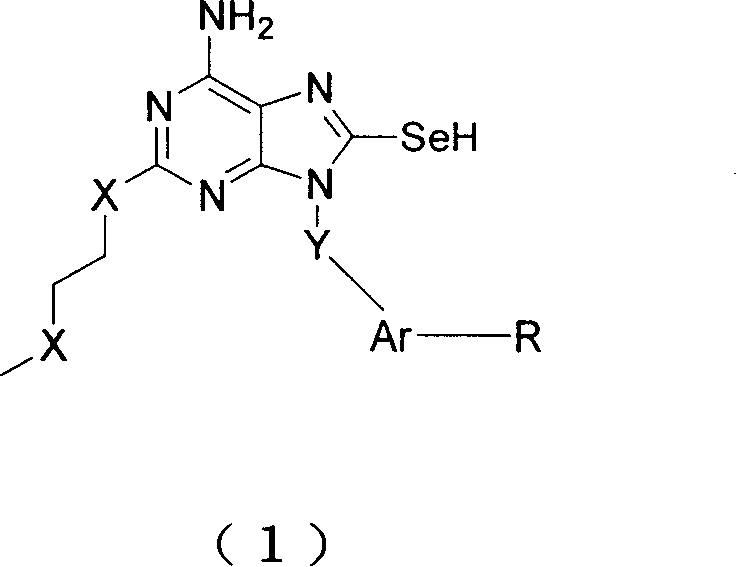
Immunity excitant containing selenium
An agonist, selenium technology, applied in antiviral, digestive system, organic chemistry, etc., can solve the problem of not including the 8-position selenol group, etc., to achieve the effect of enhancing immunity, improving activity and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0036] synthetic route:
[0037]
[0038] 3.78 grams of compound 7 and 12.3 grams of selenourea were mixed and dissolved in 150 milliliters of absolute ethanol. Heated to reflux for 24 hours, cooled to 10° C. for 4 hours, filtered, and dried at room temperature to obtain compound 1 (3.2 grams. Yield 85%) ).The melting point of the product is 283--285℃. 1 H NMR (DMSO-d 6 )δ10.38(1H, s), 7.34-7.24(5H, m), 6.44(2H, s), 4.85(2H, s), 4.24(2H, t, J=4.6Hz), 3.57(2H, t , J=4.6Hz), 3.20 (3H, s); MS (ESI) m / z 379 (MH + ); HRMS calcd for C 15 h 17 N 5 o 2 Se 378.2910, found 378.2911.
Synthetic example 2
[0040] synthetic route:
[0041]
[0042] Cool 5 ml of a 2M lithium aluminum hydride solution in tetrahydrofuran to -78°C. Slowly add 10 ml of a 1M solution of N,N'-dimethylethylenediamine in anhydrous tetrahydrofuran under cooling. The resulting solution for the following reactions.
[0043] Dissolve 1.34 g of compound 8 in 20 ml of anhydrous tetrahydrofuran solution and slowly add to the above mixture at 0°C. After stirring for 1 hour, the reaction mixture was poured into 100 ml of ice water and extracted with ethyl acetate (3X50mL) , combined the extracts, distilled under reduced pressure, and the residue was separated by silica gel column chromatography (eluent: chloroform:methanol=10:1) to obtain compound 2. (1.01 g, yield 75%). 1 H NMR (DMSO-d 6 )δ11.12(1H, s), 10.38(1H, s), 7.03-7.45(5H, m), 6.43(2H, s), 4.87(2H, s), 4.26(2H, t, J=4.6Hz ), 3.55 (2H, t, J=4.6Hz), 3.24 (3H, s); MS (ESI) m / z 407 (MH + ); HRMScalcd for C 16 h 17 N 5 o 3 Se 406.3011, found 406.301...
Synthetic example 3
[0045] synthetic route:
[0046]
[0047] Compound 8 (2 g) was dissolved in 100 ml of anhydrous methanol, 0.5 g of active nickel (R-nickel) was added, and hydrogenated at room temperature and pressure for 12 hours. Filtered, the filtrate was evaporated to dryness to obtain a solid product, and a small amount of methanol Washed and dried to obtain compound 3 (1.86 g, yield 92%). 1 H NMR (DMSO-d 6 )δ10.40(1H, s), 7.63-7.47(5H, m), 6.34(2H, s), 5.56(2H, m), 4.96(2H, s), 4.28(2H, t, J=4.6Hz ), 3.65 (2H, t, J=4.6Hz), 3.44 (2H.s), 3.25 (3H, s); MS (ESI) m / z 408 (MH + ); HRMS calcd for C 16 h 20 N 6 o 2 Se 407.3310, found 407.3011.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com