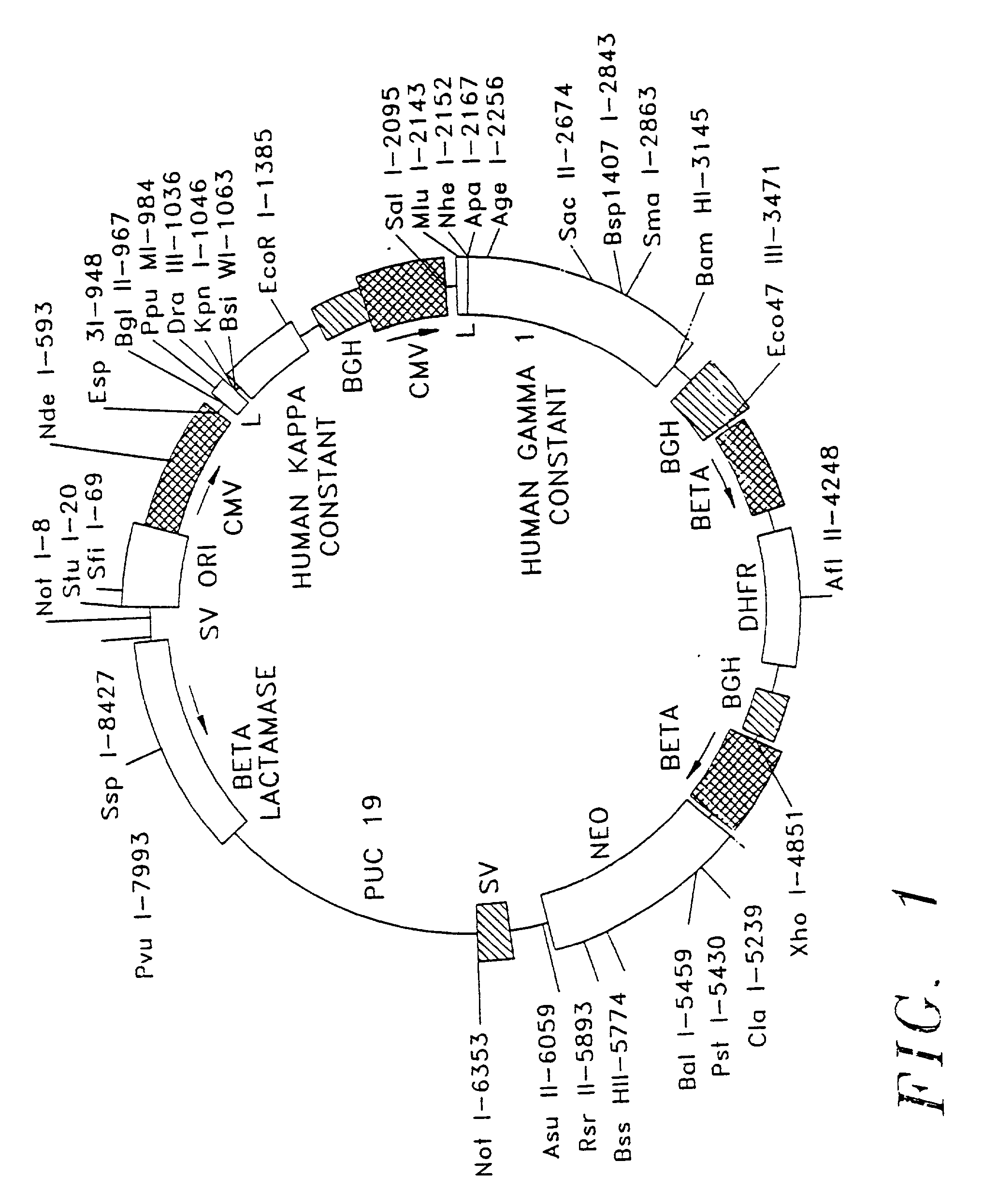
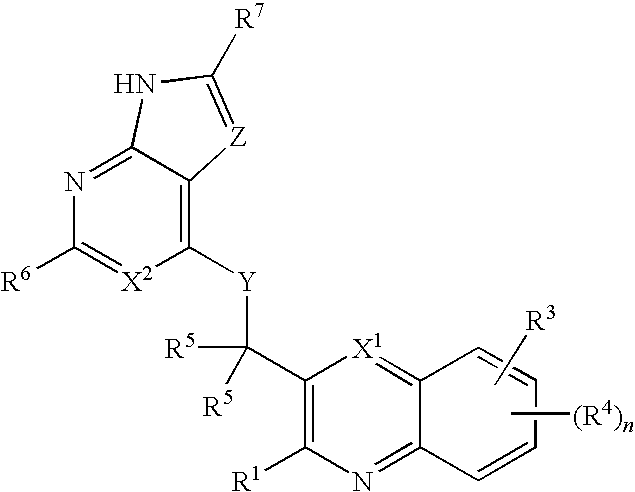
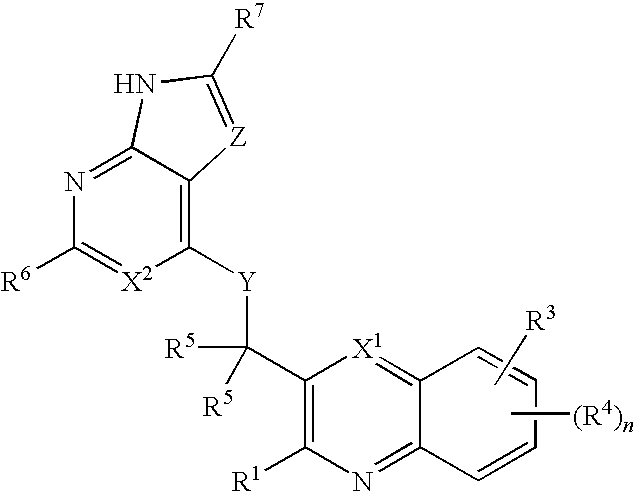
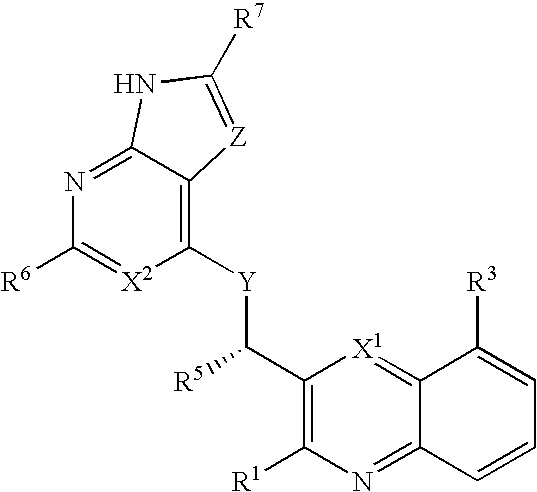
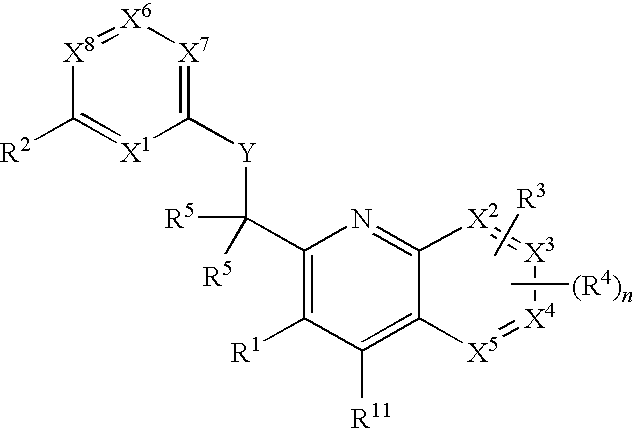
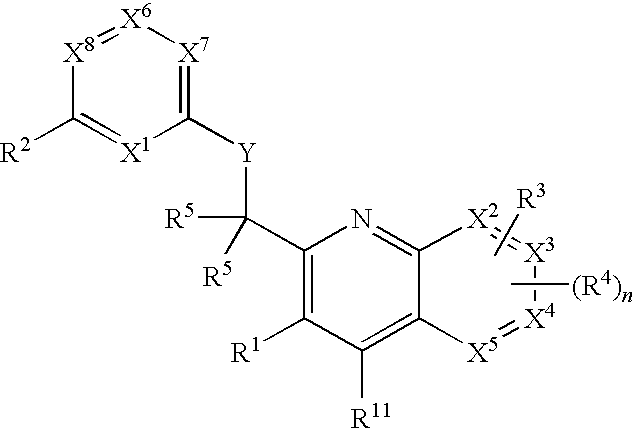
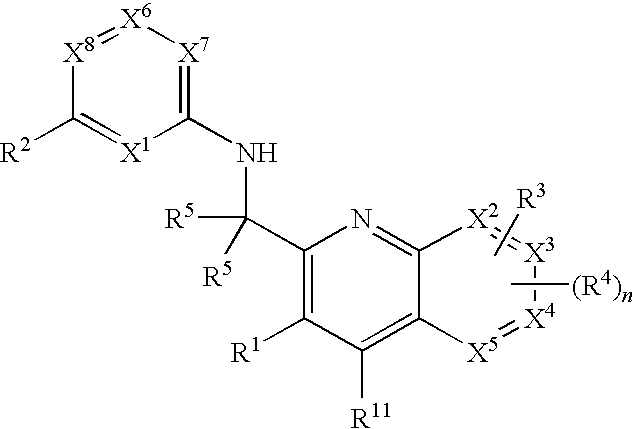
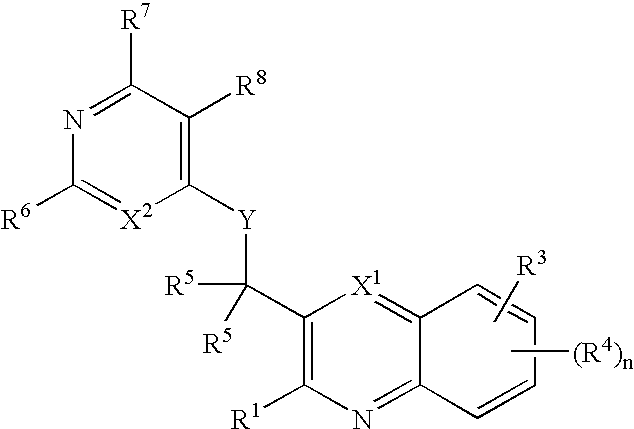
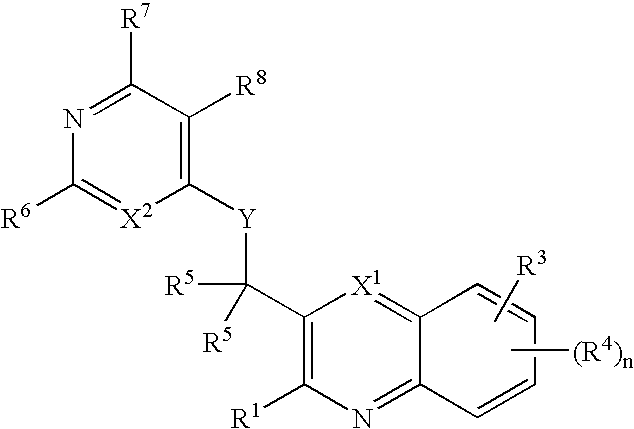
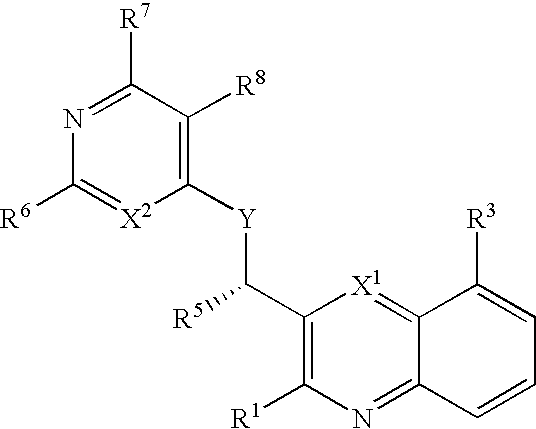
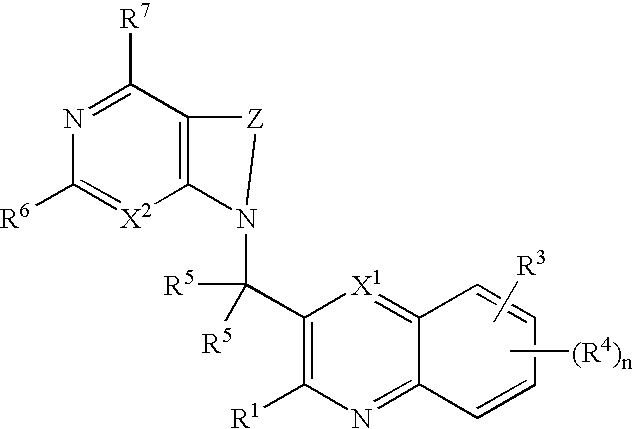
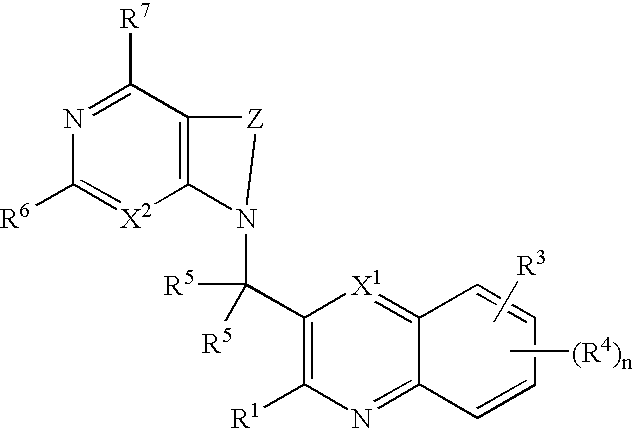
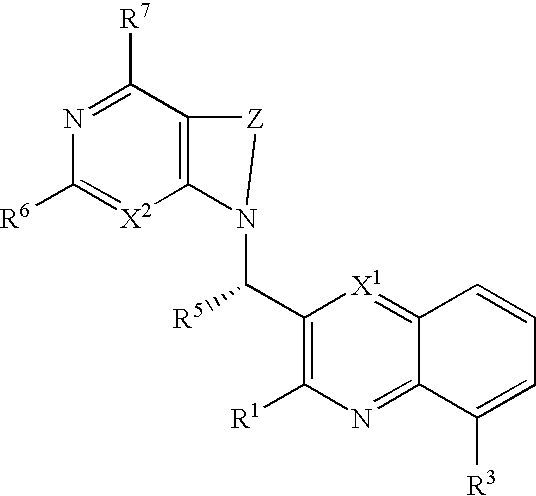
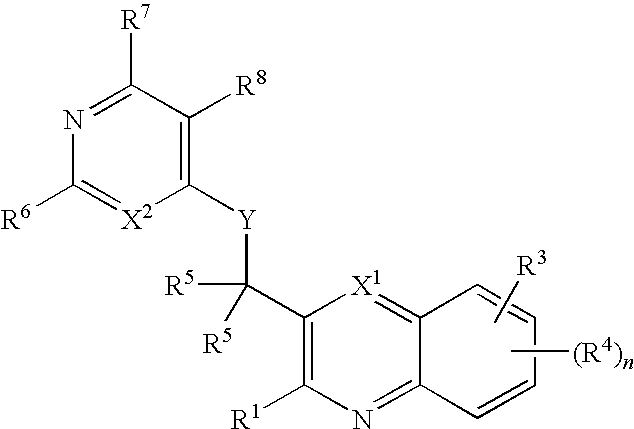
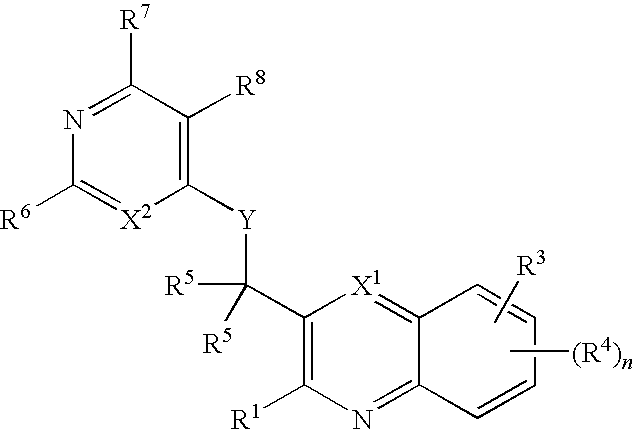
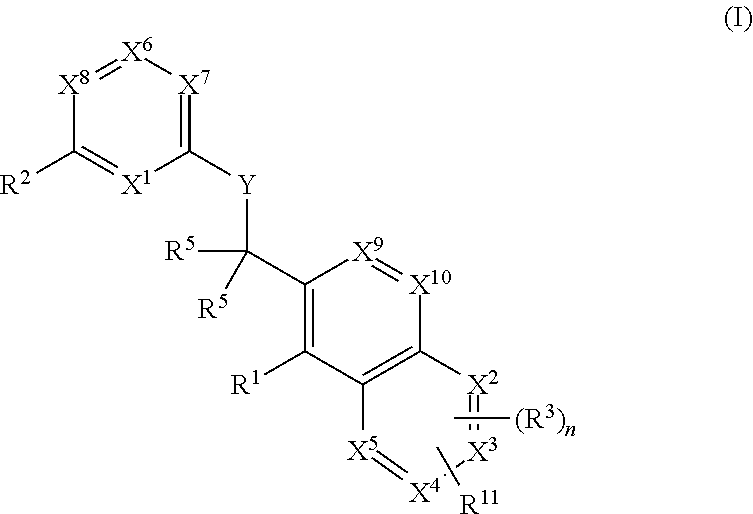
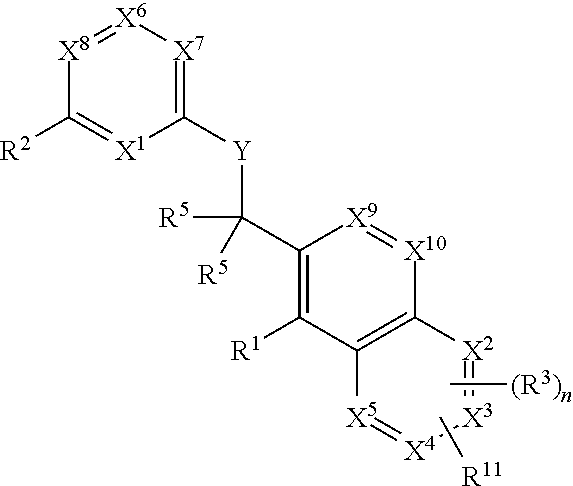
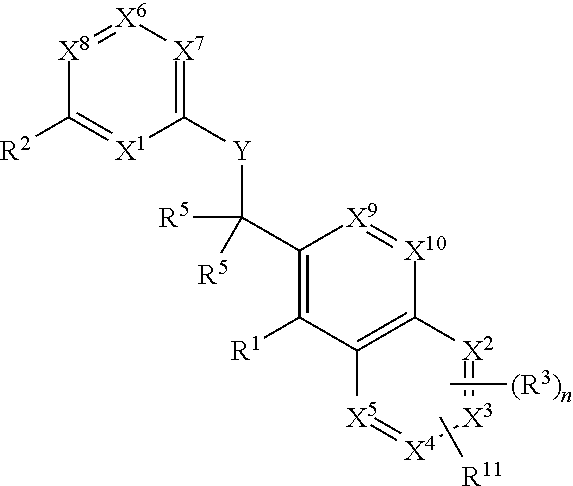
Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukemia (AML) Myelodysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Treatment of B-cell lymphoma
InactiveUS20060029543A1Increase ratingsConducive to survivalBiocideIn-vivo radioactive preparationsRegimenChemotherapy regimen
A method of treating B-cell lymphoma comprises administering to a patient a chemotherapeutic regimen, followed by treatment with a radiolabeled anti-CD20 antibody, wherein at the time of said treatment with said radiolabeled antibody said patient is not refractory to said chemotherapeutic regimen and has not relapsed.
Owner:BAYER PHARMA AG
Combination therapies for b-cell lymphomas comprising administration of Anti-cd20 antibody
InactiveUS20120258101A1Good synergyHigh response rateOrganic active ingredientsPeptide/protein ingredientsCombination therapyB cell
New combined therapeutic regimens for treatment of B-cell lymphomas are disclosed which comprise, in particular, administration of anti-CD20 antibodies to patients having low-, intermediate- or high-grade non-Hodgkin's lymphomas.
Owner:GRILLO LOPEZ ANTONIO J
Heterocyclic compounds and their uses
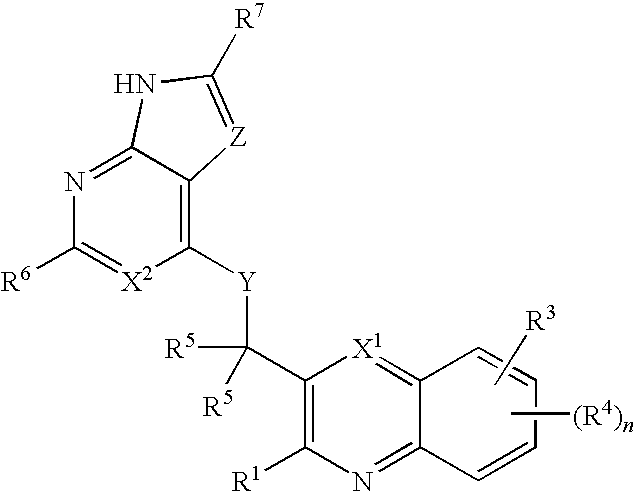
ActiveUS8193199B2Low inhibitory potencyInhibitory activityBiocideSenses disorderMyeloid leukemiaB-cell acute lymphoblastic leukaemia
Substituted bicyclic heteroaryls having the structures:and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Immunoconjugates and humanized antibodies specific for b-cell lymphoma and leukemia cells
InactiveUS20070172920A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclonal antibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com












![Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/afcc1086-f7bf-415b-a630-8356258d7962/US07919498-20110405-C00001.png)
![Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/afcc1086-f7bf-415b-a630-8356258d7962/US07919498-20110405-C00002.png)
![Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors Substituted pyrazolo[3,4-d]pyrimidines as PI3K inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/afcc1086-f7bf-415b-a630-8356258d7962/US07919498-20110405-C00003.png)