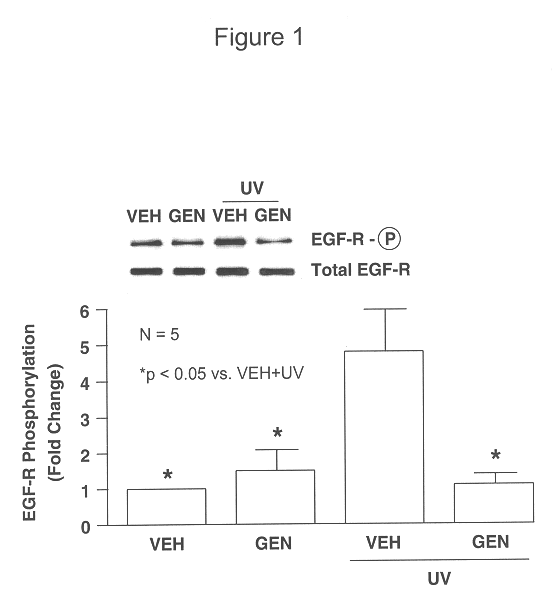
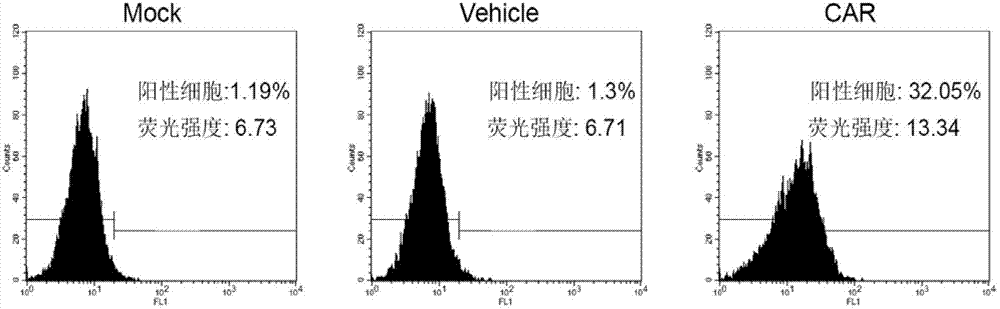
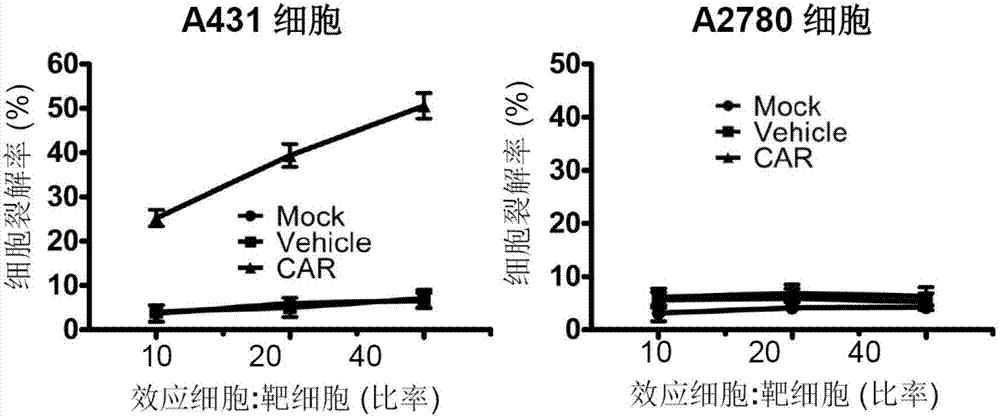
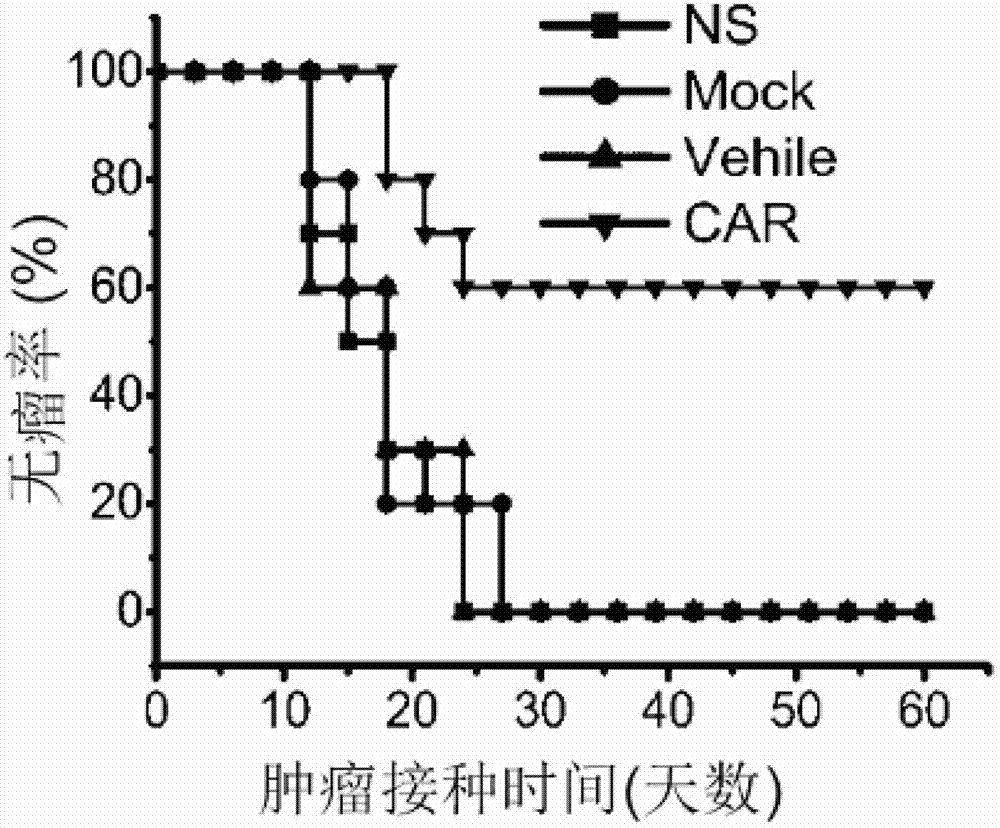
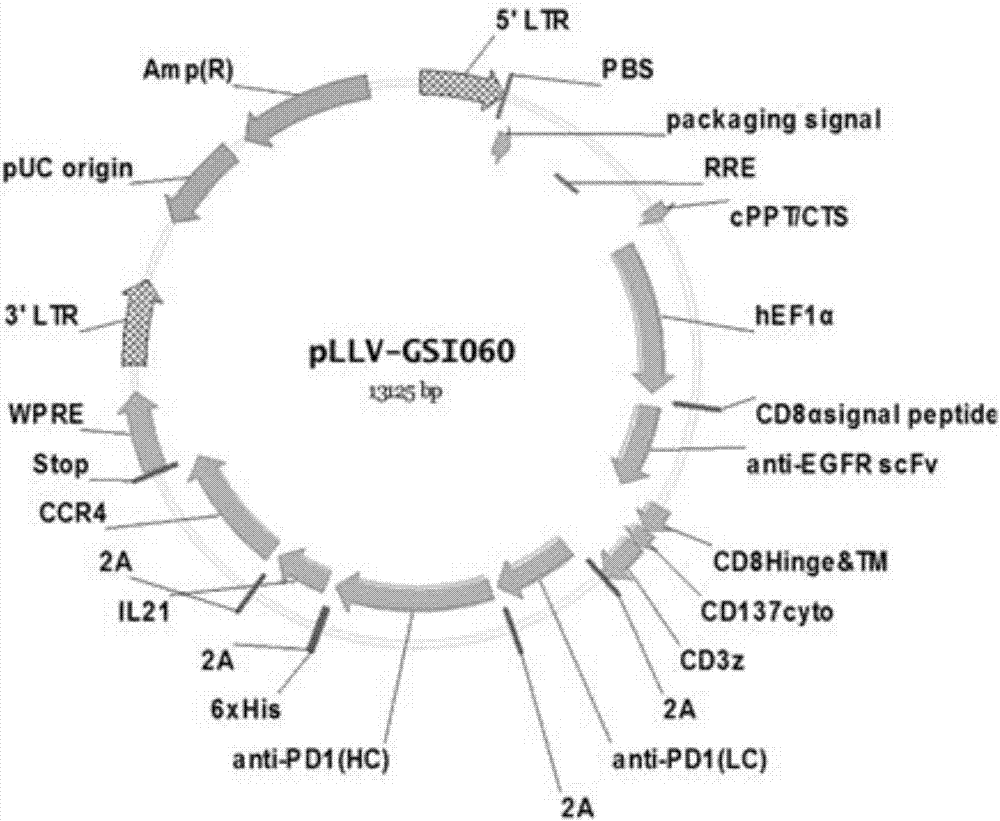
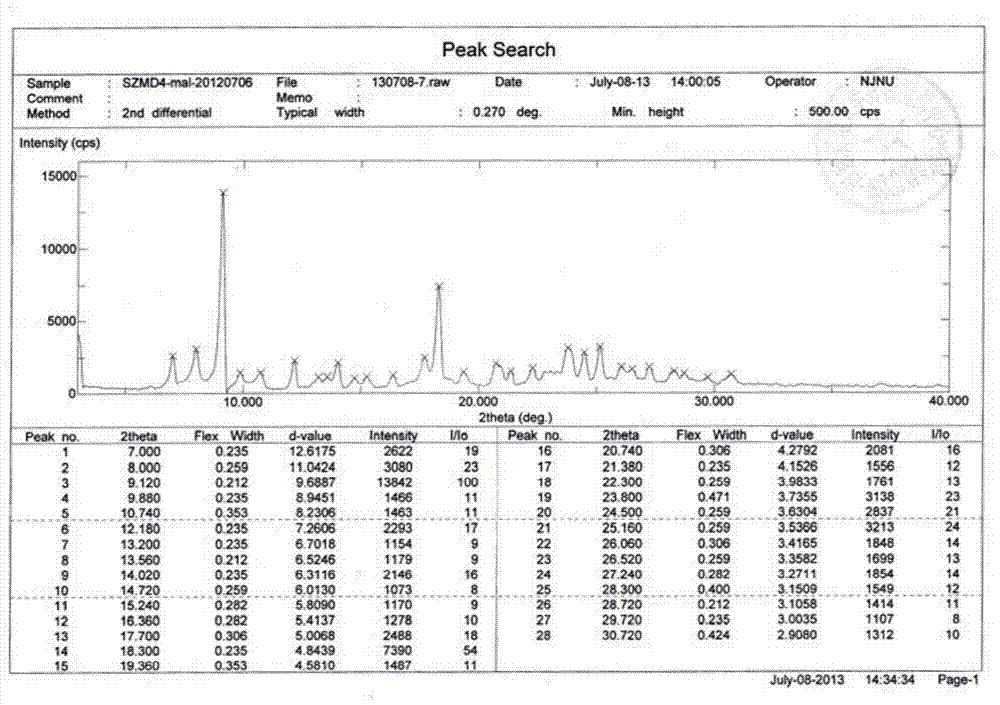
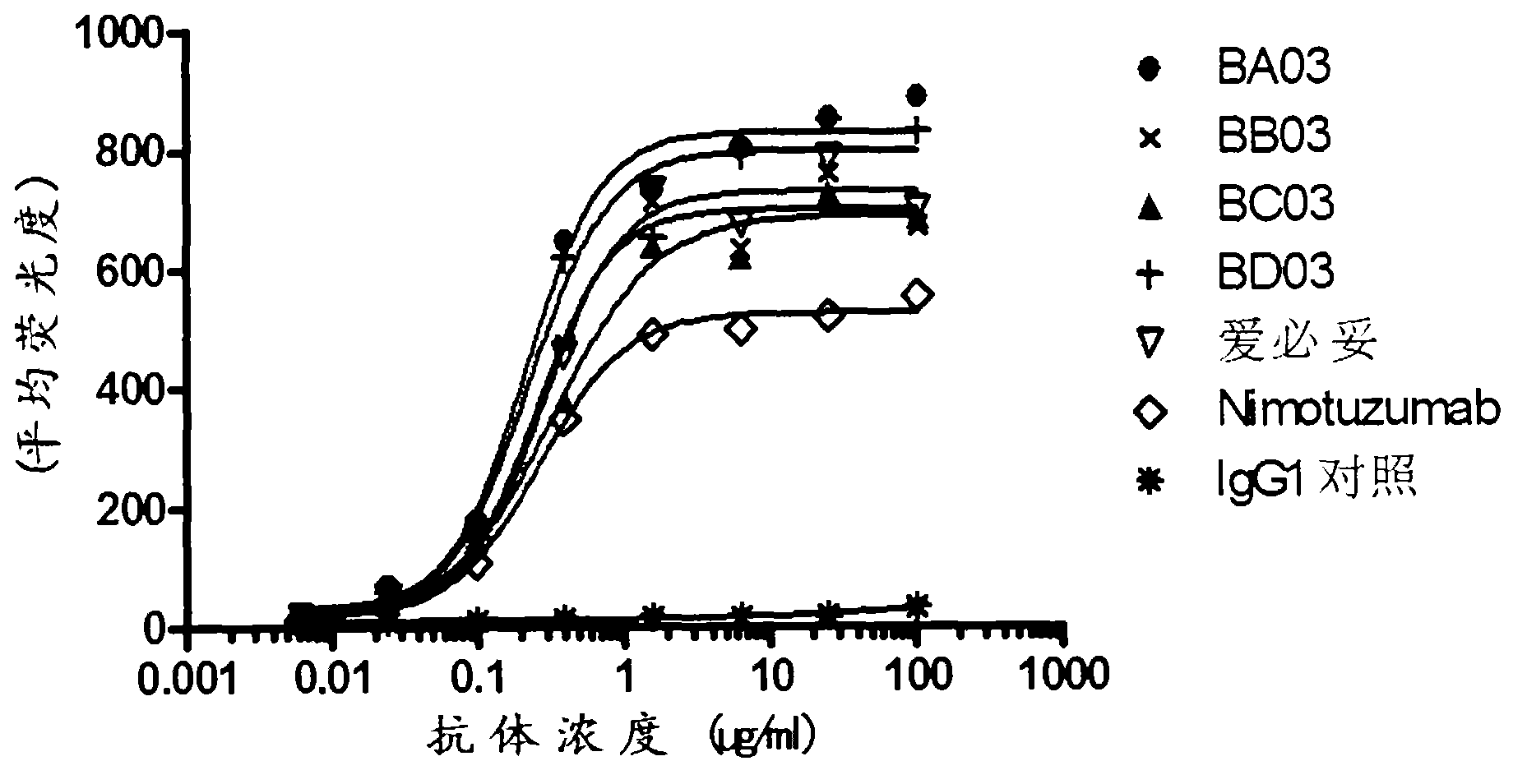
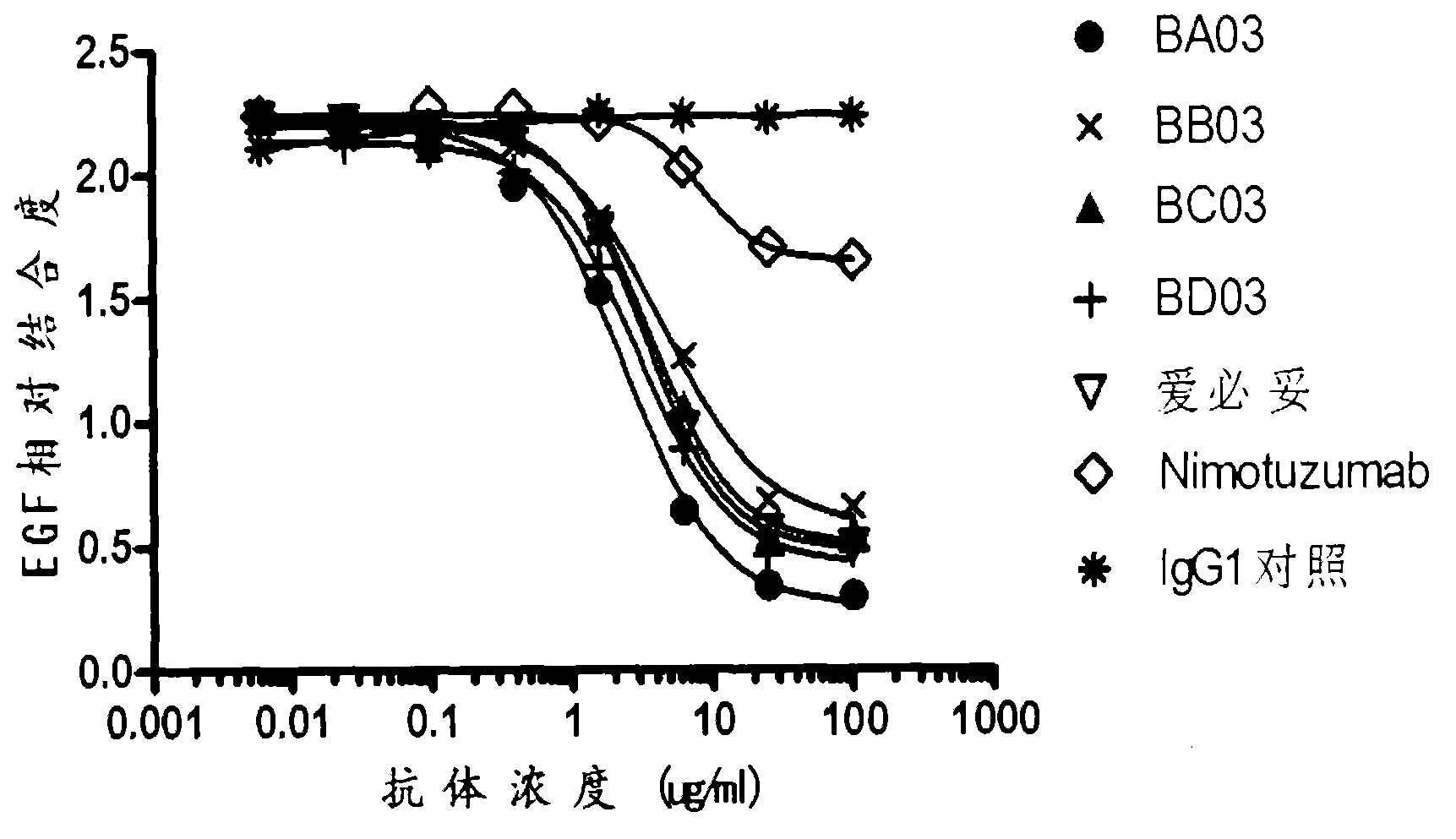
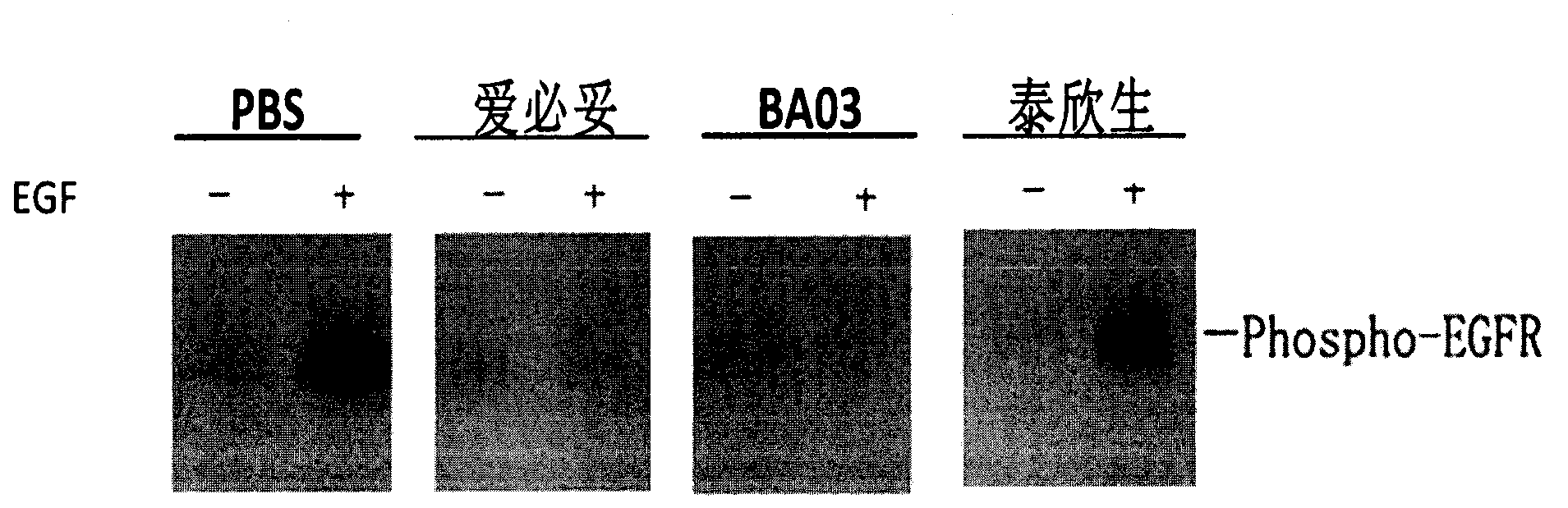
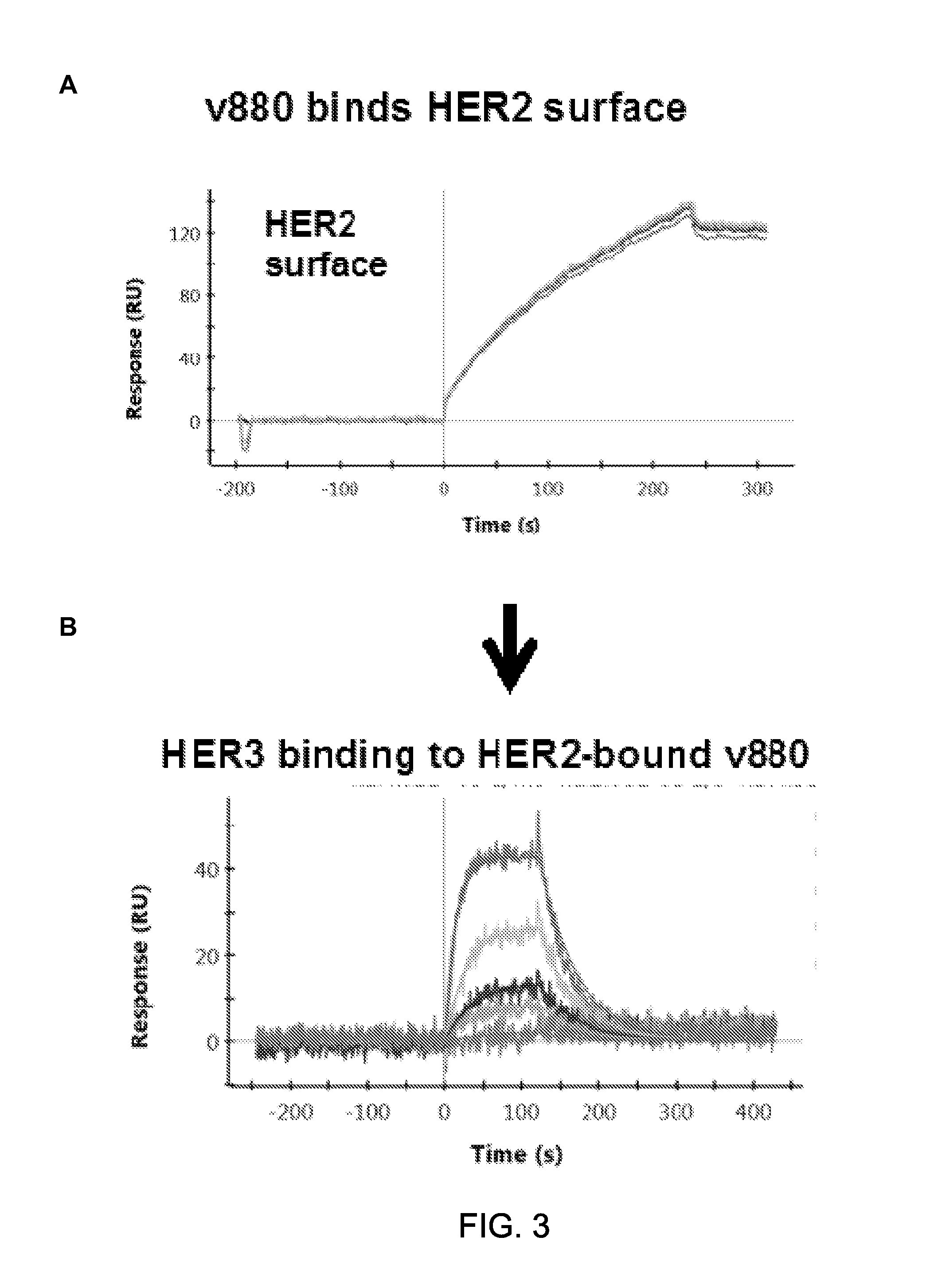
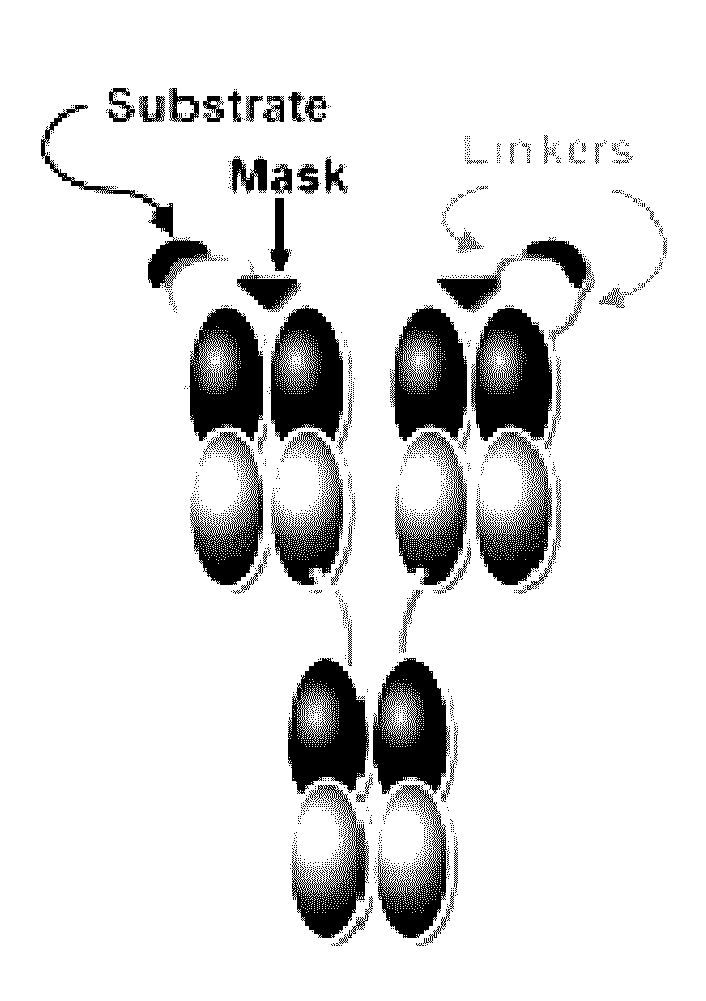
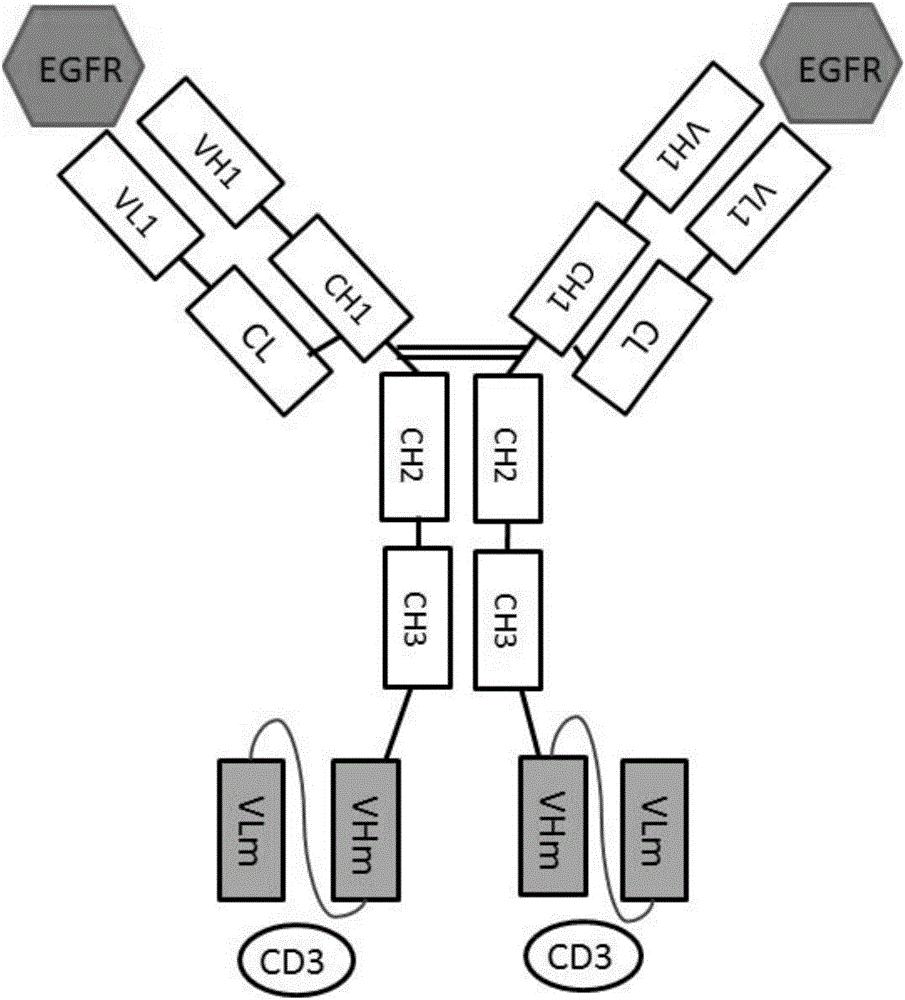
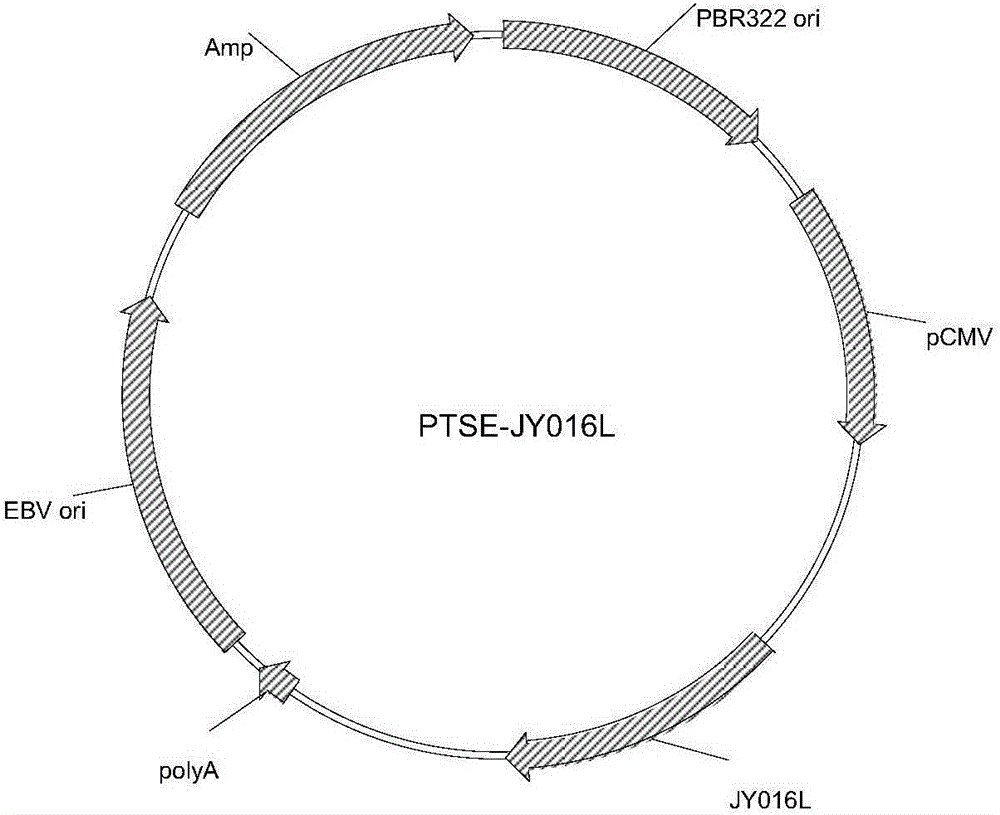
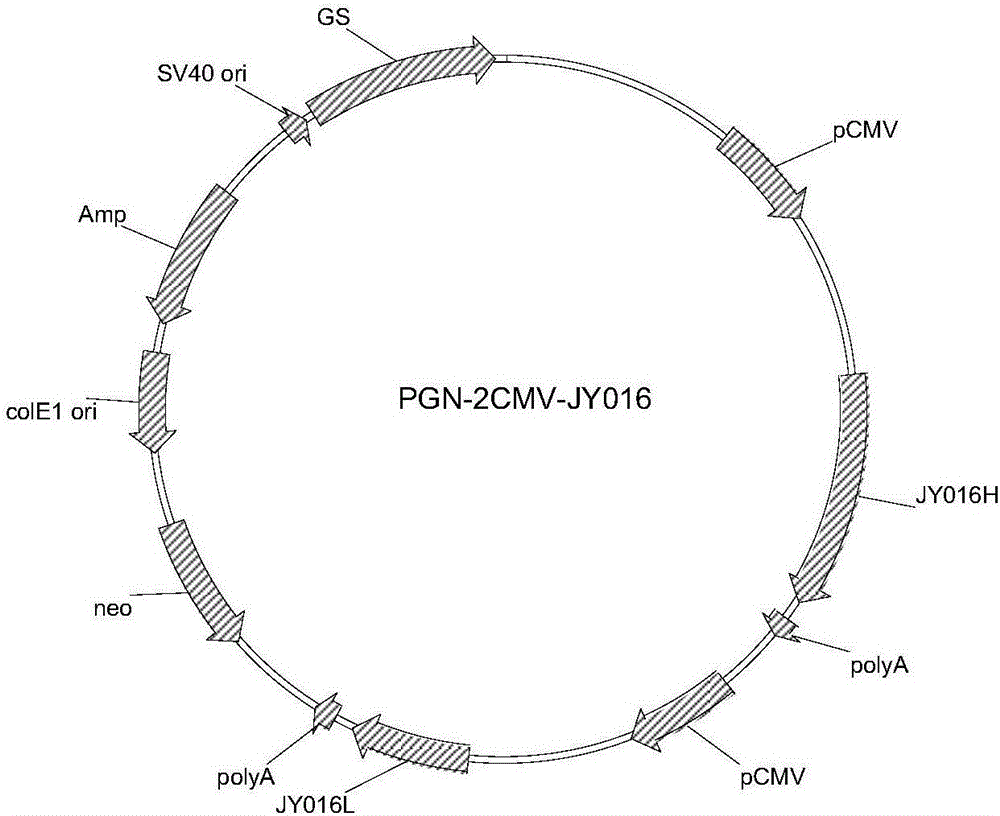
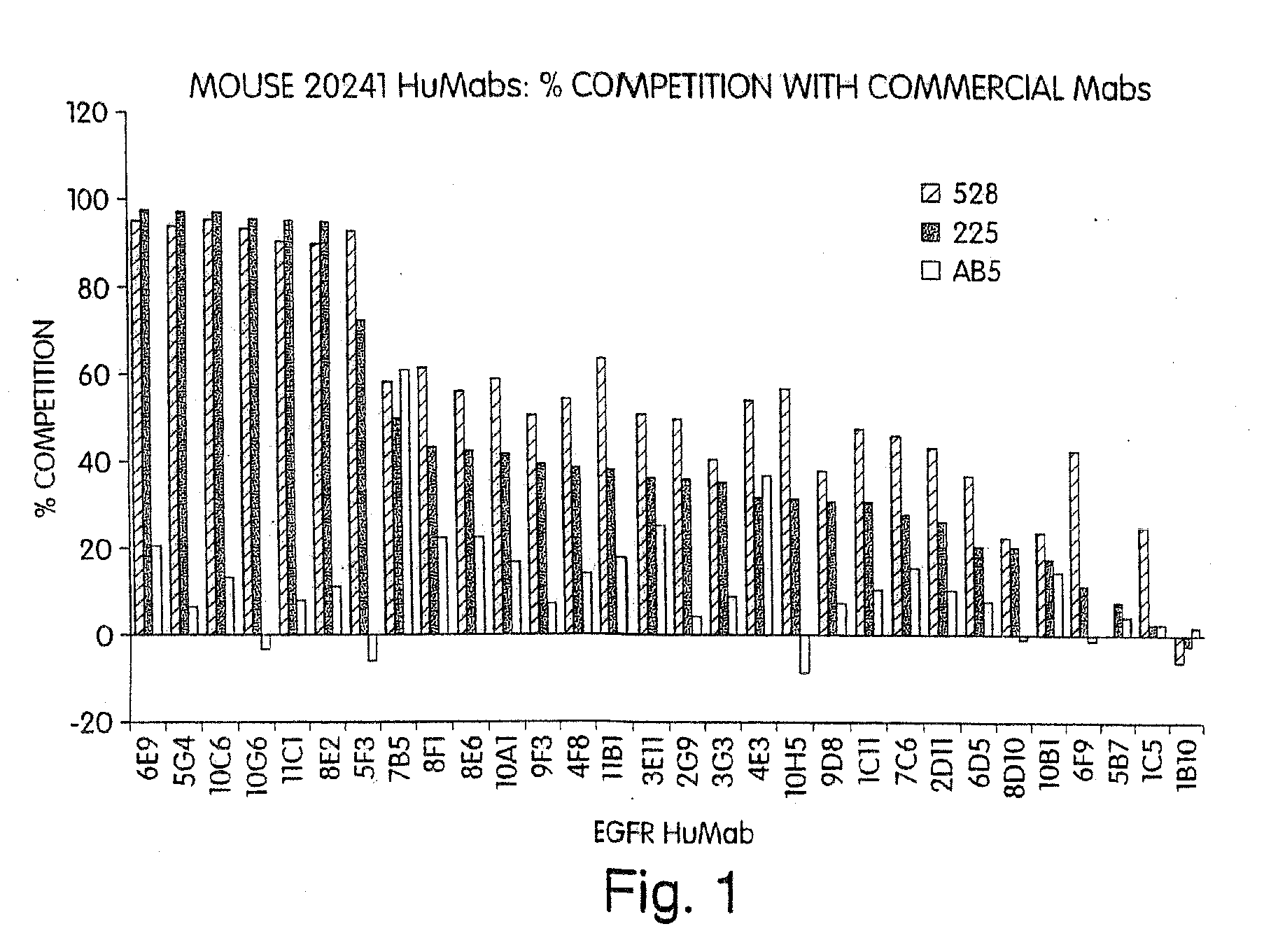
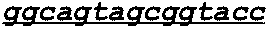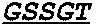Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
363 results about "Human epidermal growth factor receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Epidermal growth factor receptor (EGFR) is an important component in cancer treatment. EGFR, also called either Erb or human epidermal growth factor receptor (HER), is a protein located on the membranes of some cells.
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
InactiveUS7247301B2Less immunogenicReduce adverse side effectsInorganic active ingredientsImmunoglobulins against cytokines/lymphokines/interferonsV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB INC
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
Antibodies directed to the deletion mutants of epidermal growth factor receptor and uses thereof
ActiveUS20090155282A1Inhibit cell proliferationEnzymologyNanomedicineDeletion mutantHuman epidermal growth factor receptor
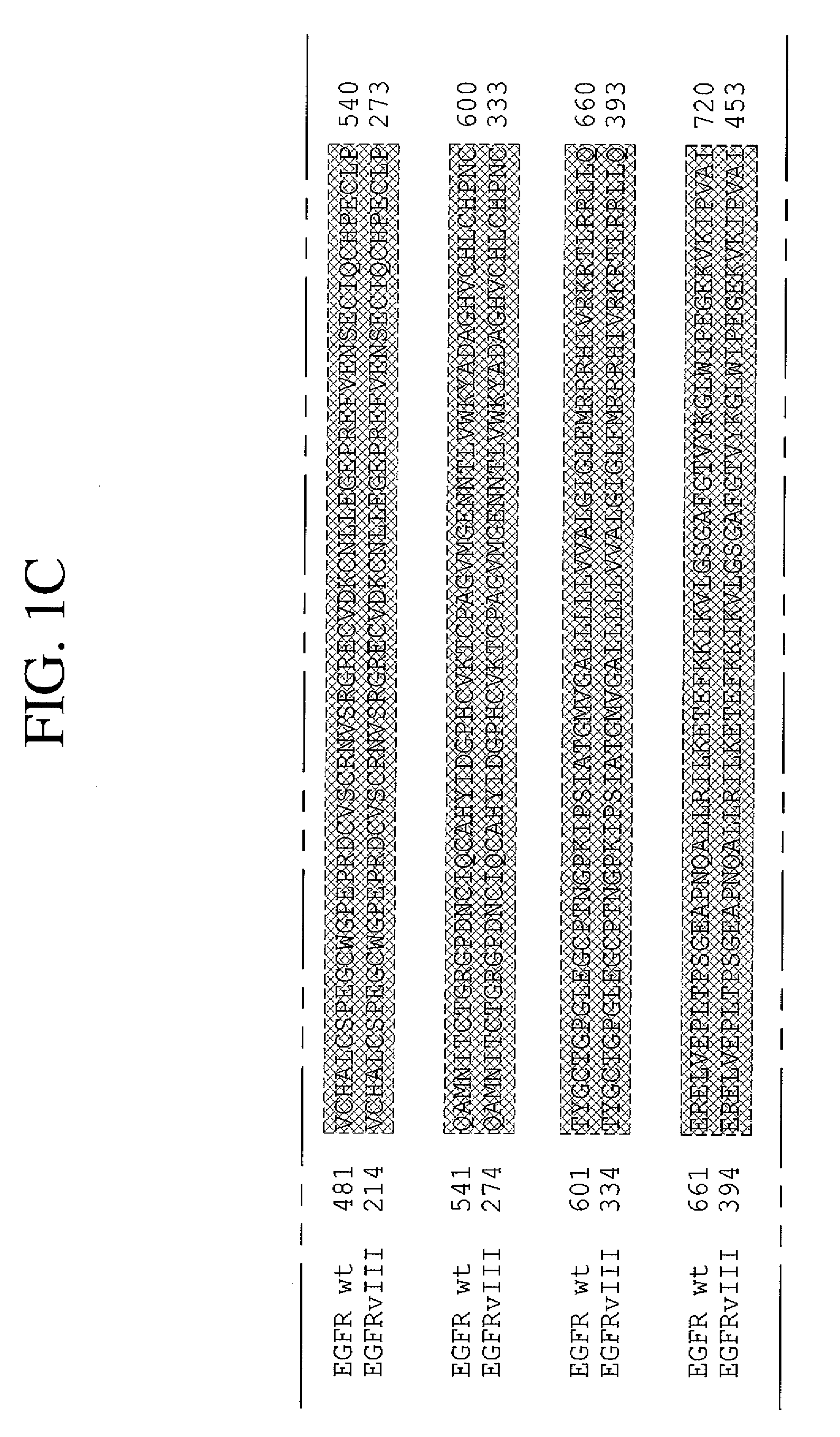
The present invention relates to novel antibodies, particularly antibodies directed against deletion mutants of epidermal growth factor receptor and particularly to the type III deletion mutant, EGFRvIII. The invention also relates to human monoclonal antibodies directed against deletion mutants of epidermal growth factor receptor and particularly to EGFRvIII. Diagnostic and therapeutic formulations of such antibodies, and immunoconjugates thereof, are also provided.
Owner:AMGEN FREMONT INC
Antibodies directed to the deletion mutants of epidermal growth factor receptor and uses thereof
ActiveUS20090175887A1Inhibit cell proliferationEnzymologyNanomedicineMonoclonal antibodyHuman epidermal growth factor receptor
The present invention relates to novel antibodies, particularly antibodies directed against deletion mutants of epidermal growth factor receptor and particularly to the type III deletion mutant, EGFRvIII. The invention also relates to human monoclonal antibodies directed against deletion mutants of epidermal growth factor receptor and particularly to EGFRvIII. Diagnostic and therapeutic formulations of such antibodies, and immunoconjugates thereof, are also provided.
Owner:AMGEN FREMONT INC
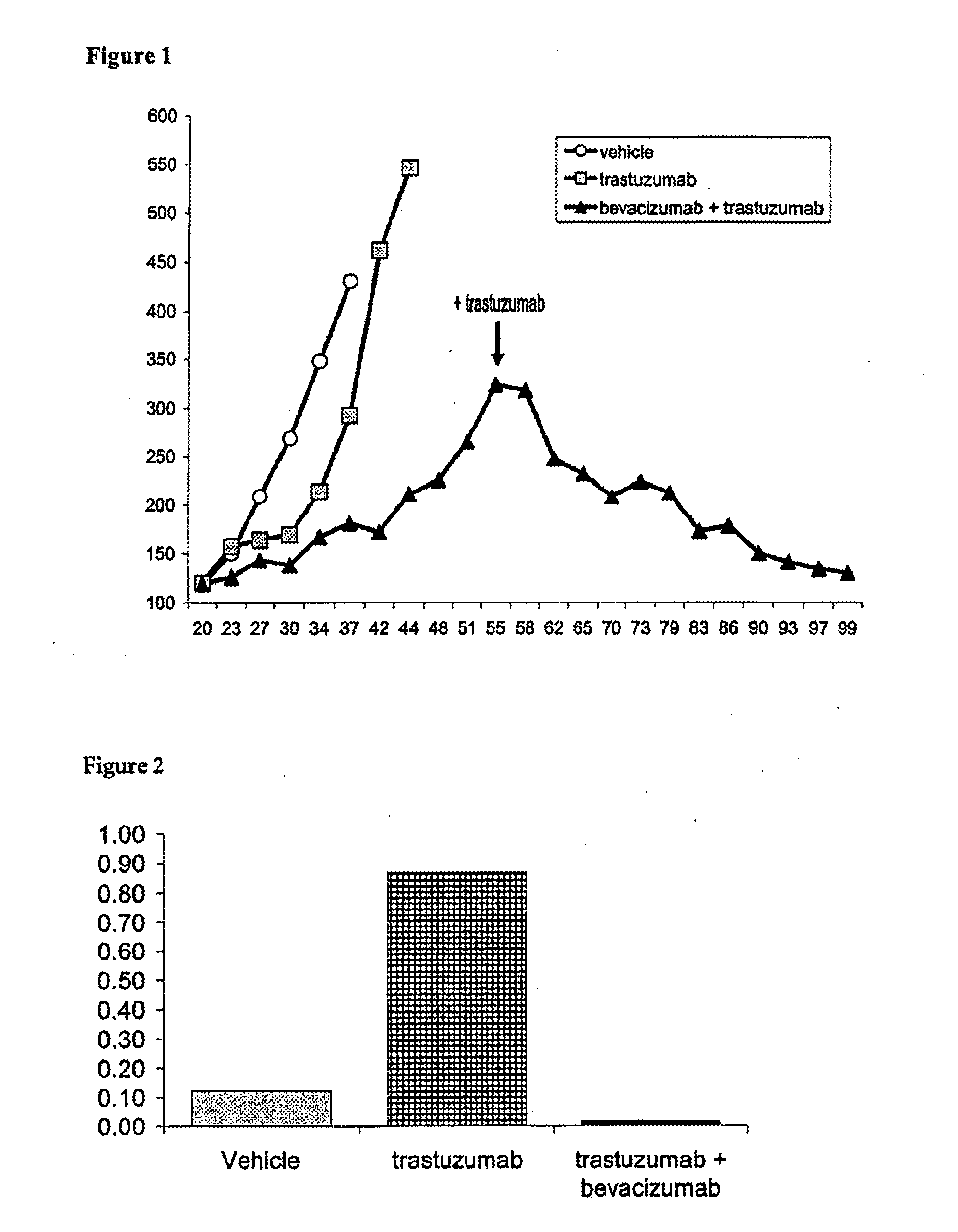
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20070224203A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseTumor therapy
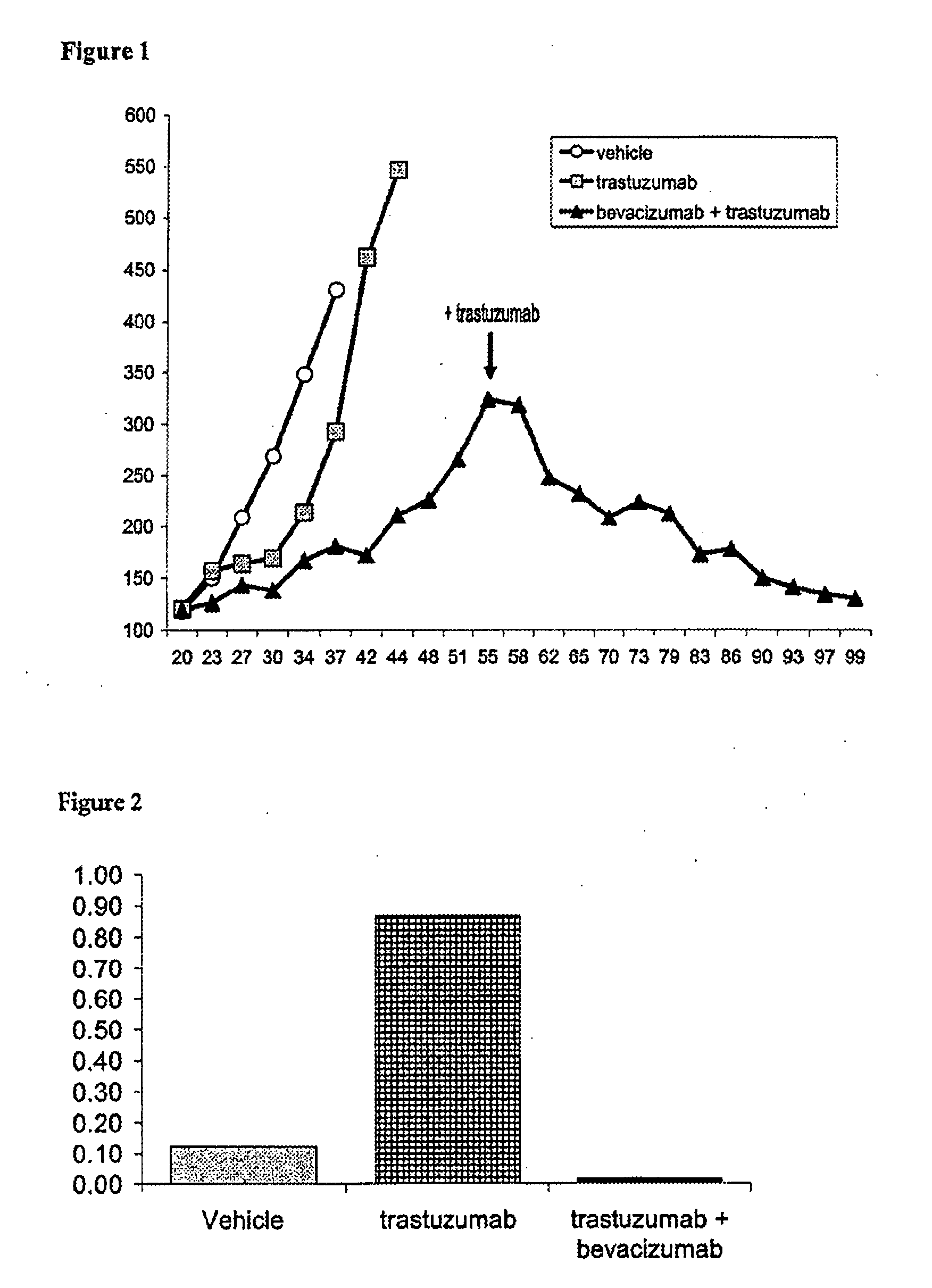
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
Human anti-epidermal growth factor receptor antibody
ActiveUS7598350B2Neutralize EGFRInhibit bindingSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesHuman epidermal growth factor receptor
Owner:IMCLONE SYSTEMS
Chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and composition and uses thereof
ActiveCN103483453AFix security issuesImprove proliferative abilityPeptide/protein ingredientsGenetic material ingredientsEpidermal Growth Factor Receptor KinaseAntigen
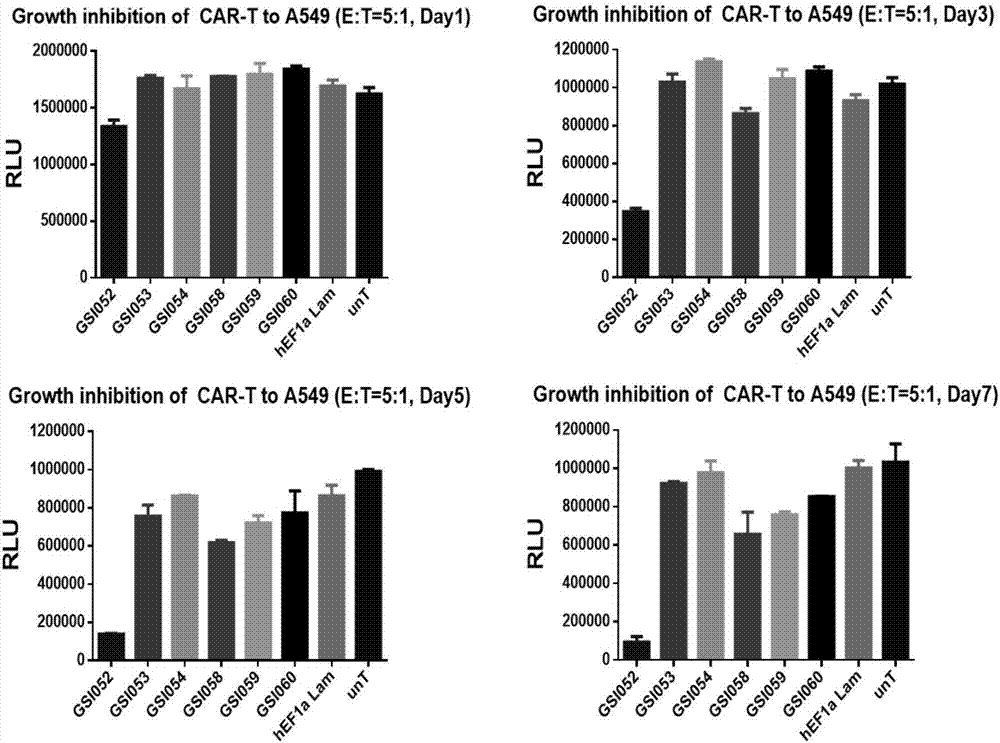
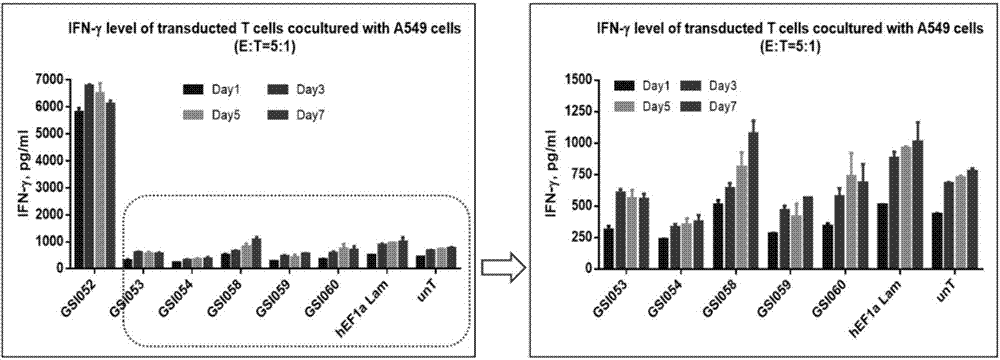
The present invention belongs to the fields of molecular biology and immunology and relates to a chimeric antigen receptor combining EGFR (epidermal growth factor receptor) family proteins and a composition and uses thereof The present invention particularly relates to the chimeric antigen receptor comprising polypeptides efficiently combining the EGFR family proteins and transmembrane domains, wherein the polypeptides efficiently combining EGFR family proteins comprise HERIN. The chimeric antigen receptor can promote the proliferation ability and killing effect of T cell at the premise of maintaining the killing specificity of the T cell.
Owner:SHANGHAI CELL THERAPY GRP CO LTD
Treatment of refractory human tumors with epidermal growth factor receptor and HER1 mitogenic ligand (EGFRML) antagonists
InactiveUS20030202973A1Reduce HAMA responseMinimize side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman tumorHuman epidermal growth factor receptor
A method of inhibiting the growth of refractory tumors that are stimulated by mitogenic ligands of epidermal growth factor receptor in human patients, comprising treating the human patients with an effective amount of a mitogenic ligand antagonist.
Owner:DR GEORGE PIECZENIK
Use of natural EGFR inhibitors to prevent side effects due to retinoid therapy, soaps, and other stimuli that activate the epidermal growth factor receptor
InactiveUS6638543B2Diminishing desired therapeutic effectPrevent drynessBiocideCosmetic preparationsSide effectRetinoid
Many human conditions, often skin conditions, are treated topically or orally with a retinoid such as retinoic acid or acetretin, which treatment often has the side effect of dry, irritated, and / or peeling skin. The use of soaps, detergents, chemical irritants, and such can also cause these same side effects. These side effects can be reduced or eliminated by the topical administration of an inhibitor, especially a natural inhibitor, of the epidermal growth factor receptor (EGFR), administered concomitantly with the retinoid, separately from the retinoid (such as on an as needed basis), or both. Administration of the two together is facilitated by a composition suitable for topical application and comprising both the retinoid and a natural EGFR inhibitor. Preferred natural inhibitors are genistein and other isoflavones extracted from natural occurring substances, or simple derivatives of such substances.
Owner:RGT UNIV OF MICHIGAN
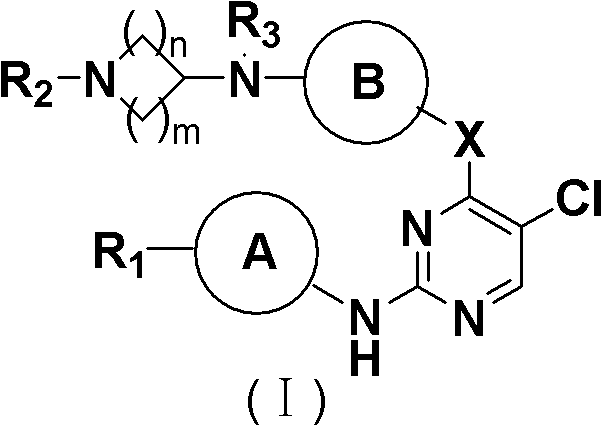
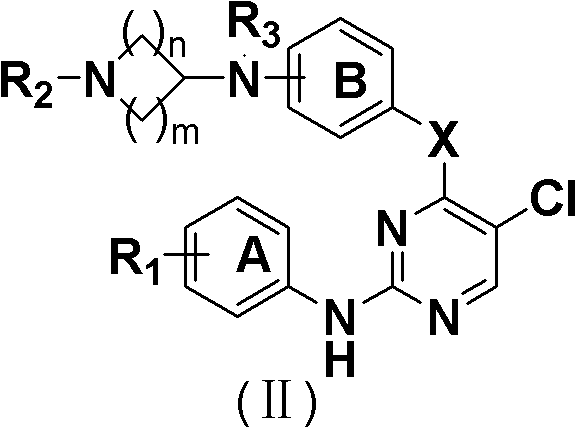
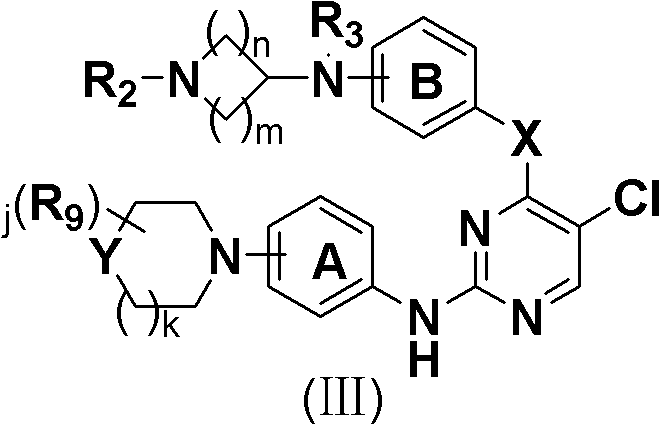
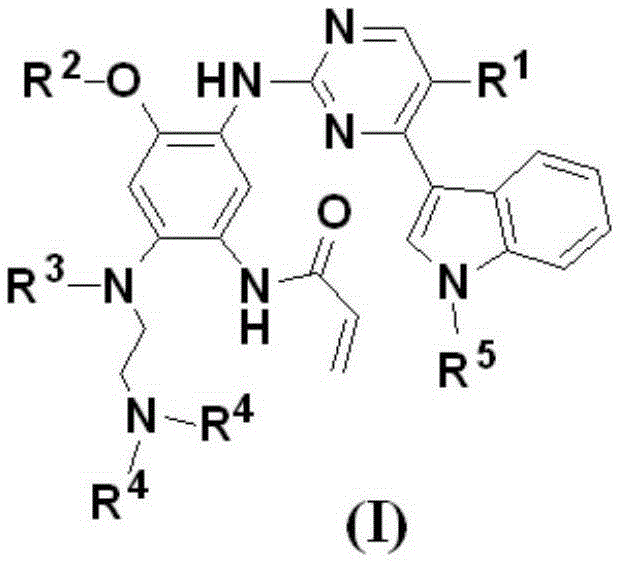
5-Chloropyrimidine compound and application of 5-Chloropyrimidine compound serving as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor
ActiveCN103159742AHigh inhibitory strengthSmall toxicityNervous disorderOrganic chemistryEGFR Tyrosine Kinase InhibitorsHuman epidermal growth factor receptor
The invention discloses an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, and the structural formula of the inhibitor is shown as in the formula (I). The invention further discloses an application of any compound shown as in the formula (I) and pharmacy-acceptable salt of the compound. The invention further provides a medicine compound for curing, and the medicine compound comprises the compound shown as in the formula (I) and an EGFR modifier.
Owner:BEIJING HANMI PHARMA CO LTD
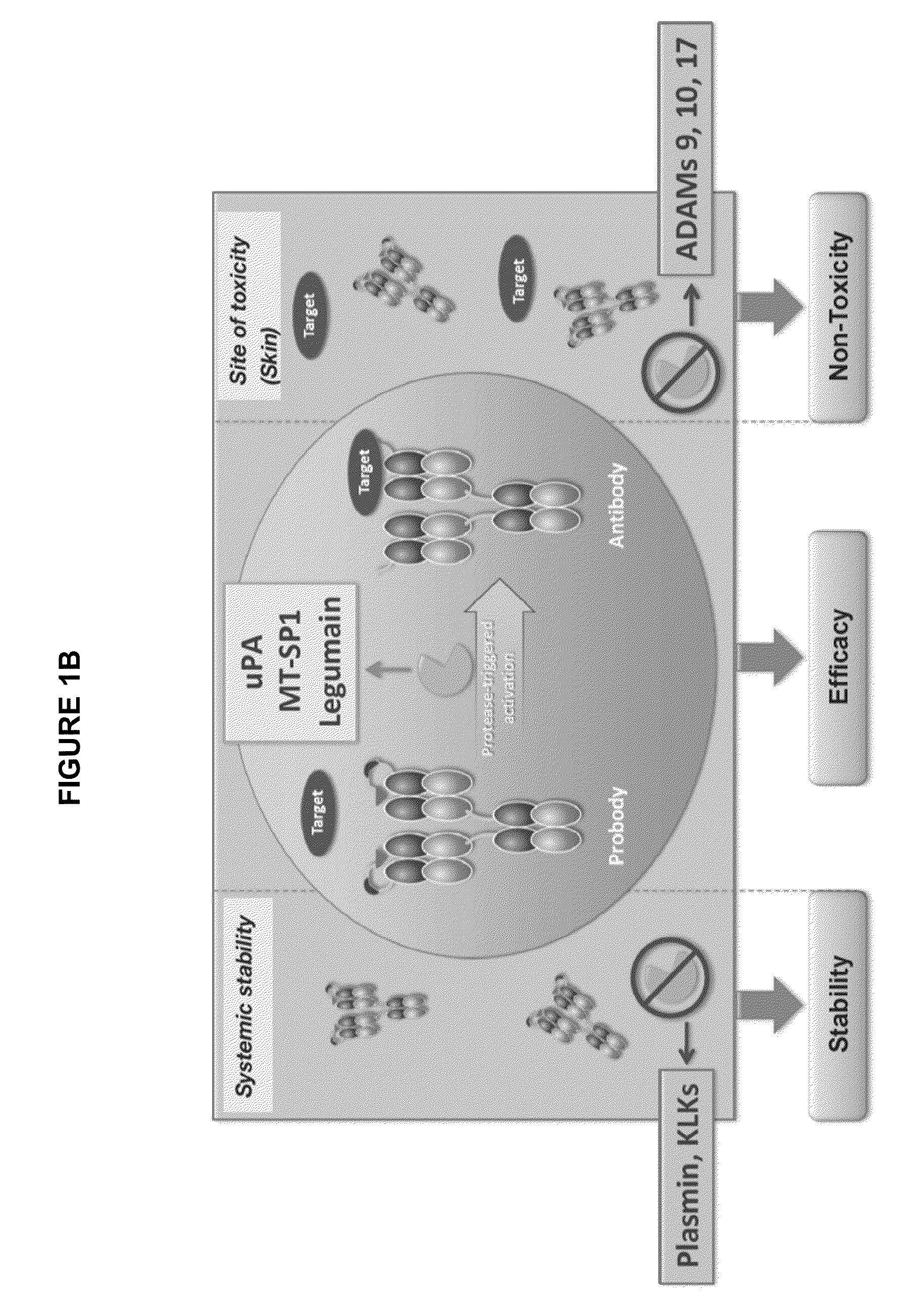
Activatable antibodies that bind epidermal growth factor receptor and methods of use thereof
Owner:CYTOMX THERAPEUTICS INC
Single domain antibodies directed against epidermal growth factor receptor and uses therefor
InactiveUS20110123529A1Preventing and alleviating symptomPeptide/protein ingredientsFermentationInhalationHuman epidermal growth factor receptor
The present invention relates to polypeptides derived from single domain heavy chain antibodies directed to Epidermal Growth Factor Receptor. It further relates to single domain antibodies that are Camelidae VHHs. It further relates to methods of administering said polypeptides orally, sublingually, topically, intravenously, subcutaneously, nasally, vaginally, rectally or by inhalation. It further relates to protocols for screening for agents that modulate the Epidermal Growth Factor Receptor, and the agents resulting from said screening. The invention further a method for delivering therapeutic molecules to the interior of cells.
Owner:ABLYNX NV
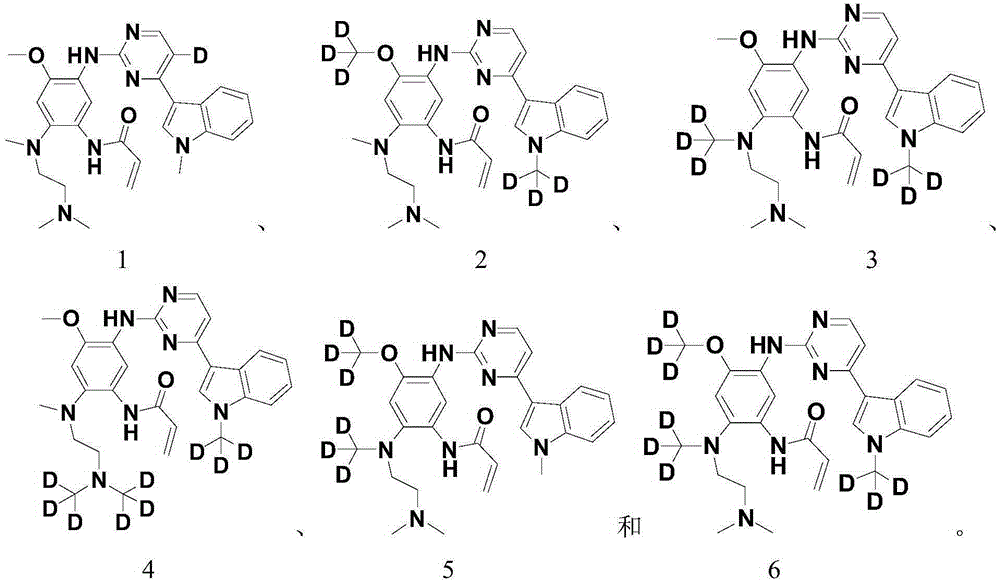
Pentadeuteropyridine compounds, and preparation method, pharmaceutical compositions and uses thereof
ActiveCN105237515AImprove securityStrong inhibitory activityOrganic active ingredientsOrganic chemistry methodsDiseaseErlotinib
The invention relates to pentadeuteropyridine compounds represented by the following formula (I) and pharmaceutically acceptable salts, stereoisomers, prodrugs and solvates thereof, and a preparation method, pharmaceutical compositions and uses thereof. The compounds can generate an inhibitory effect on variation forms of epidermal growth factor receptor (EGFR) protein kinase, thereby effectively inhibiting the growth of a variety of tumor cells; the compounds can be used for preparation of antitumor drugs, are used for treatment or prevention of a plenty of different cancers, and moreover, can overcome the drug resistance induced by conventional drugs gefitinib, erlotinib and other first-generation EGFR inhibitors. More specifically, the compounds can be used for preparation of drugs for treatment or prevention of diseases, obstacles, disorders or illness conditions mediated by certain variation-form epidermal growth factor receptors (such as L858R activated mutants, Exon19 deletion activated mutants, and T790M resistance mutants).
Owner:INVENTISBIO CO LTD +1
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20110064736A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseFactor ii
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
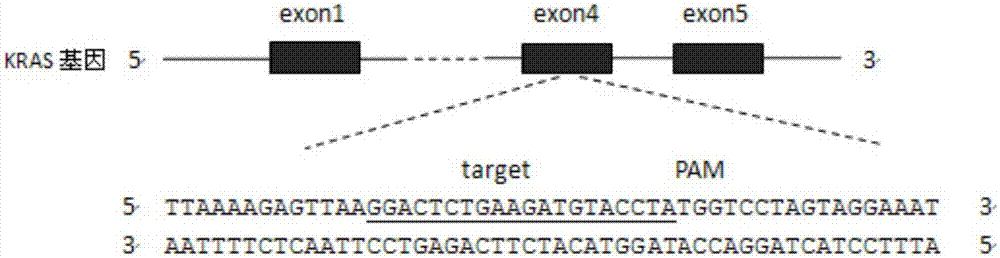
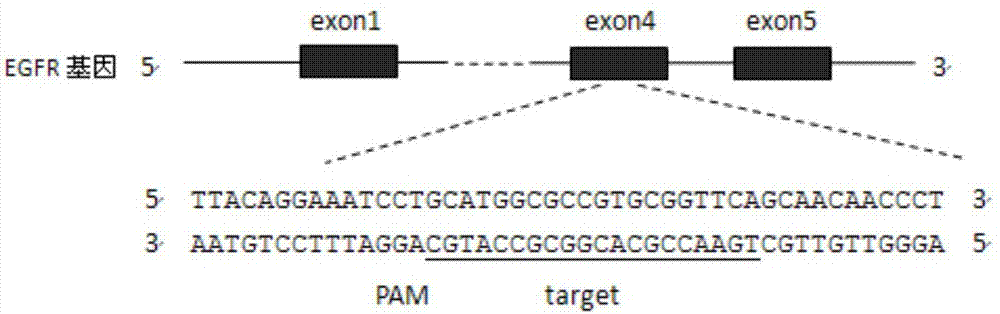
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system capable of simultaneously knocking out KRAS genes and EGFR (Epidermal Growth Factor Receptor) genes and application thereof
ActiveCN107130000AHigh knockout efficiencyEasy to operateOrganic active ingredientsHydrolasesAbnormal expressionKRAS
The invention discloses a CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-Cas9 system capable of simultaneously knocking out KRAS genes and EGFR genes. The system comprises sgRNA for specifically targeting KRAS genes and sgRNA for specifically targeting EGFR genes, wherein a corresponding DNA sequence of the sgRNA for specifically targeting KRAS genes is shown as SEQ ID NO.1 or / and SEQ ID NO.2; and a corresponding DNA sequence of the sgRNA for specifically targeting EGFR genes is shown as SEQ ID NO.11 or / and SEQ ID NO.12. The invention further discloses application of the system in preparation of medicines for treating cancers. The CRISPR-Cas9 system disclosed by the invention is capable of simultaneously and efficiently knocking out two cancer driving factors KRAS and EGFR which are highly-expressed in lung cancer. The system is simple in operation and high in knockout efficiency and is expected to be applied to treatment of the lung cancer. The system disclosed by the invention is applicable to multiple cancers with abnormal expressions of the EGFR and KRAS.
Owner:浙江卫未生物医药科技有限公司
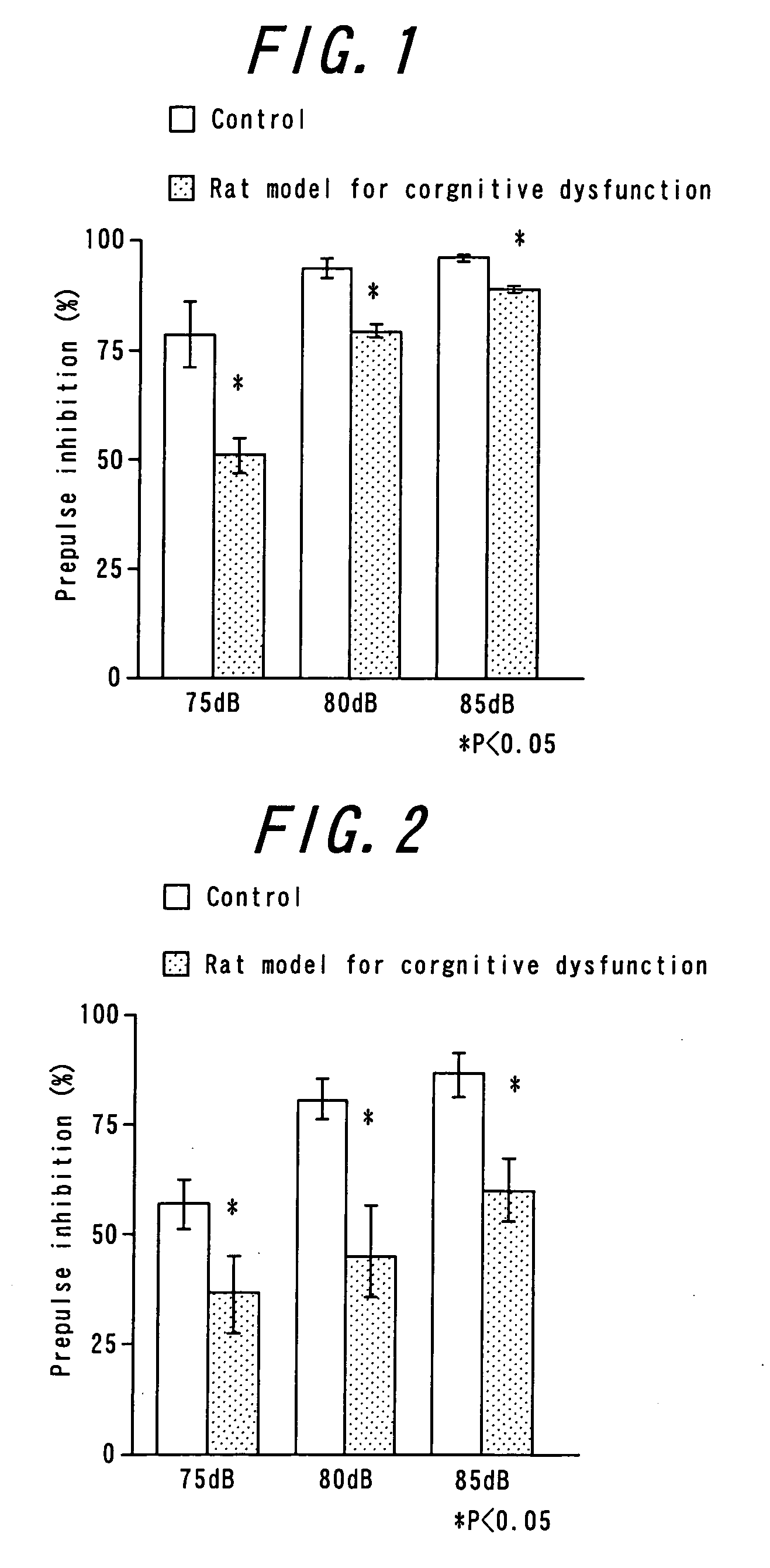
Antipsychotic molecular-targeting epithelial growth factor receptor
The purpose of this invention is to provide an agent useful for prevention and / or treatment of psychosis, schizophrenia and cognitive impairments. To solve this problem, this invention provides epidermal growth factor receptor inhibitors as therapeutic agents for psychosis, schizophrenia and cognitive impairments.
Owner:HIROYUKI NAWA
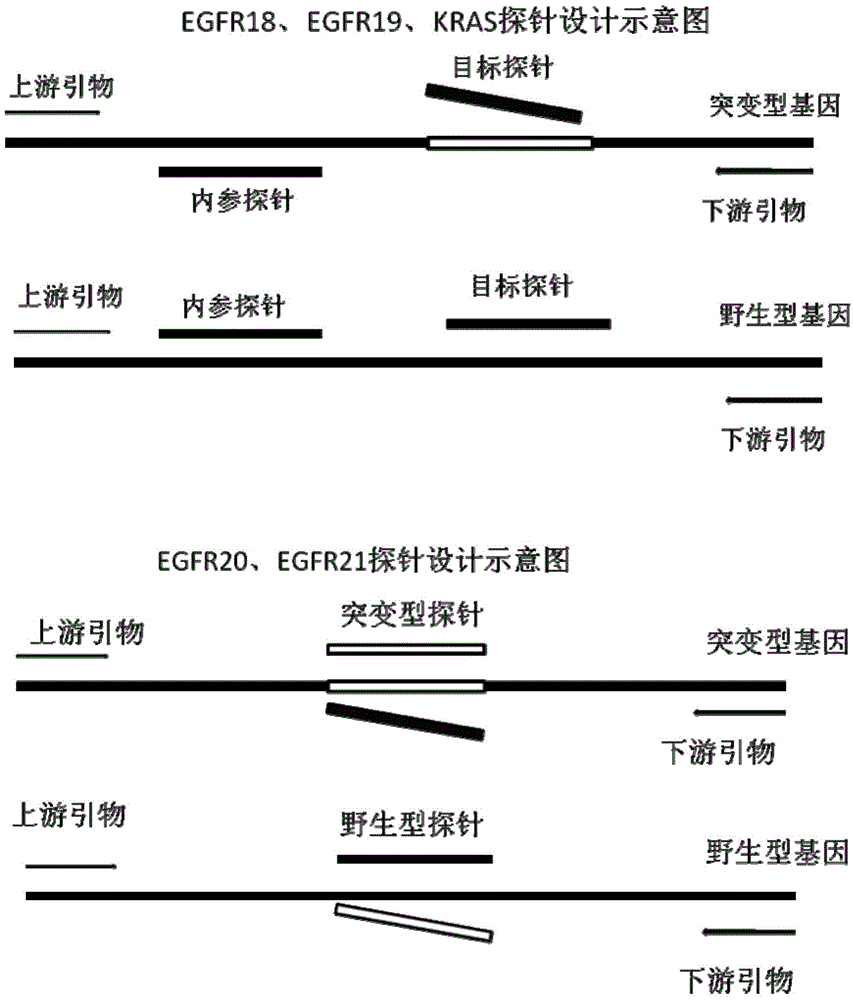
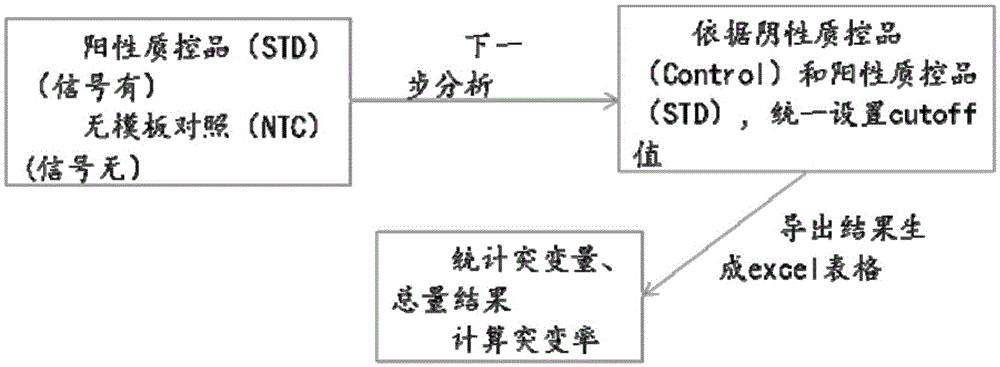
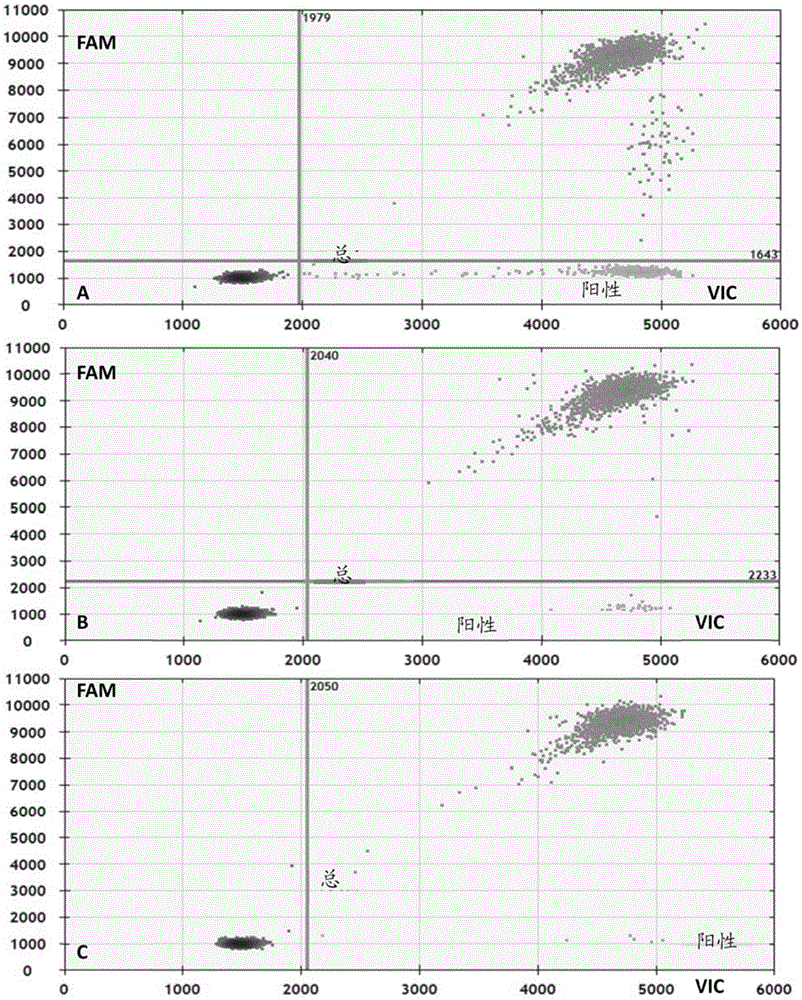
Primer, probe and kit for detecting EGFR and/or K-ras genetic mutation
ActiveCN105624309AEasy to optimizeMeet the requirements of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationK-ras GenesFluorescence
The invention discloses a primer and probe for detecting a human epidermal growth factor receptor (EGFR) gene and / or a K-ras gene, a kit containing the primer and the probe and a device for detecting genetic mutation on the basis of a digital PCR platform.The method for detecting the genetic mutation by means of the primer and the probe comprises the steps that the prime and the probe are provided; DNA of a sample to be detected is extracted; a fluorescent PCR reaction system capable of amplifying a mutant gene sequence is prepared; a target probe and an internal reference probe are utilized to be hybridized with amplified products respectively, and fluorescent signals of corresponding fluorescent groups are detected; existence of the genetic mutation is judged and / or the mutation rate is calculated according to the strength and proportion of the fluorescent signals of the target probe and the internal reference probe.According to the method for detecting the genetic mutation, the needed primers and probes are small in number, the optimization procedure is simple, related mutation of EGFR and / or K-ras gene can be qualitatively or quantitatively detected, and the detection sensitivity is high; a DNA sample with low initial amount can also be detected stably.
Owner:SHENZHEN HUADA GENE INST
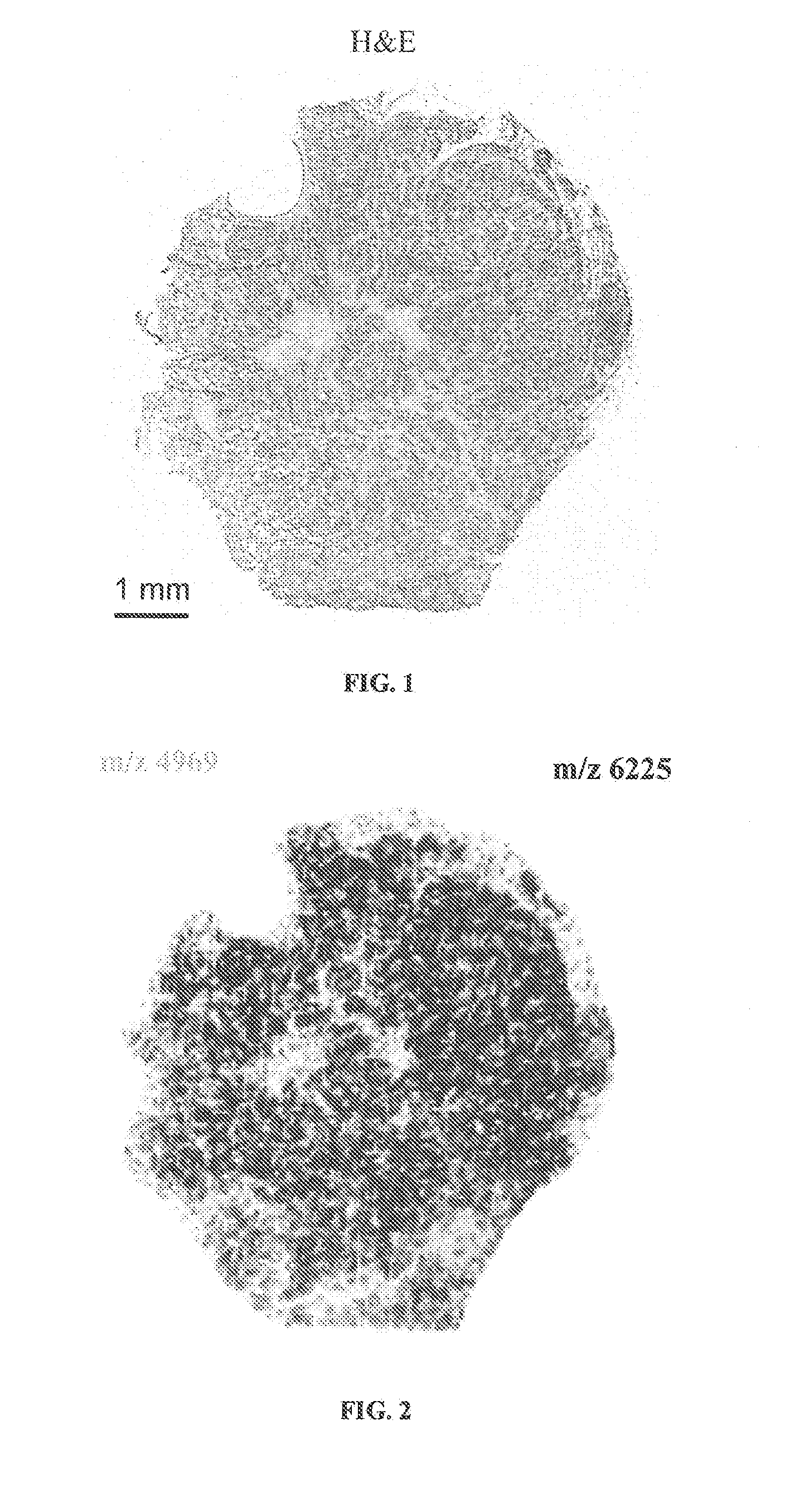
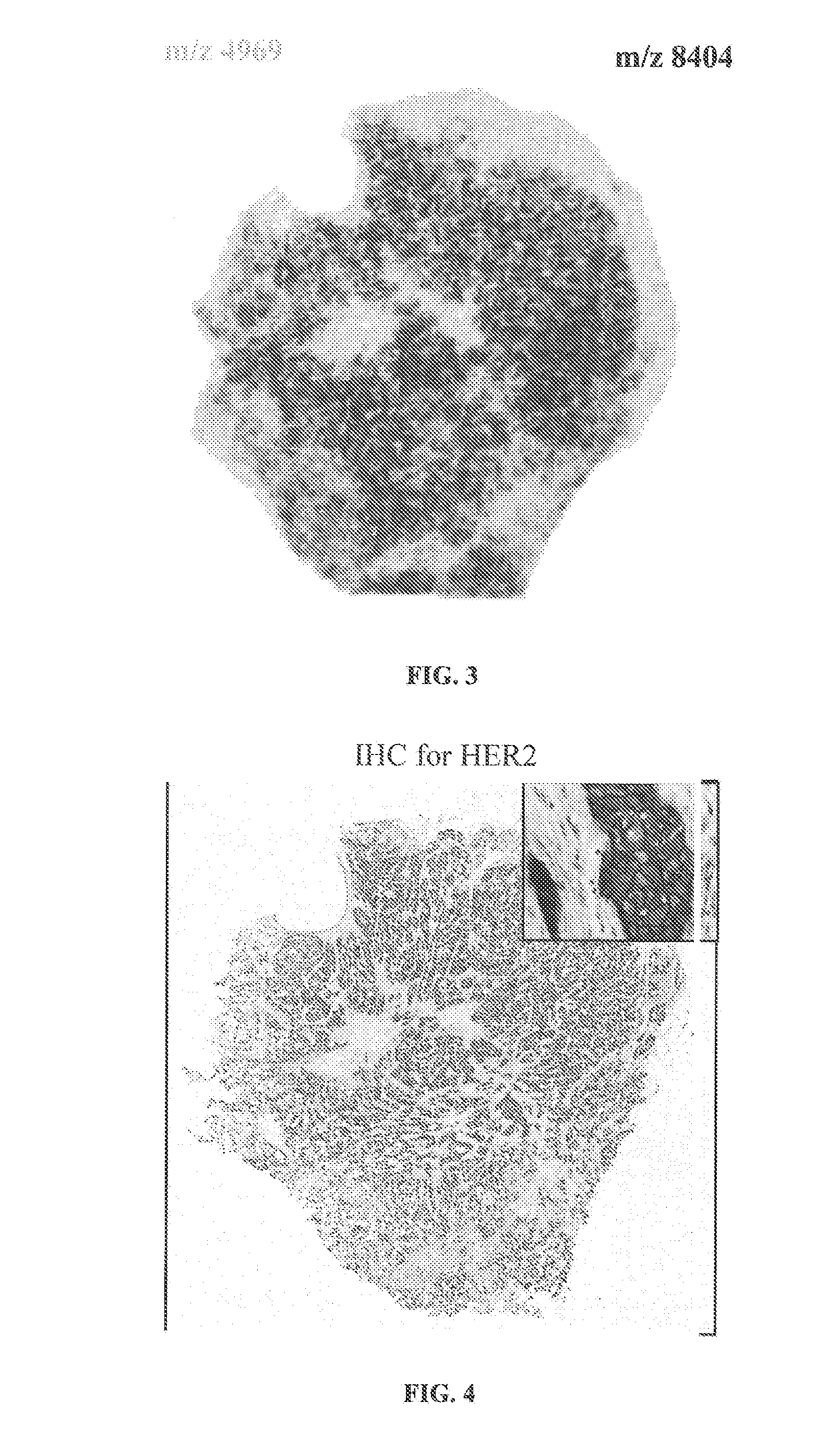
Determining an expression status of human epidermal growth factor receptor 2 (HER2) in a biological sample
A method for determining an expression of human epidermal growth factor receptor 2 (HER2) of a subject. The method includes providing a sample from the subject; measuring one of (i) amounts of two or more proteins in the sample, each protein having a molecular weight substantially equal to 4740, 8404, 8419, 8435, 8450, 8455, 8465, 8570, 8607 or 8626 atomic mass units, and (ii) amounts of at least one of human cystein-rich intestinal protein 1 (CRIP1), one or more variants of the human cystein-rich intestinal protein 1 (CRIP1 variants), and proteolytic digestion products thereof in the sample; and comparing the amounts of the proteins to control amounts, which control amounts are determinative of the expression of the human epidermal growth factor receptor 2.
Owner:BRUKER DALTONIK GMBH & CO KG
Antibody fragment-polymer conjugates and uses of same
Described are conjugates formed by an antibody fragment covalently attached to a non-proteinaceous polymer, wherein the apparent size of the conjugate is at least about 500 kD. The conjugates exhibit substantially improved half-life, mean residence time, and / or clearance rate in circulation as compared to the underivatized parental antibody fragment. Also described are conjugates directed against human vascular endothelial growth factor (VEGF), human p185 receptor-like tyrosine kinase (HER2), human CD20, human CD18, human CD11a, human IgE, human apoptosis receptor-2 (Apo-2), human tumor necrosis factor-α (TNF-α), human tissue factor (TF), human α4β7 integrin, human GPIIb-IIIa integrin, human epidermal growth factor receptor (EGFR), human CD3, and human interleukin-2 receptor α-chain (TAC) for diagnostic and therapeutic applications.
Owner:GENENTECH INC
Genetically engineered lymphocyte targeting Human EGFR (Epidermal Growth Factor Receptor), preparation method and application of genetically engineered lymphocyte
ActiveCN103113470AGood effectInhibit tumor formationGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-Chain AntibodiesTumor antigen
The invention relates to the field of genetic engineering, and in particular relates to an anti-EGFR ((Epidermal Growth Factor Receptor) chimeric antigen receptor, and a lymphocyte for expressing the antigen receptor. The technical solution of the invention provides a new effective selection for the anti-tumor technical field. The technical scheme is that a single-chain antibody targeting the EGFR is provided. The single-chain antibody is further designed to be constructed into the chimeric antigen receptor by means of a genetic engineering technology, and the structure of the gained chimeric antigen receptor is anti-EGFR (scFv)-IgG2 (Fc)-CD28-CD3 Zeta. According to the genetically engineered lymphocyte targeting the human EGFR, the chimeric antigen receptor is transfected into the lymphocyte by the nucleofaction technology, and thus the nonspecific lymphocyte can be endowed with the capacity of specifically distinguishing the EGFR tumor antigen and targeting and killing the EGFR over-expression tumor cells. According to the technical scheme, the adoptive cell therapy and the genetic therapy are organically combined, so that remarkable effect of killing the tumor is gained, and a novel effective selection is provided for the field.
Owner:WEST VAC BIOPHARMA CO LTD
Tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor)
InactiveCN107034235AIncrease lethalityImprove homingGenetically modified cellsMammal material medical ingredientsTumor targetEGFR Antibody
The invention discloses a tumor immunization method combining with chimeric antigen T cells targeting at PD-1 (programmed cell death protein 1) and EGFR (epidermal growth factor receptor) and also discloses a plasmid vector for implementing the method. In combination with fourth-generation CAR-T (chimeric antigen receptor T) cells targeting at PD-1 and EGFR, a lentiviral vector is used as a CAR-T vector basic structure, and truncated EGFR antibody is selected as a CAR core to give tumor targeted enrichment to play, tumor-killing effect is given to play in conformity with overexpressed immune checkpoint inhibitor PD-1 monoclonal antibody; by constructing the fourth-generation CAR-T vector for co-expressing various regulatory factors such as IL21, CCR4 and Bcl2, the killing, homing and persistent proliferating abilities of CAR-T cells are improved. EP-CAR T (esophageal papilloma chimeric antigen receptor T) cells are treated by transducing patient's autologous T-lymphocytes in vitro, amplifying suitably and transducing back to the patient's body via autologous transfusion, and no reports on similar designs of CAR-T cells are provided at present.
Owner:尹荣
Substituted crotonamide maleate and crystal forms thereof
ActiveCN104513200ATumor suppressionOrganic active ingredientsCarboxylic acid salt preparationHuman epidermal growth factor receptorKinase inhibition
The invention discloses a substituted crotonamide maleate, a crystal form thereof, a method for preparing all crystal forms of crystallized maleate, relevant compounds, and a medicinal composition including the compounds. (E)-N-{4-[3-acetylenylphenylamino]-3-cyan-7-ethoxy-6-quinolyl}}-4(dimethylamino)-2-crotonamide maleate, a crystal form I, a crystal form II or a crystal form III respectively have EGFR or ErbB2 kinase inhibition activity, can be used to prevent, treat or inhibit tumors, and can be especially to prepare drugs for preventing, treating or inhibiting epidermal growth factor receptor family related tumors.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD
Humanized antibody against epidermal growth factor receptor and application thereof
The invention relates to a humanized antibody against an epidermal growth factor receptor. The invention further relates to a nucleic acid molecule coding the antibody, a vector, a host cell and a composition containing the nucleic acid molecule, a detection reagent and a kit. The invention also relates to application of the antibody in preparation of drugs used for prevention / treatment of diseases related to an epidermal growth factor or the epidermal growth factor acceptor and preparation of the detection reagent or kit. The molecule of the antibody provided by the invention has lower immunogenicity and higher target binding activity, so the antibody has stronger biological activity and in-vitro and in-vivo antineoplastic activity, and it is expected that the antibody has a smaller side-effect and a better curative effect in clinic application.
Owner:SHANGHAI JMT BIO INC
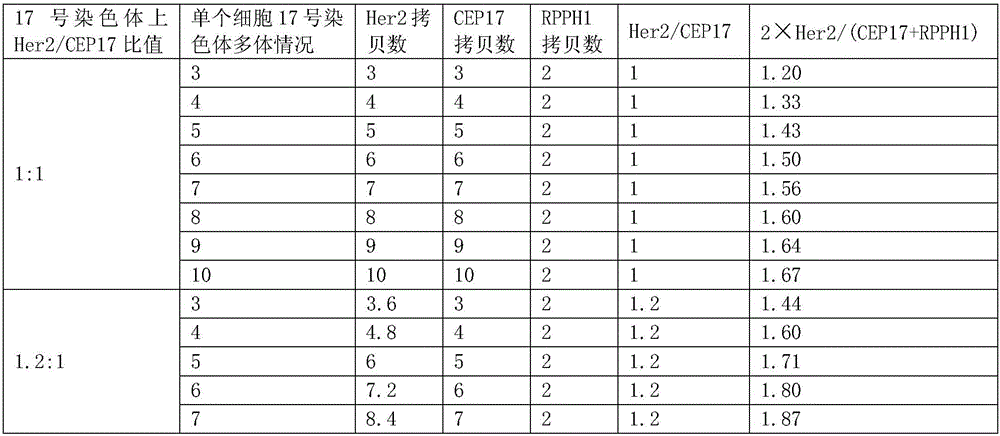
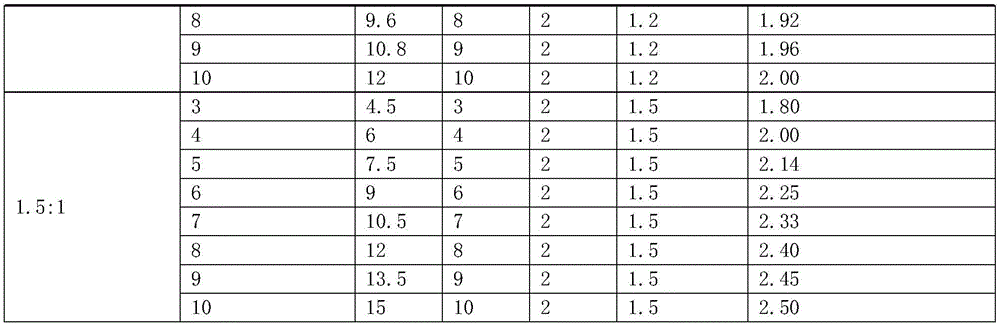
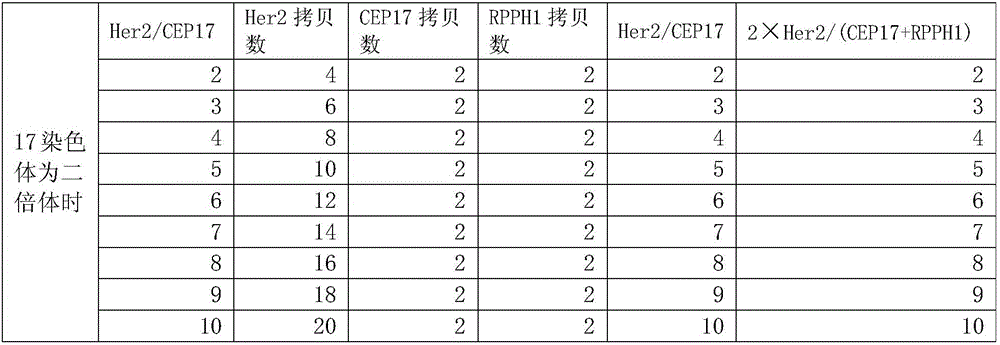
Nucleic acid combination for detecting Her2 gene, kit and application
ActiveCN106591438AFast analysisImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationBiologyPcr method
The invention discloses a nucleic acid combination for detecting Her2 (human epidermal growth factor receptor-2) gene, a kit and application. The nucleic acid combination comprises two pairs of Her2 gene primers and two primers of internal reference primers. According to the invention, two pairs of primers and two pairs of internal references are employed to detect Her2 gene by digital PCR method, absolute quantification can be performed on Her2 gene, full automatic result interpretation can be carried out by means of software, and the analysis speed is fast. Use of two pairs of primers is conducive to enhancing the accuracy of a detection result, especially for a blood sample with low DNA content in itself. Simultaneous use of two pairs of internal reference primers reduces the possibility of false negative and false positive.
Owner:领航医学科技(深圳)有限公司
Bispecific HER2 and HER3 Antigen Binding Constructs
InactiveUS20160083480A1Ultrasonic/sonic/infrasonic diagnosticsVaccination/ovulation diagnosticsHuman epidermal growth factor receptorExtracellular Structure
Described herein are isolated bi-specific antigen binding constructs, e.g., antibodies. The bi-specific antigen binding constructs include two antigen binding polypeptide constructs, e.g., a Fab and an scFv. The first antigen-binding polypeptide construct monovalently and specifically binds to extracellular domain 4 (ECD4) of HER2 (human epidermal growth factor receptor 2); the second antigen-binding polypeptide construct monovalently and specifically binds to an extracellular domain (ECD) of HER3 (human epidermal growth factor receptor 3). One antigen binding polypeptide construct is a Fab format and the other antigen binding polypeptide construct is an scFv format. The bi-specific antigen binding constructs includes an Fc having two Fc polypeptides each having a CH3 domain for dimerization. Each Fc polypeptide is linked to the C-terminus of one of the antigen binding polypeptide constructs with or without a linker.
Owner:ZYMEWORKS INC
Activatable antibodies that bind epidermal growth factor receptor and methods of use thereof
ActiveUS9540440B2Reduce capacityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman epidermal growth factor receptorAntibody
The invention relates generally to variant activatable antibodies that include a masking moiety (MM), a cleavable moiety (CM), and an antibody (AB) that specifically binds to epidermal growth factor receptor (EGFR), and to methods of making and using these variant anti-EGFR activatable antibodies in a variety of therapeutic, diagnostic and prophylactic indications.
Owner:CYTOMX THERAPEUTICS
Anti-EGFR (epidermal growth factor receptor) and anti-CD3 (cluster of differentiation 3) bispecific antibody and application thereof
ActiveCN106632681AConducive to biological functionsConducive to biological functionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntigen
Owner:BEIJING DONGFANG BIOTECH +1
Human monoclonal antibodies to epidermal growth factor receptor (EGFR)
ActiveUS20100008929A1Less immunogenicPreventing growth and functionAntibacterial agentsNervous disorderV(D)J recombinationHuman epidermal growth factor receptor
Isolated human monoclonal antibodies which specifically bind to human EGFR, and related antibody-based compositions and molecules, are disclosed. The human antibodies can be produced by a transfectoma or in a non-human transgenic animal, e.g., a transgenic mouse, capable of producing multiple isotypes of human monoclonal antibodies by undergoing V-D-J recombination and isotype switching. Also disclosed are pharmaceutical compositions comprising the human antibodies, non-human transgenic animals and hybridomas which produce the human antibodies, and therapeutic and diagnostic methods for using the human antibodies.
Owner:GENMAB AS
Quantitative detection kit and method for exon mutation of epidermal growth factor receptor (EGFR) relevant to lung cancers
ActiveCN102140518AReduce material requirementsReduce false positivesMicrobiological testing/measurementLife qualityMagnetic bead
The invention discloses a quantitative detection kit for exon mutation of epidermal growth factor receptor (EGFR) relevant to lung cancers, which comprises a primer for amplifying exons of EGFR genes 18, 19, 20, 21, a sequencing primer and a magnetic bead marked by avidin. The invention also discloses a preparation method and a using method of the kit. The detection method in the invention not only has high sensitivity so that 1% of mutation can be detected, but also has visual result and simple, accurate and rapid interpretation, greatly reduces the false negative rate of the detection result and avoids invalid targeted medicine selections of clinical patients, thereby saving valuable treating time for the patients and improving the life quality of the patients.
Owner:北京迪安医学检验实验室有限公司
Epidermal Growth Factor Receptor-Derived Peptides
ActiveUS20080119399A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHuman epidermal growth factor receptorTransit Peptide
The object of the invention is to provide an EGFR-derived peptide useful for EGFR-based immunotherapy.The invention provides an EGFR-derived peptide capable of inducing both cellular and humoral immune responses and mutant peptide thereof and a polypeptide comprising said peptide, a nucleic acid molecule encoding the same, and a pharmaceutical composition comprising the same.
Owner:BRIGHTPATH BIOTHERAPEUTICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com