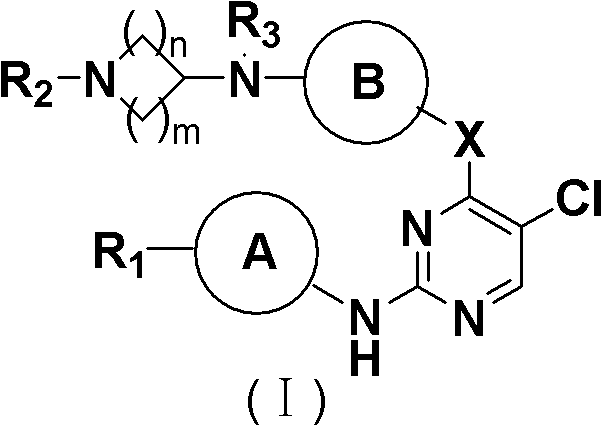
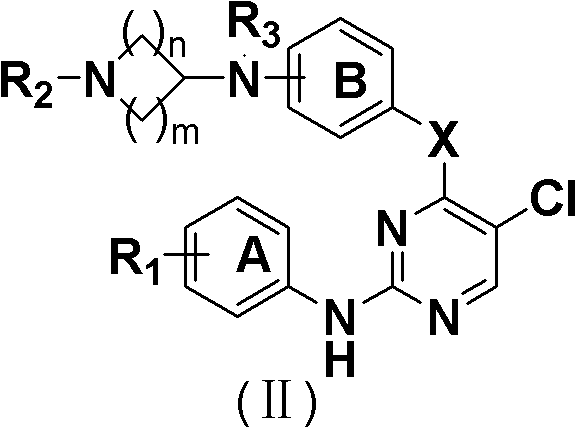
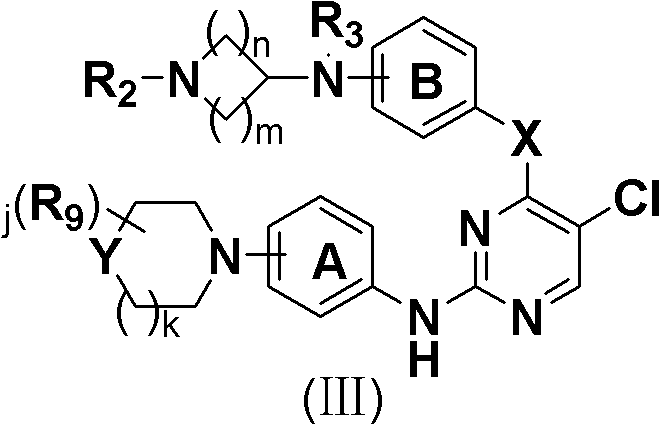
5-Chloropyrimidine compound and application of 5-Chloropyrimidine compound serving as epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor
A compound and solvate technology, applied in the field of EGFR tyrosine kinase inhibitors, can solve the problems of drug resistance, difficult to achieve concentration, etc., and achieve the effect of reducing mutation selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Embodiment 1, the preparation of compound
[0136] 1-(3-(3-(5-chloro-2-(4-(4-methylpiperazin-1-yl)anilino)pyrimidin-4-yloxy)anilino)azetidin-1 -yl)prop-2-en-1-one
[0137]
[0138] 1: 3-(2,5-Dichloropyrimidin-4-yloxy)aniline
[0139] 2,4,5-Trichloropyrimidine (3.67g, 20mmol), m-hydroxynitrobenzene (2.78g, 20mmol) and diisopropylethylamine (7mL, 40mmol) were added to 30mL absolute ethanol. After stirring at room temperature, the system gradually became turbid, and a large amount of off-white precipitates could be seen. After 2 hours, TLC monitoring showed that the raw materials had been basically completely converted. Suction filtration under reduced pressure, the filter cake was washed twice with n-hexane, and dried to obtain 5.32 g (93%) off-white solid.
[0140]Dissolve all the white solid in 30mL tetrahydrofuran, add zinc powder (6g, 93mmol), water (8mL) and ammonium chloride (5g, 93mmol) and heat to reflux for 5 hours, TLC monitoring shows no raw material poin...
Embodiment 2
[0151] Embodiment 2, the preparation of compound
[0152] 4-(4-(3-(1-acryloylazetidin-3-ylamino)phenoxy)-5-chloropyrimidin-2-ylamino)-N-(1-ylpiperazine -4-yl)benzamide
[0153]
[0154] 1: 4-Amino-N-(1-methylpiperidin-4-yl)benzamide
[0155] 1-Methylpiperidin-4-amine (228mg, 2mmol), p-nitrobenzoic acid (335mg, 2mmol) and diisopropylethylamine (1.04mL, 6mmol) were dissolved in N,N-dimethyl In acetamide (10 mL), HATU (1.14 g, 3.0 mml) was dissolved in N,N-dimethylacetamide (10 mL), added to the above reaction solution, and reacted at room temperature for 3 hours. Evaporate N,N-dimethylacetamide under reduced pressure, add saturated aqueous sodium bicarbonate solution (100mL), remove insoluble matter by filtration, neutralize the aqueous phase with 2N hydrochloric acid and extract with dichloromethane, and use saturated brine for the organic phase After washing, drying over anhydrous sodium sulfate, and purification by normal-pressure column chromatography, 500 mg (95.0%) o...
Embodiment 3
[0170] Embodiment 3, cell growth inhibition experiment
[0171] 1. Materials and methods
[0172] Cell growth medium: RPMI1640 (Gibco, 22400089) plus 10% FBS (Gibco, 10099141)
[0173] Detection kit: CCK-8 detection reagent (DojinDo, CK04-20)
[0174] Cell lines: A431 (EGFR WT, Shanghai Cell Bank, Chinese Academy of Sciences), NCI-H1975 (EGFR L858R / T790M, Shanghai Cell Bank, Chinese Academy of Sciences), HCC827 (EGFR Del E746_A750,
[0175] Korea Hanmi Pharmaceutical), Hs-27 (human skin fibroblasts, Korea Hanmi Pharmaceutical)
[0176] TECANIF200 microplate reader
[0177] Invitrogen Countess Cell Counter (C10227)
[0178] 2. Cell Seeding
[0179]Cells in logarithmic growth phase were digested with 0.25% trypsin. A single cell suspension was prepared with RPMI1640 medium containing 10% FBS. Invitrogen Countess for cell counting. A431, NCI-H1975, HCC827 and Hs-27 were seeded in 96-well plates (experimental plate and control plate) at 5000 cells / well / 100uL. Add 100uL me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com