Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
a technology of chimeric virus and gene sequence, applied in the field of virus vaccines, therapeutics, diagnostics, etc., can solve the problems of reducing antigen and immunogenity, reducing the number of recombinant peptides, and affecting the normal gene regulation of host cells, so as to reduce the number of dna structures in the further-modified nucleotide sequence, the effect of minimizing the number of transcription and post-transcription repressor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Establishment of Serum-free SF-9 Insect Cell Line
[0181] A new insect cell line designated Sf-9S was derived from the parent S. frugiperda Sf-9 cell line (ATCC CRL-1771) by several rounds of selective processes based on serum-independent growth and enhanced expression of secreted recombinant proteins from baculovirus vectors. Specifically, Sf-9 cells were cultivated to passage 38 in Grace's insect media (Life Technologies, Grand Island, N.Y. 14072) supplemented with 10% fetal bovine serum (Life Technologies, Grand Island, N.Y. 14072) as monolayer cultures in T-75 flasks (Corning, Inc., Corning, N.Y.). The master cell bank of Sf-9 cells was stored at passage 38 in serum-containing media at −70° C. and in liquid nitrogen. A working cell bank was established from a single cryovial of the Sf-9 master cell bank and cultivated in serum-containing insect media for an additional five (5) passages.
[0182] Initially, cell clones capable of growing in commercial serum-free media as suspension...
example 2
Establishment of Transformed SF-9S Cell Line
[0184] In a second selection process, one of the serum-free cell clones developed in Example 1 was chosen to select cell clones that may produce enhanced levels of recombinant extracellular proteins and VLPs from several viruses including rotaviruses and human papillomaviruses by successive rounds of clonal selection of cells infected with recombinant baculoviruses and expressing extracellular self-assembled VLPs.
[0185] This process involved the plating of cell aliquots (200 μl) from a cell suspension (one cell per 200 μl) of the parent cell clone (#23) in serum-free media onto 96-well dishes at a ratio of 200 μl per well. From wells containing a single cell in the original seeding, cells were grown to confluency and subcultured into six replica-plates (96-well). The first round of selection was performed when a total cell density of 2-4×103 cells / well was obtained; the cells were infected with recombinant baculoviruses encoding human r...
example 3
Cloning Codon-optimized HPV-16 L1 Genes and Establishment of Recombinant Baculovirus Stocks
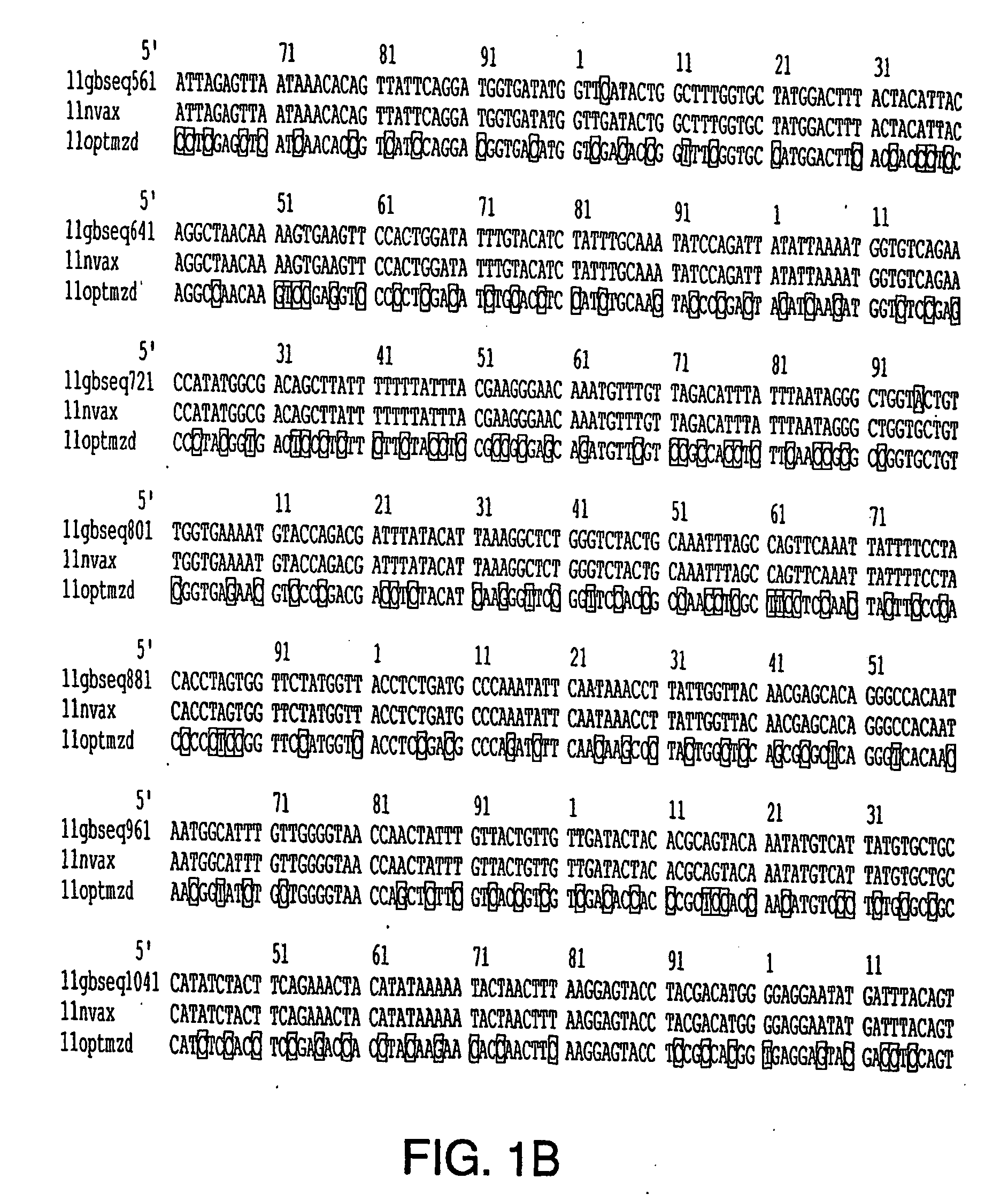
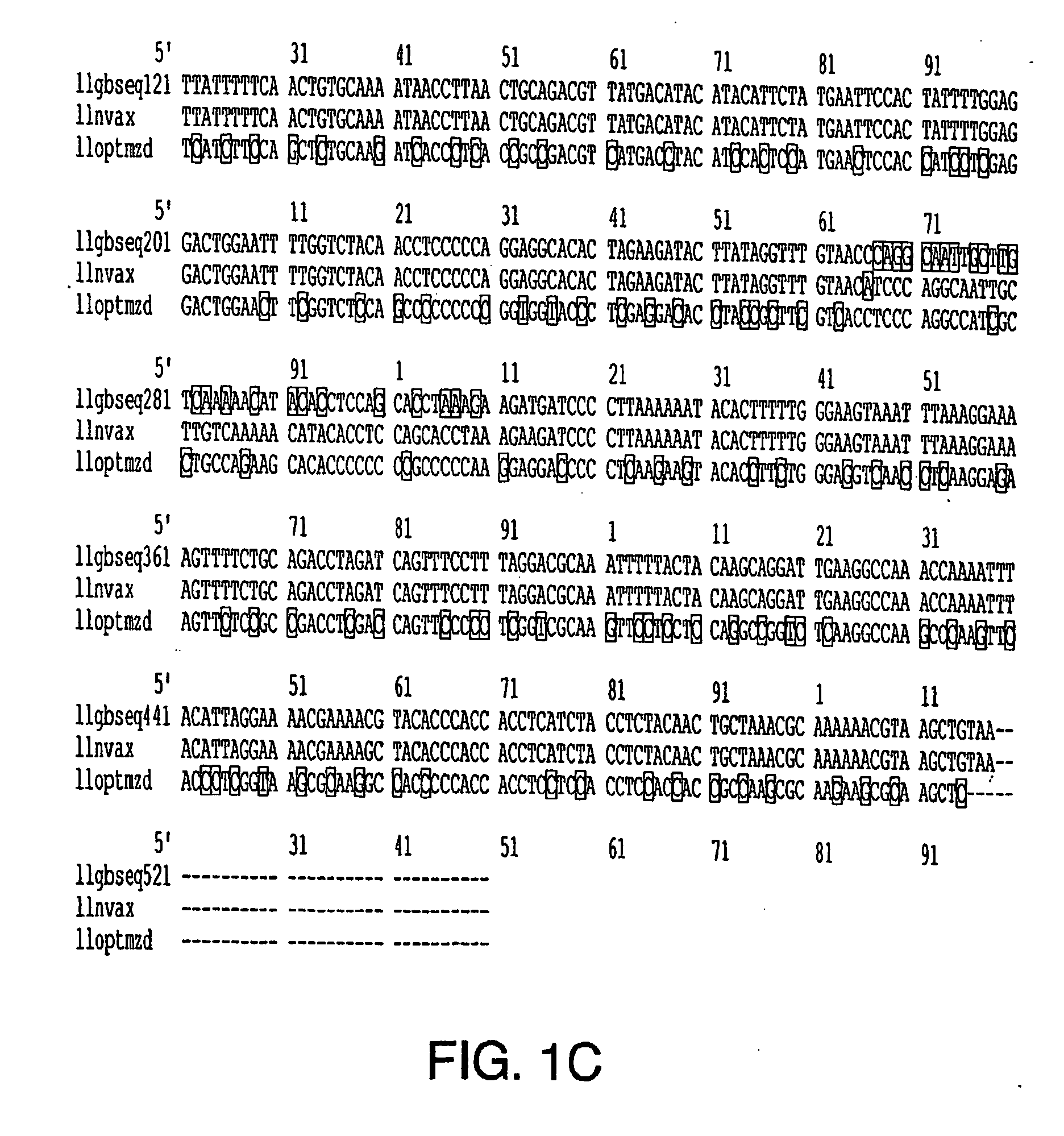
[0188] A BPV-16 L1 prototype (GenBank Accession No. K02718) and modified in U.S. Pat. No. 5,985,610, was optimized for codon usage in insect cells of the Lepidopteran family. The HPV-16 L1 gene was optimized (FIG. 1A) in this embodiment of the present invention for codon usage based on the following criteria: (1) abundance of aminoacyl-tRNAs for a particular codon in Lepidopteran species of insect cells for a given amino acid as described by Levin and Whittome (2000); (2) maintenance of GC-AT ratio in L1 gene sequence at approximately 1:1; (3) minimal introduction of palindromic or stem-loop DNA structures, and (4) minimal introduction of transcription and post-transcription repressor element sequences.
[0189] The optimized gene sequence was synthesized in vitro as overlapping oligonucleotides, cloned into a subcloning plasmid vector, and then cloned into a bacmid transfer vector (i.e., Luck...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com