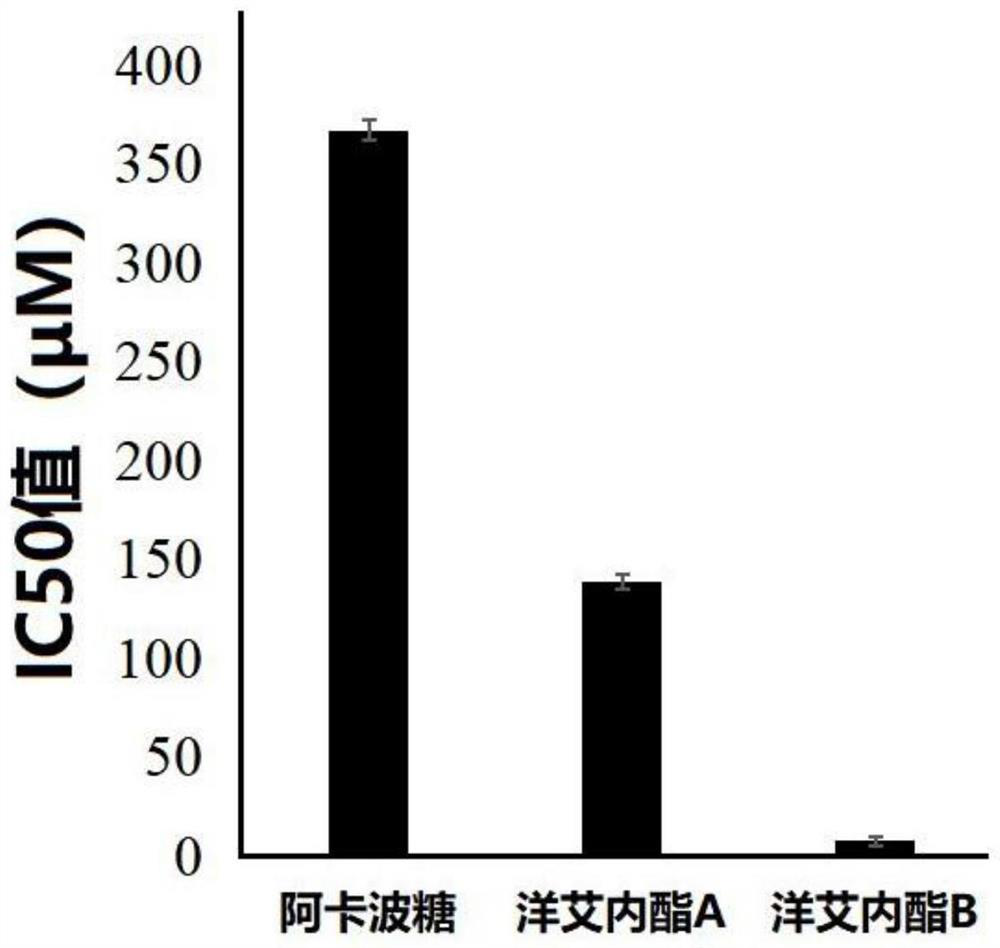
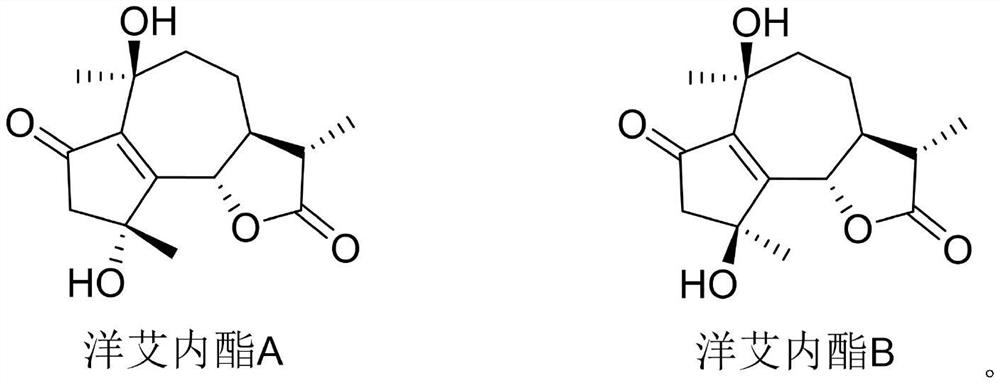
The medicinal use of enigninactone a and b as an α-glucosidase inhibitor and then for the preparation of hypoglycemic drugs
A kind of technology of engeninolactone and hypoglycemia, which is applied in the field of medicine to achieve the effect of high inhibition intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Test materials
[0019] α-glucosidase (yeast) was purchased from Shanghai Jingke Chemical Technology Co., Ltd.
[0020] p-nitrobenzene-β-D-galactopyranoside and DMSO were analytically pure.
[0021] The purity of acarbose, enininactone A and enininactone B is not less than 98%.
[0022] The brand of 96-well plate is Corning.
[0023] 2. Test method
[0024] According to the method disclosed in the literature (Study on lanolin-type triterpenoids in Chizhi and its α-glucosidase inhibitory activity, Chinese herbal medicine, 2017):
[0025] Solution preparation: take α-glucosidase to prepare 0.2U / mL enzyme solution; take p-nitrophenyl-β-D-galactopyranoside (PNPG) to prepare 2.5mM PNPG solution; prepare pH6 according to conventional formula .8 potassium phosphate buffer solution.
[0026] The inhibition test was carried out in a 96-well plate, and the 140 μL potassium phosphate buffer solution (pH6.8) reaction system in each well contained 20 μL of 0.2 U / mL α-glucosi...
Embodiment 2
[0033] A hypoglycemic tablet, the formula of which comprises 10 mg of cyanolactone A or 10 mg of cyanolactone B, 90 g of starch, and 3 g of magnesium stearate.
[0034] Preparation process: Take enigninactone A or enigninactone B, add starch and magnesium stearate, mix evenly, granulate, dry, and compress into tablets.
Embodiment 3
[0036] A hypoglycemic capsule, the formula of which comprises 10 mg of enigninactone A or enigninactone B, 90 g of starch and 3 g of magnesium stearate.
[0037] Preparation process: Take enigninactone A or enigninactone B, add starch and magnesium stearate, mix evenly, granulate, dry, and pack into capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com