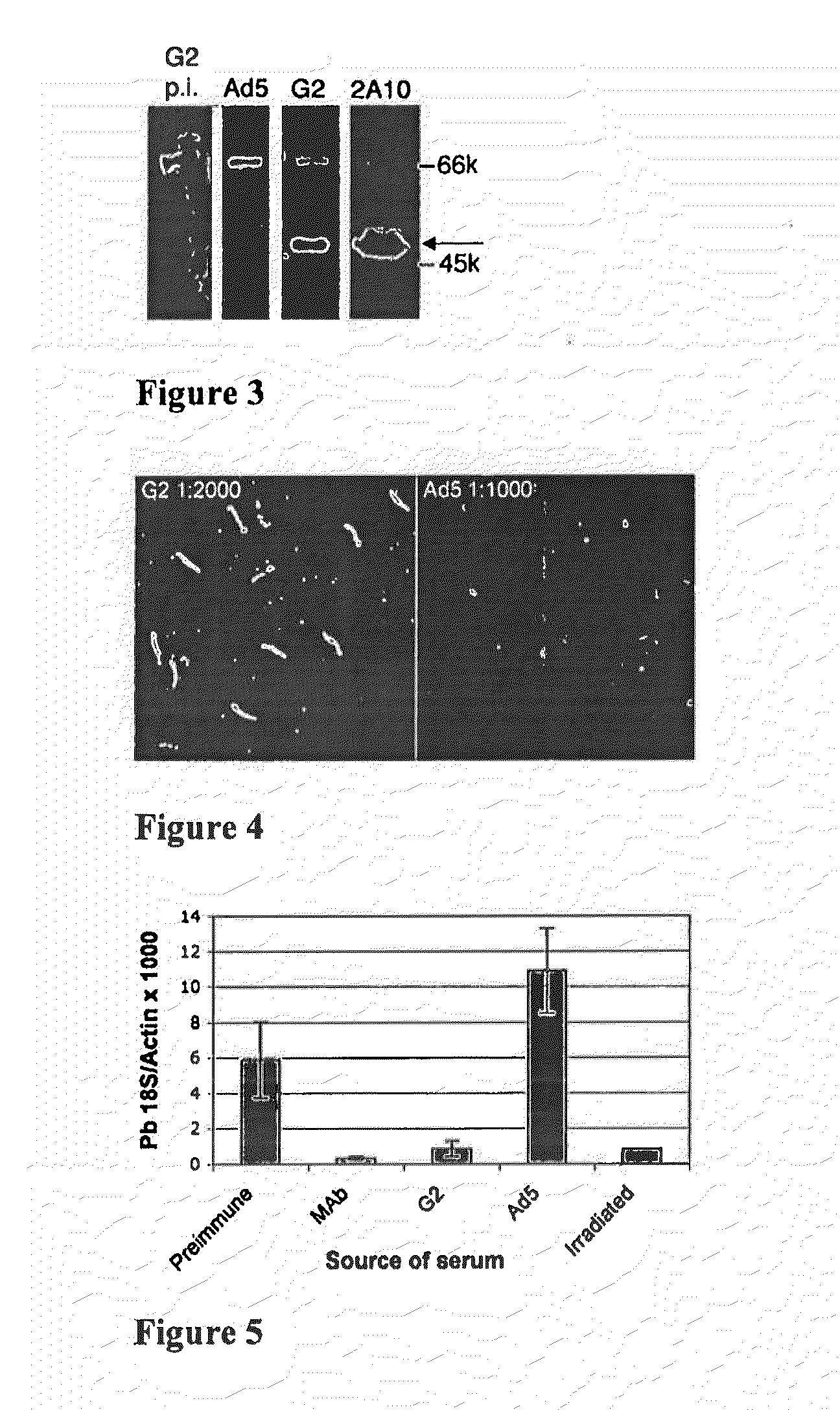
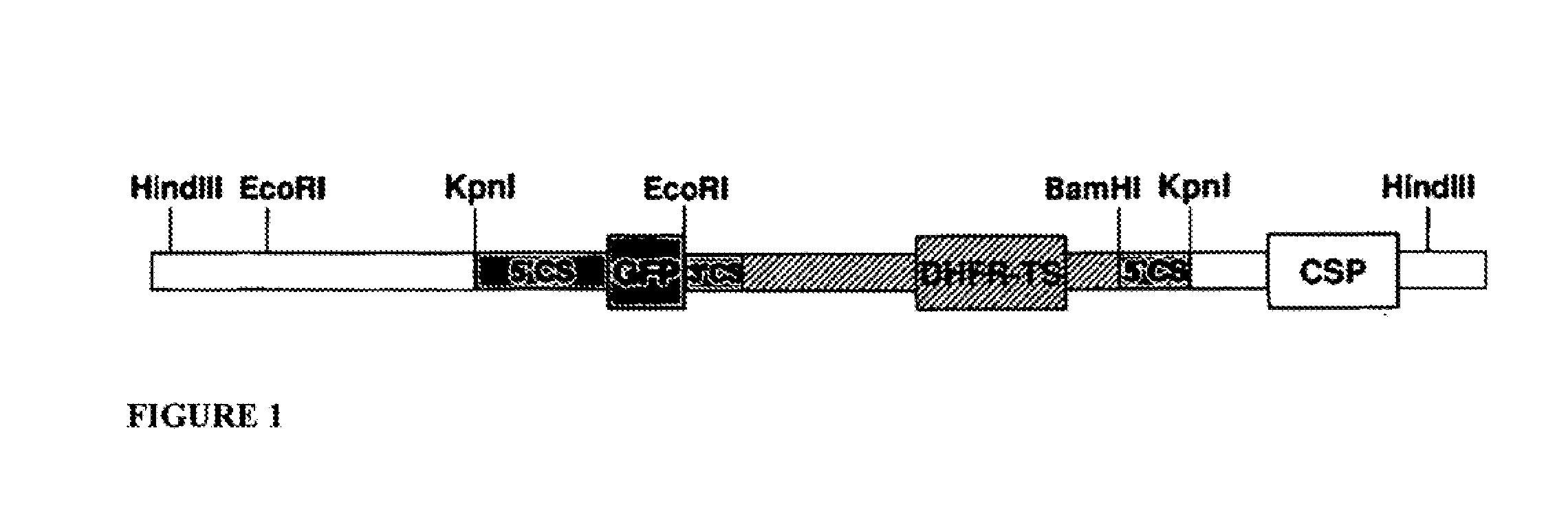
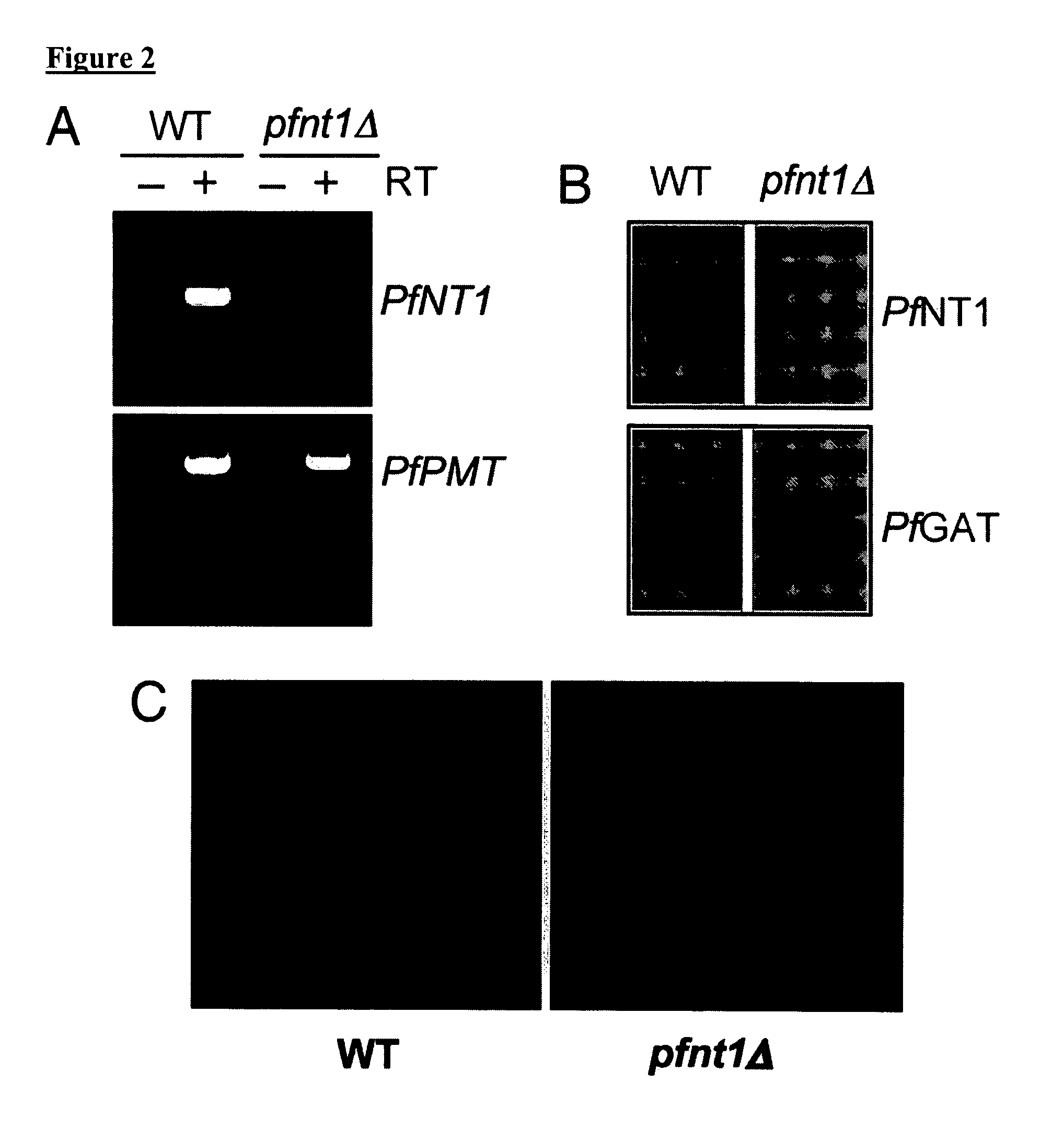
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69 results about "Malaria vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
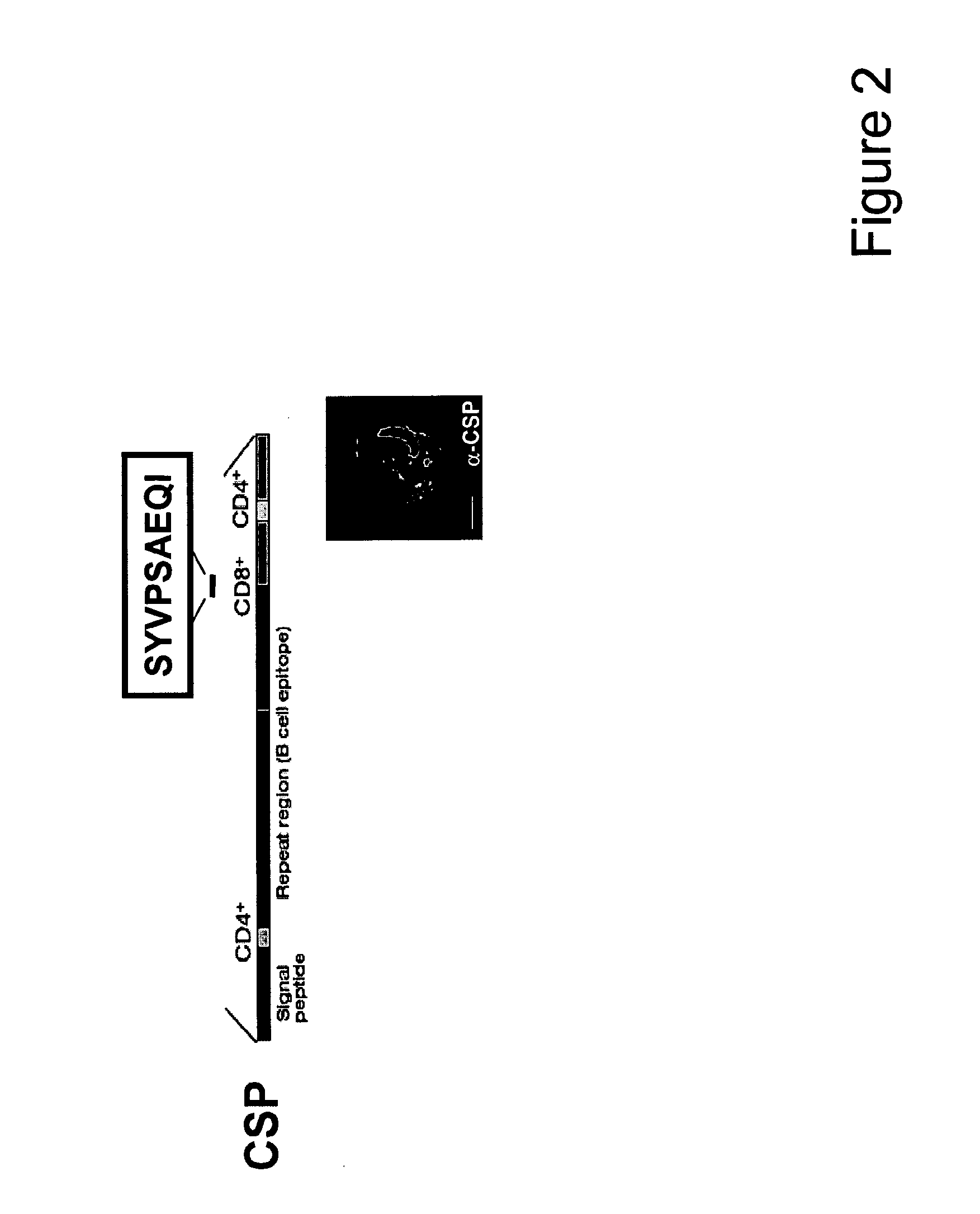
Malaria vaccine is a vaccine that is used to prevent malaria. The only approved vaccine as of 2015 is RTS,S, known by the trade name Mosquirix. It requires four injections, and has a relatively low efficacy. Due to this low efficacy, the World Health Organization (WHO) does not recommend the routine use of the RTS,S vaccine in babies between 6 and 12 weeks of age.
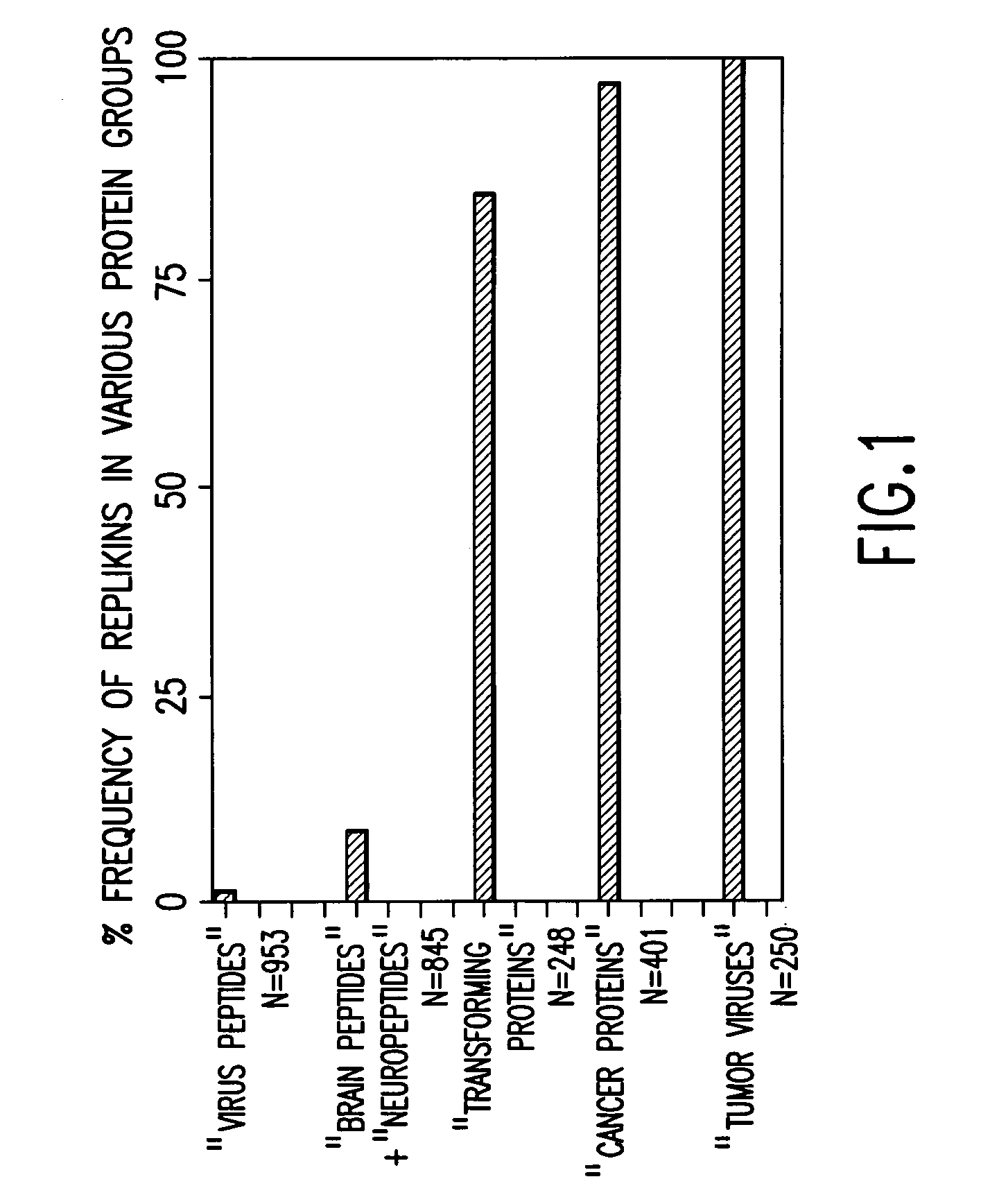
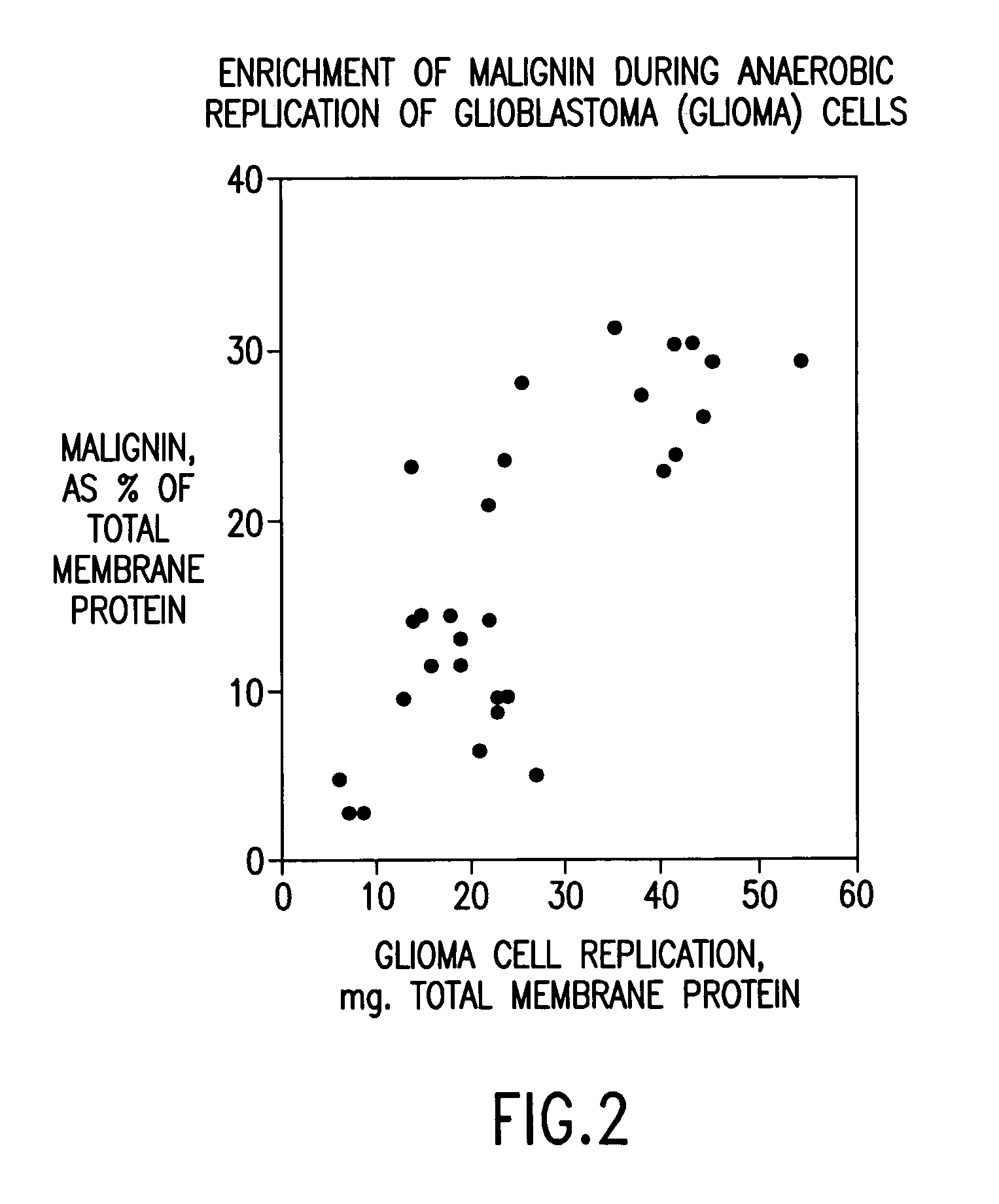
Replikin peptides in rapid replication of glioma cells and in influenza epidemics
Peptides of influenza virus hemagglutinin protein and Plasmodium falciparum malaria antigen, antibodies specific for the peptides, influenza vaccines, malaria vaccines and methods of stimulating the immune response of a subject to produce antibodies to influenza virus or malaria are disclosed. Also disclosed are methods for formulating vaccines for influenza virus.
Owner:BOGOCH SAMUEL +1
Adenoviral vector-based malaria vaccines
The invention provides adenoviral vectors comprising an adenoviral genome comprising heterologous antigen-encoding nucleic acid sequences, such as Plasmodium nucleic acid sequences, operably linked to promoters. The invention further provides a method of inducing an immune response against malaria in a mammal comprising administering the adenoviral vectors to the mammal.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Methods for vaccinating against malaria
ActiveUS20060188527A1Reduce chanceReduce severityBiocideGenetic material ingredientsVaccinationA-DNA
The invention pertains to methods for protecting against malaria infection by vaccination. The method of the invention involves priming an anti-malaria immune response with a DNA-based vaccine and boosting that response with a protein-based a vaccine. The method of the invention also relates to broadening the resulting immune response by boosting with a protein-based vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Recombinant viral-based malaria vaccines
Owner:JANSSEN VACCINES & PREVENTION BV
Plasmodium axenic liver stages as a noninfectious whole organism malaria vaccine
The present invention relates to the treatment and prevention of malaria infection. In particular, the present invention provides novel noninfectious, whole organism vaccines for malaria, which vaccines comprise a Plasmodium axenic liver stage. The invention also provides methods to treat and prevent malaria by administering such Plasmodium axenic liver stage vaccines, as well as methods to generate Plasmodium axenic liver stages.
Owner:NYU MEDICAL CENT
Malaria Vaccines
InactiveUS20120328645A1ConfidenceEfficient protective immunityProtozoa antigen ingredientsAntiparasitic agentsProtection sexProtective immunity
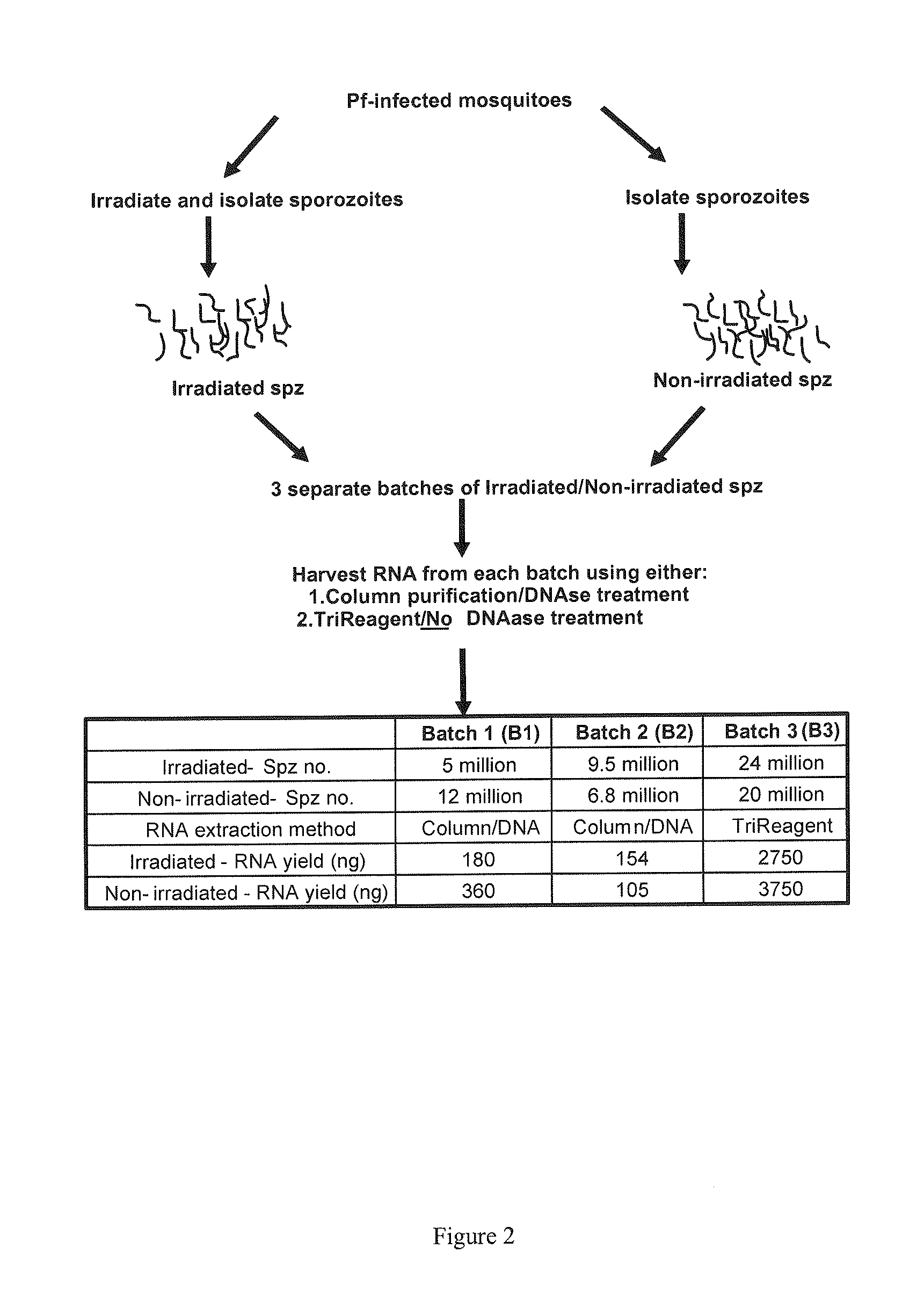
Transcript profiles for irradiated and non-irradiated P. falciparum sporozoites were compared in an attempt to identify transcripts that are reproducibly perturbed by irradiation. Four loci were identified with high confidence. Three of these transcripts were derived from 3 different known gene families, and the fourth from a gene encoding a conserved hypothetical protein of unknown function. All four loci are up-regulated in radiation attenuated sporozoites which have been shown to be very effective in establishing protective immunity against malaria. Up-regulation of these transcripts contributes directly to protective immunity. The polypeptides encoded by the transcripts, individually and / or in combination, are incorporated into subunit vaccines, useful for the prevention of malaria and the establishment of protective immunity against malaria.
Owner:SANARIA INC
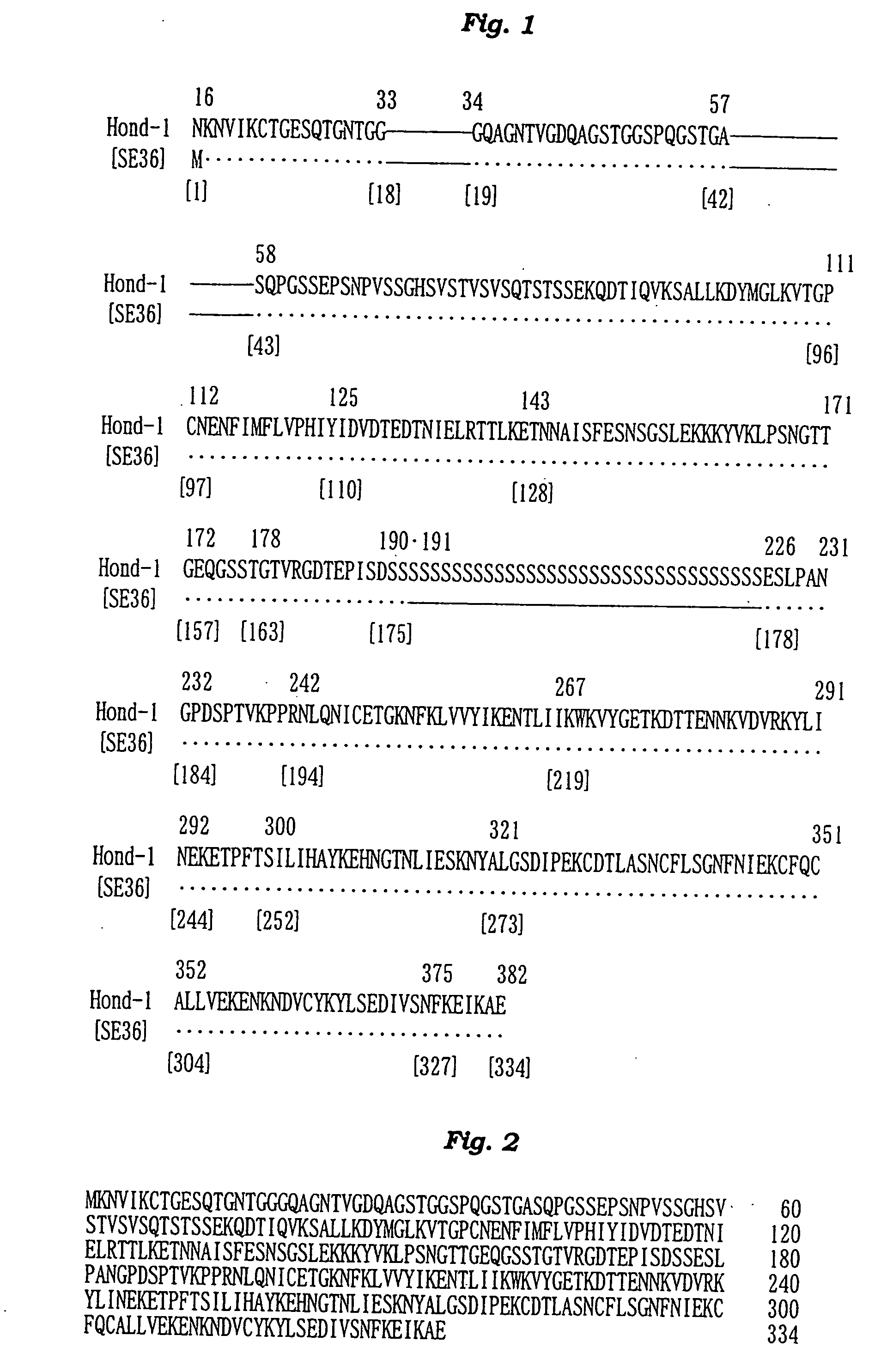
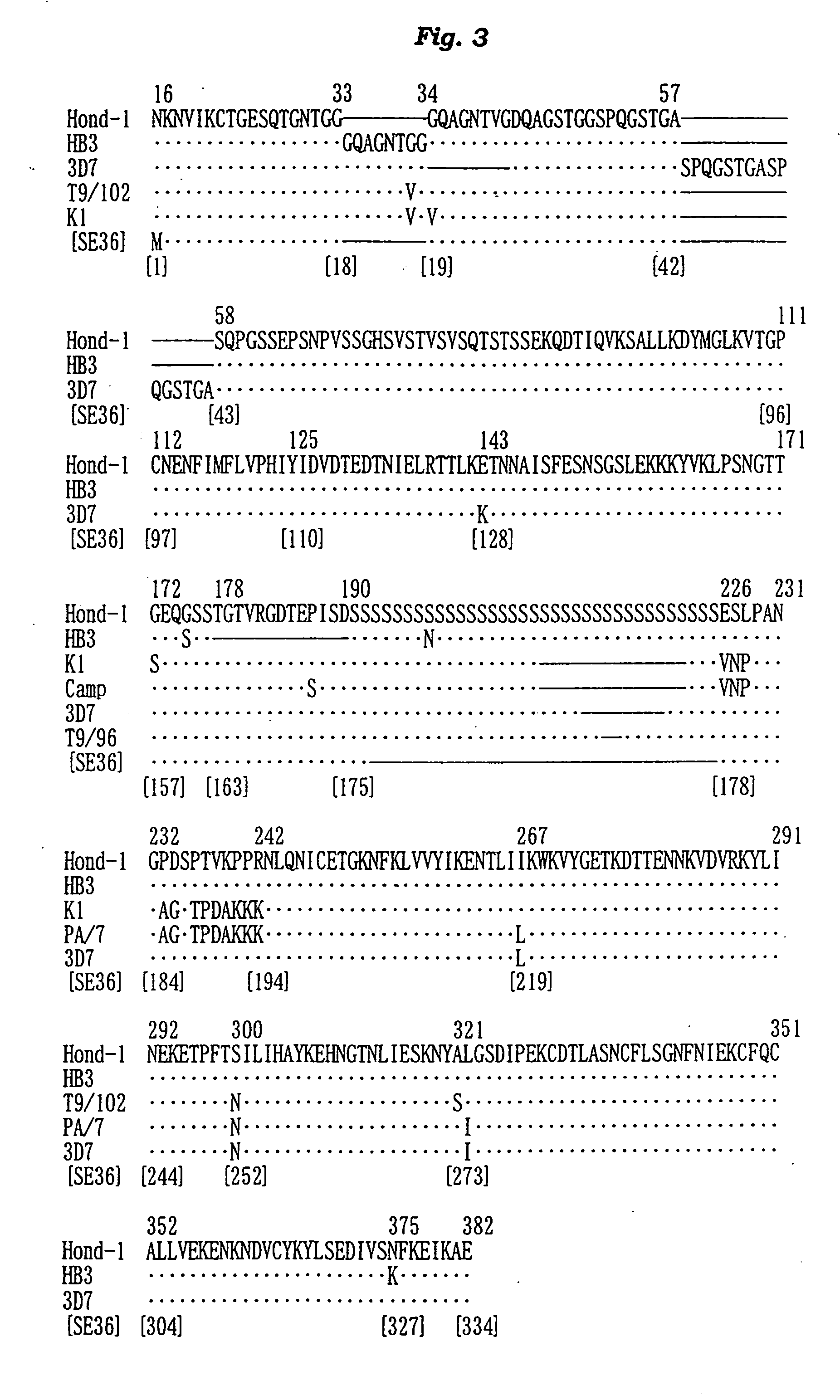
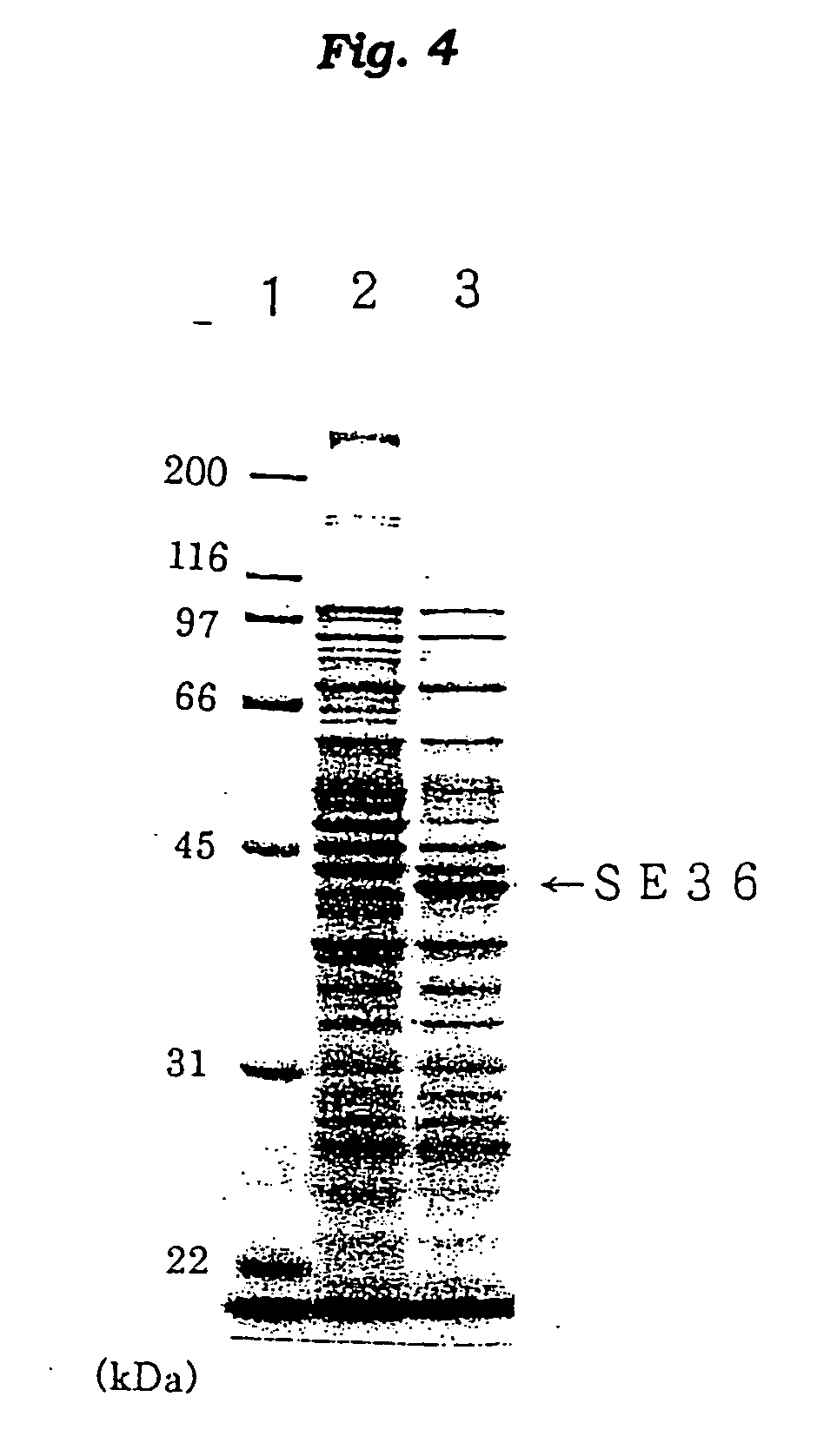
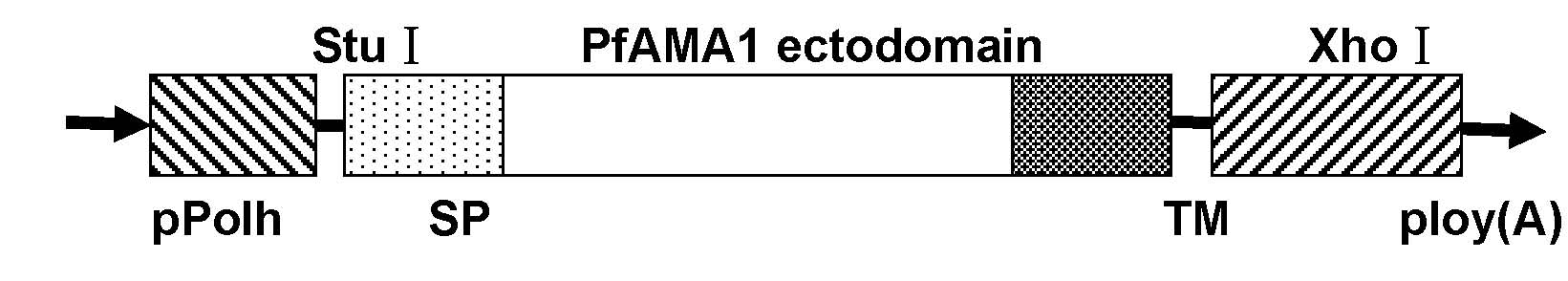
Antigenic polypeptide SE36 of malaria plasmodium, process for purification thereof, and vaccine and diagnostic agent using the antigen
The present invention provides a polypeptide SE36 derived from the N-terminal domain (47 kd) of SERA (serine-repeat antigen) produced by malaria parasite, Plasonodium falciparum, at the erythrocyte stage, a process for purifying said polypeptide, and a malaria vaccine and diagnostic agent using as an active component said purified antigen obtained therefrom. SE36 can be produced in Escherichia coli on a large scale by deleting all or part of polymerized serines of the 47 kd serine-repeat region, whereby high purification is permitted. The human IgG3 antibodies specifically binding to SE36 prevents highly effectively growth of the protozoa in the red blood cells to inhibit fever and cerebral malaria, and further prevent the death.
Owner:VACCINE BIOTECH CO
Malignant malaria vaccine and preparation method thereof
ActiveCN102559613AHigh clinical application valueIncrease productionMicroorganism based processesDepsipeptidesAnti-mitochondrial antibodyAntigen
The invention relates to malignant malaria vaccine and a preparation method thereof, which belong to the technical field of biological medicine. A silkworm recombinant baculovirus is constructed, the surface of the recombinant baculovirus is used for showing technical construction and expressing a major antigen anti-mitochondrial antibody 1 (AMA1) extracellular domain of plasmodium falciparum, silkworm chrysalises are used as bio-reactors to generate the recombinant baculovirus, and the recombinant baculovirus is used as original production vaccine of the malaria vaccine. Compared with the traditional vaccine, the malignant malaria vaccine has the advantages of being good in safety, low in production cost, high in yield, easy to operate and suitable for mass production.
Owner:特菲(天津)生物医药科技有限公司 +1
Recombinant Adenovirus Vaccines
Recombinant adenovirus vaccines comprising recombinant adenoviruses whose hexon, fiber or protein IX capsid proteins are engineered to include exogenous peptide segments, e.g. vaccines for human papillomavirus (HPV) and malaria.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Malaria vaccine of self-assembling polypeptide nanoparticles
The invention is directed to functionalized self-assembling polypeptide nanoparticles, and to methods of using these nanoparticles to vaccinate against malaria. The functionalized SAPN comprises a self-assembling core, and at least one epitope fused to the self-assembling core. The self-assembling core comprises a pentameric coiled-coil domain, a trimeric coiled-coil domain, and a linker. The linker joins the pentameric coiled-coil domain and the trimeric coiled-coil domain. Particular sequences of the epitopes used in the vaccine are from the Plasmodium parasite.
Owner:LANAR DAVID +1
Malaria vaccine and preparation method thereof
InactiveCN102533677AMaintain antigenicityImprove securityMicroorganism based processesDepsipeptidesSurface displayMicrobiology
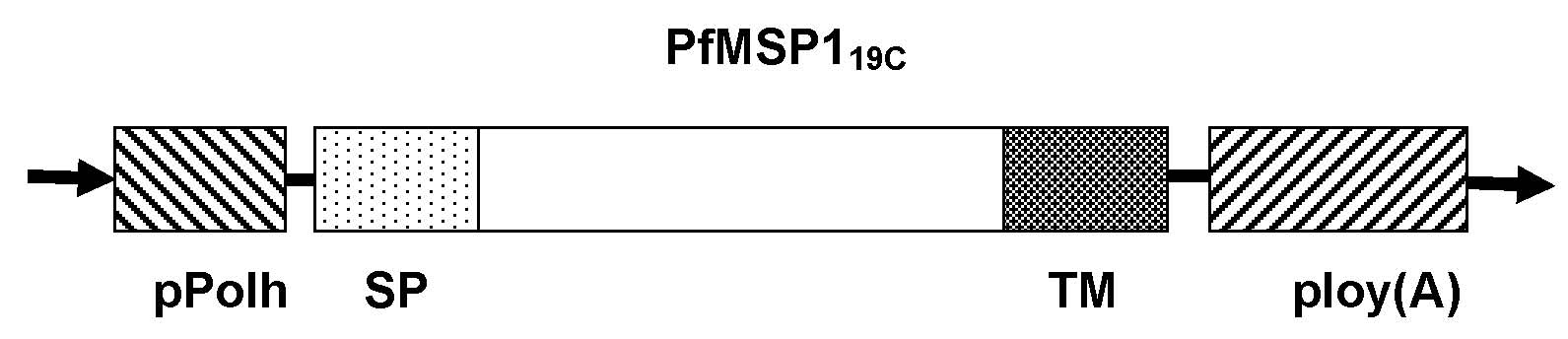
The invention relates to a malaria vaccine and a preparation method thereof and belongs to the technical field of medicaments. A recombinant silkworm baculovirus is constructed, the 19kD segment (MSP119C) at a tail end of a plasmodium falciparum merozoite surface membrane protein 1C is expressed on the surface of the silkworm baculovirus by baculovirus surface display technology, a recombinant baculovirus is generated by using a silkworm pupa as a biological reactor, and the malaria vaccine is prepared from the recombinant baculovirus. Compared with the traditional malaria vaccine production method, the method has the characteristics of high safety, low production cost, high yield, easiness in operation and suitability for mass production.
Owner:特菲(天津)生物医药科技有限公司 +2
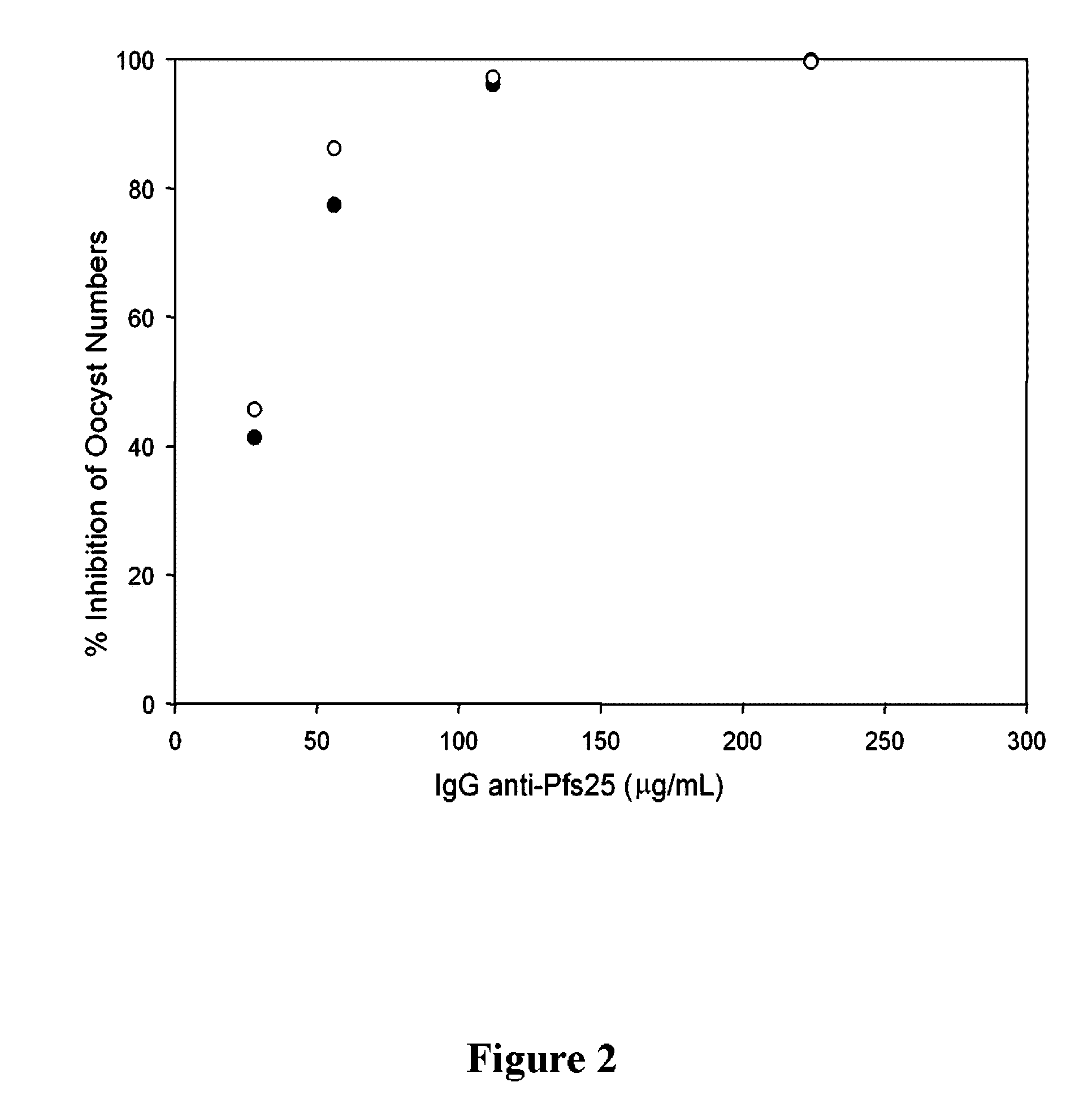
Malaria vaccines based on apicomplexan ferlins, ferlin-like proteins and other c2-domain containing proteins
The present invention relates to peptides comprising at least one antigenic determinant or epitope of an apicomplexan Ferlin, Ferlin-like protein and / or another C2-domain containing protein for use as malaria vaccines. It further relates to compositions comprising said peptides and to the use of such compositions as malaria vaccines.
Owner:MALVA GMBH
Chloroplast-derived human vaccine antigens against malaria
InactiveUS20090297550A1Low costImprove expression levelVaccinesImmunoglobulinsVaccine antigenMalaria
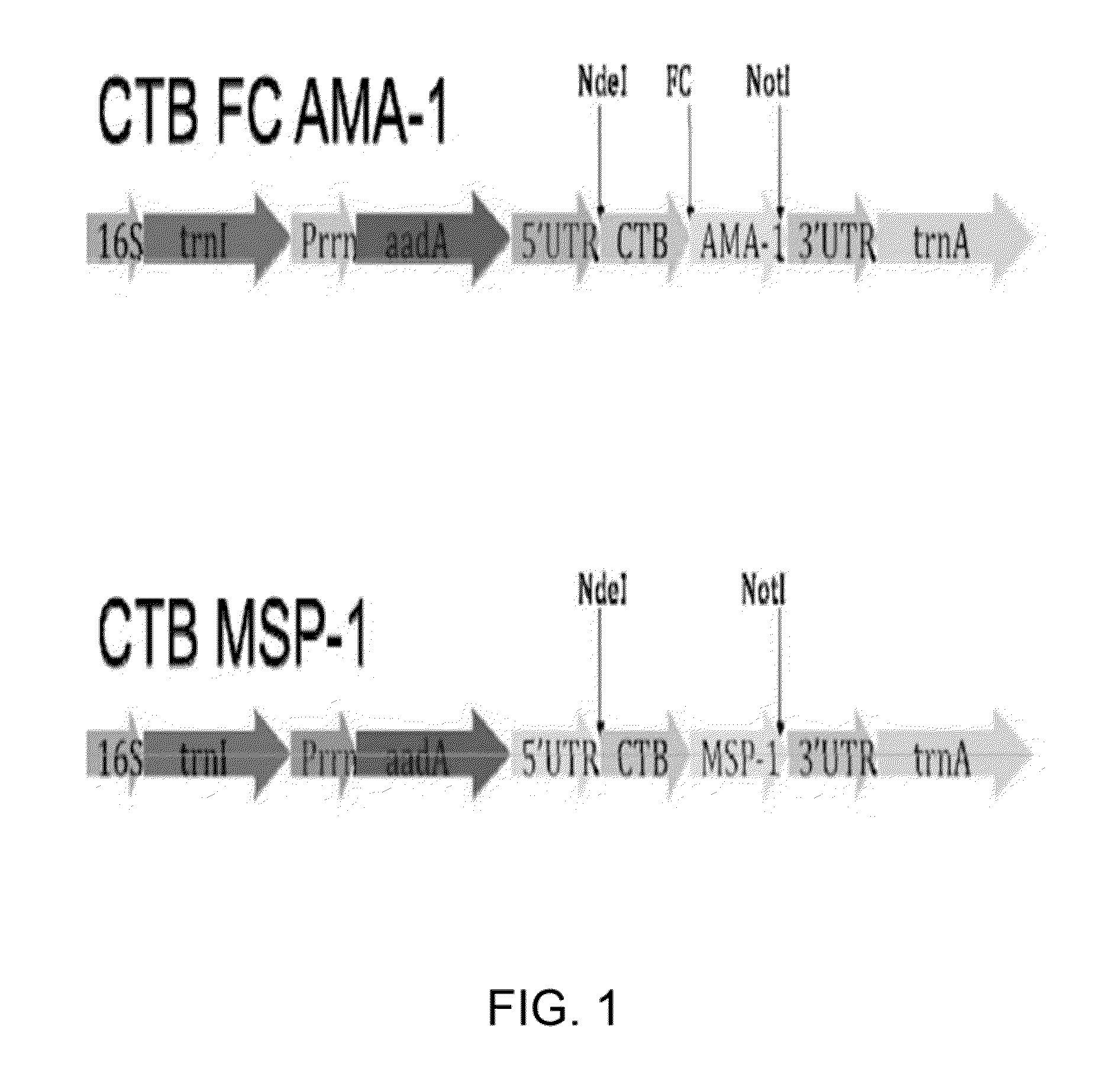
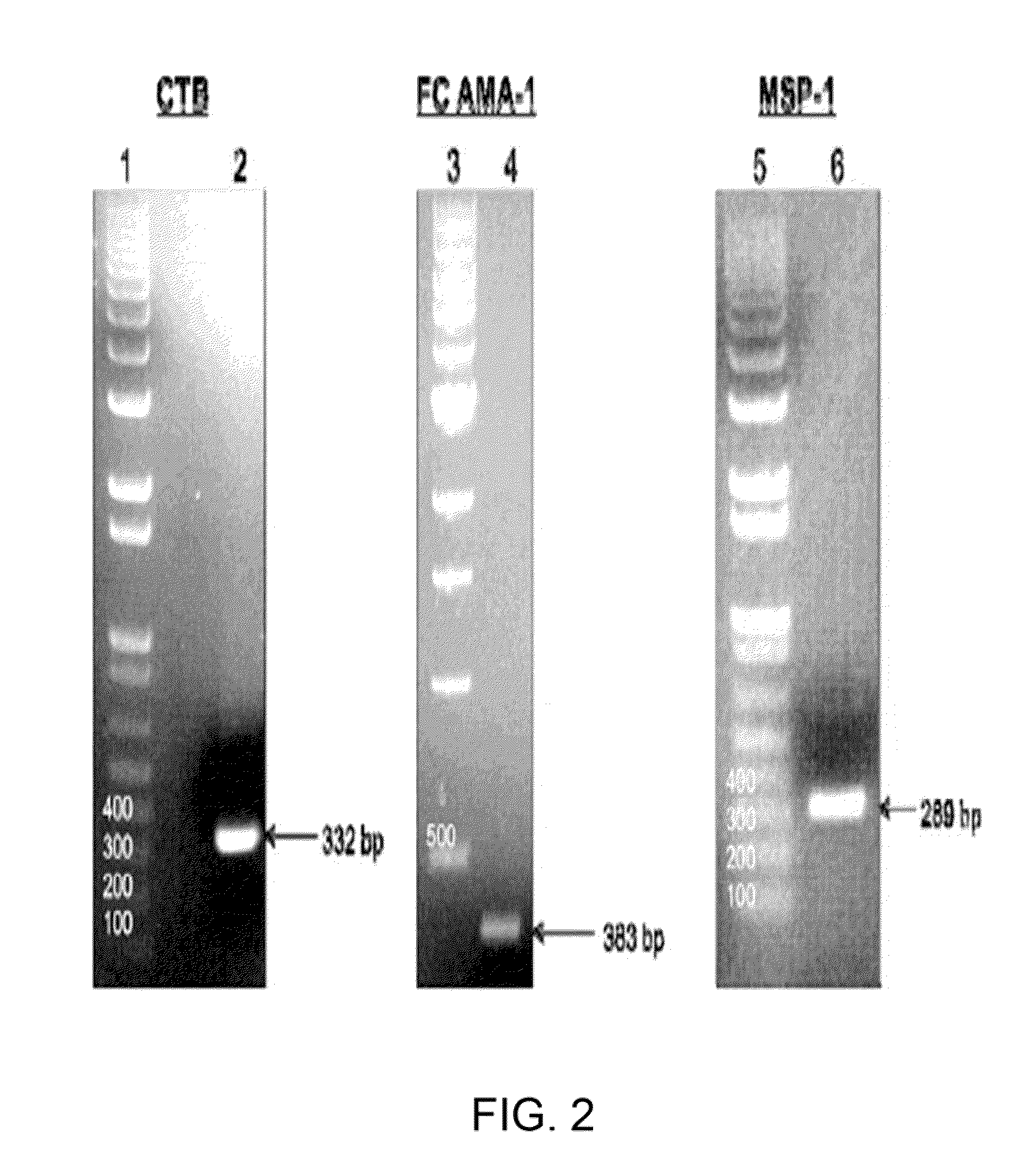
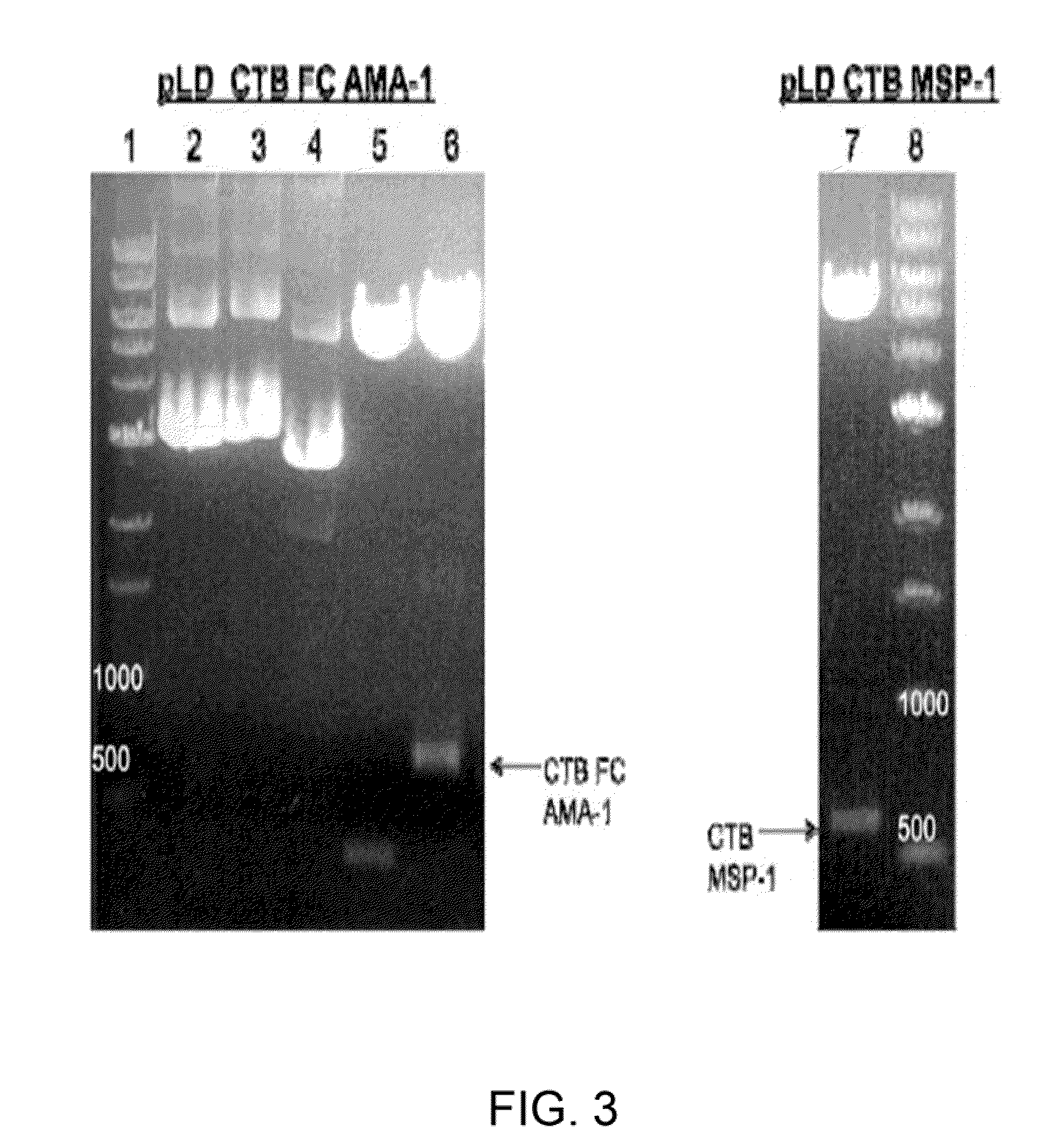
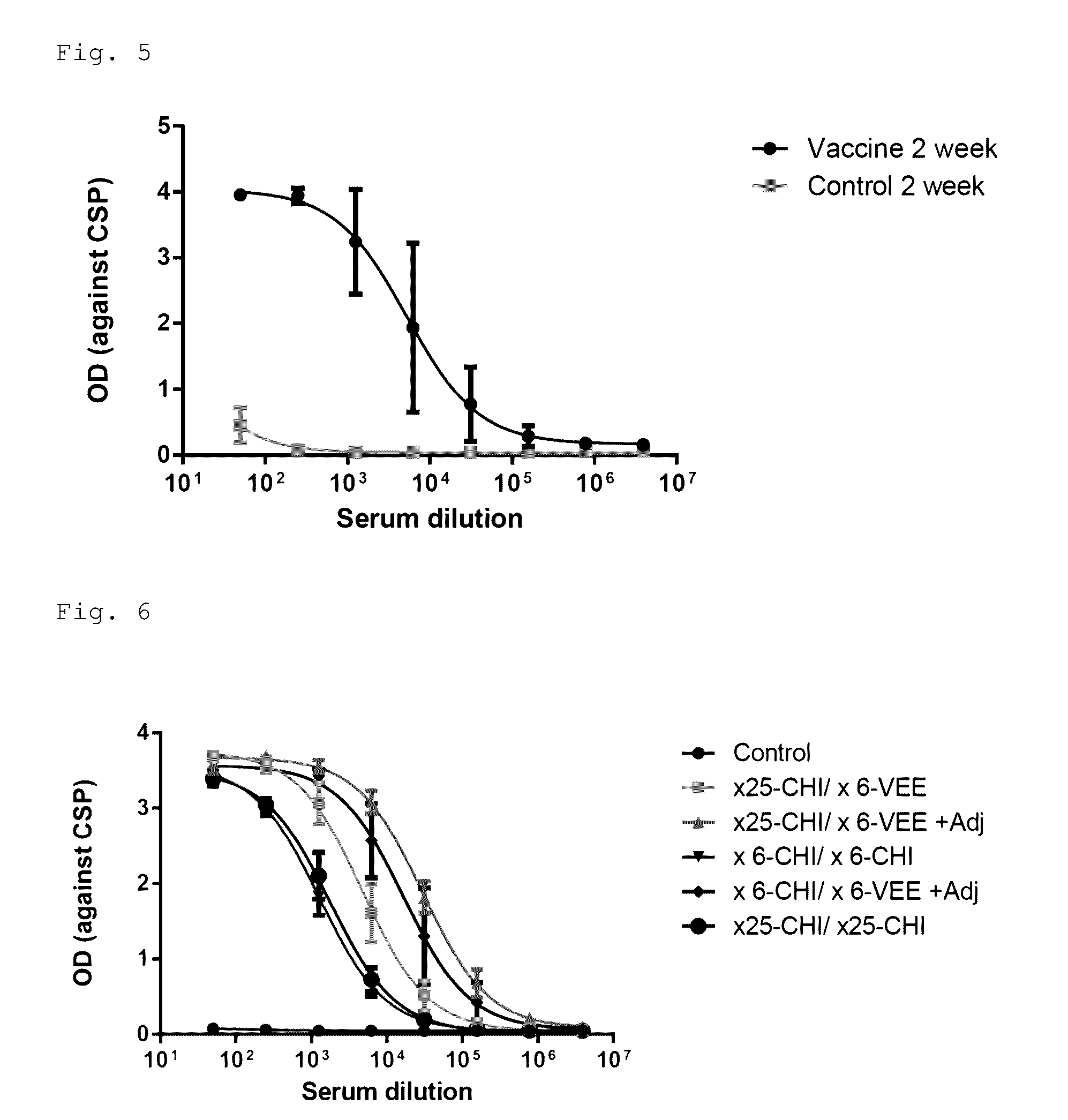
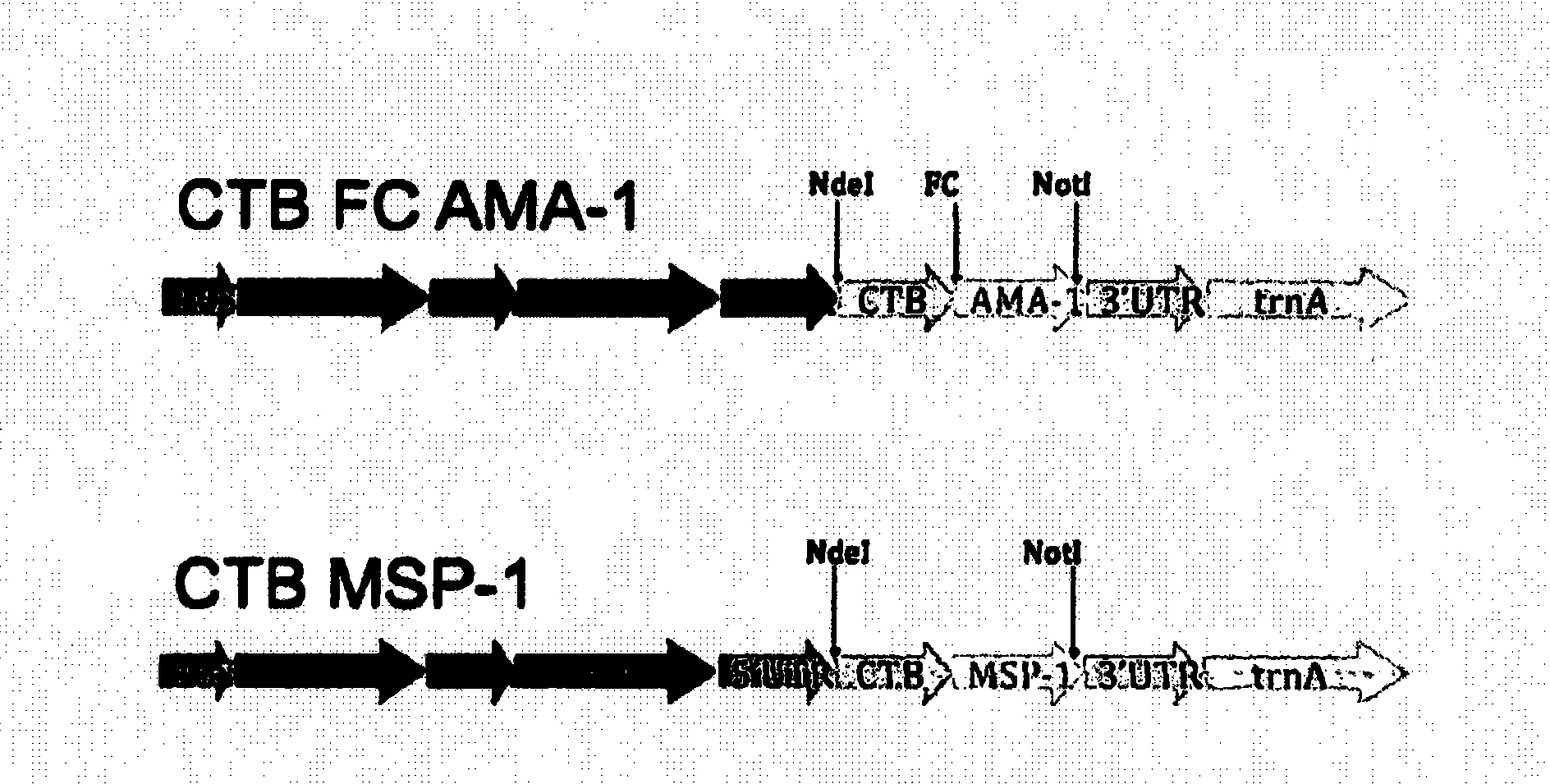
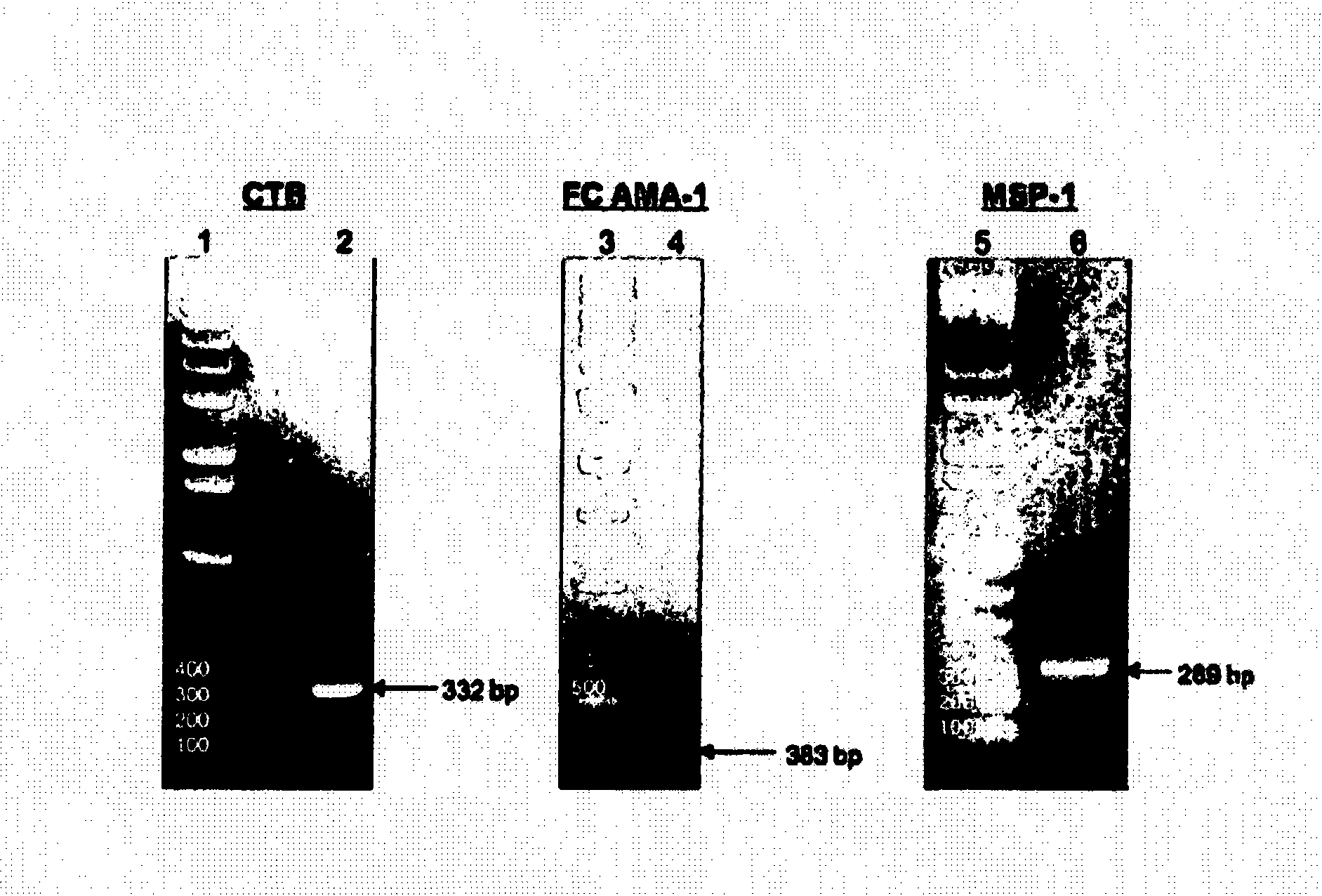
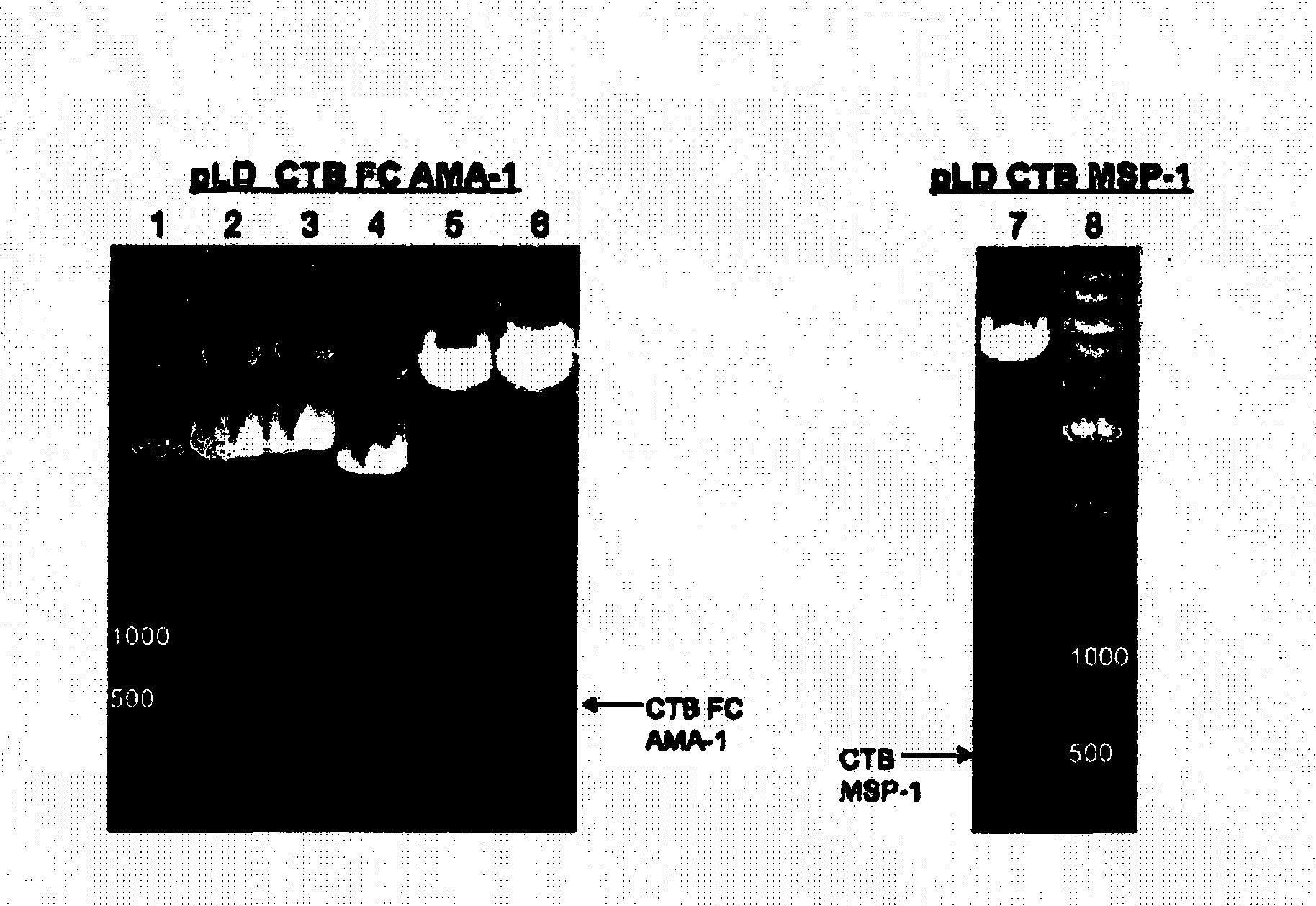
Disclosed is a method of making a malaria vaccine, the method comprising stably transforming a plant by inserting into its plastid genome a nucleic acid sequence encoding and operable to constitutively express a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both; harvesting the stably transformed plant in whole or in part; purifying the expressed malaria antigenic polypeptide from the harvested plant; and packaging the purified antigenic polypeptide under sterile conditions in an amount for a predetermined dosage. Also disclosed is an oral vaccine effective in raising malaria antibodies in a susceptible host, the vaccine comprising leaf material from an edible plant containing plastids stably transformed to constitutively express a fusion polypeptide consisting essentially of cholera toxin B subunit and a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both.
Owner:DANIELL HENRY +1
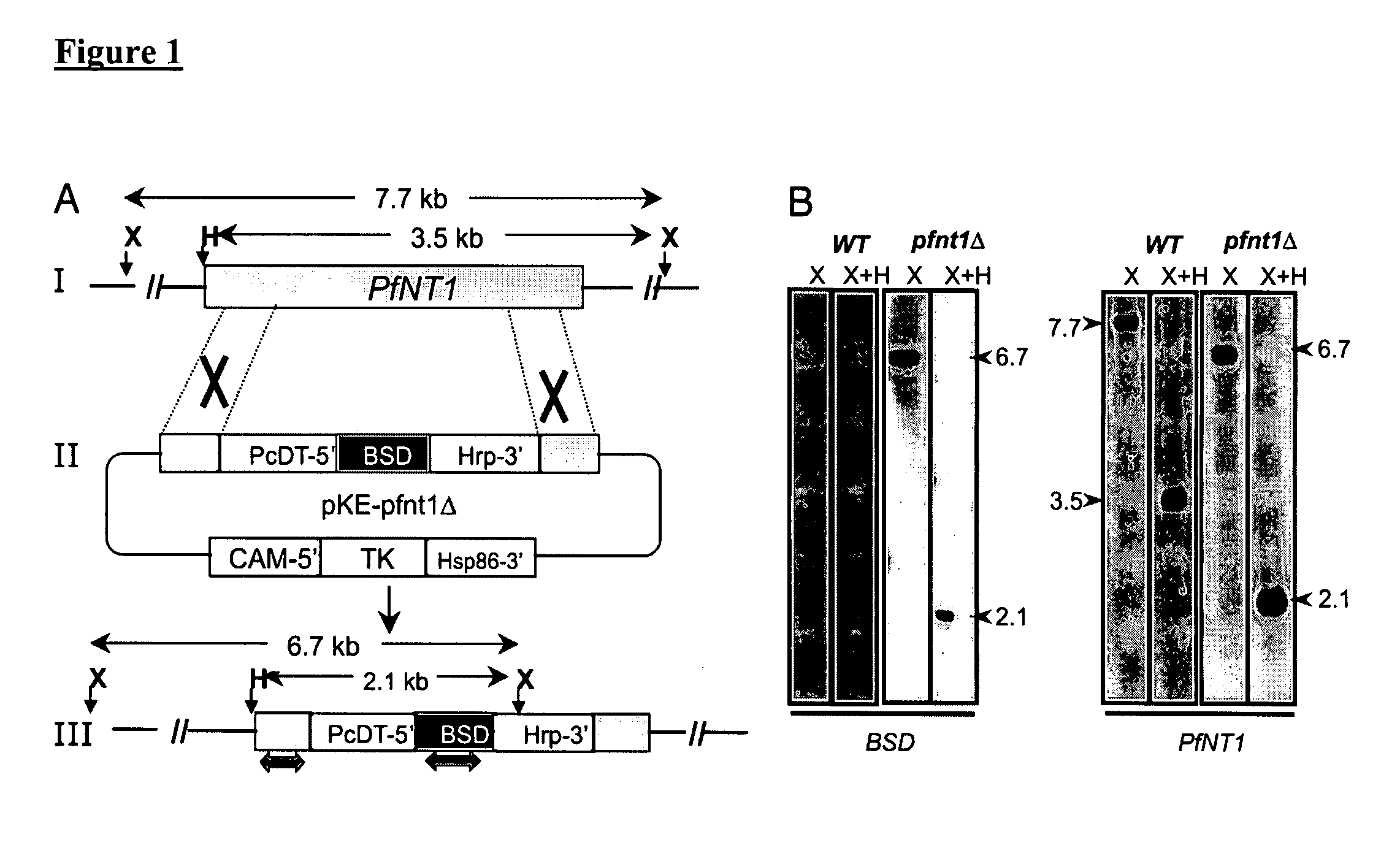
Use of conditional plasmodium strains lacking nutrient transporters in malaria vaccination
InactiveUS20080026010A1Great degree of controlImprove predictabilityBiocideProtozoa antigen ingredientsVaccinationRed blood cell
The present invention relates to a malaria vaccine for administration to a host comprising an attenuated malarial parasite with a gene which has been rendered non-functional, wherein the gene, when present in naturally occurring form, encodes a protein necessary for continued in vivo survival and proliferation of the parasite and for infection of host red blood cells. The invention further relates to a malaria vaccine in which a gene that encodes a nutrient transporter protein has been rendered non-functional.
Owner:UNIV OF CONNECTICUT
Recombinant vector, recombinant baculovirus prepared from the same and application of virus in preparation of malaria vaccines
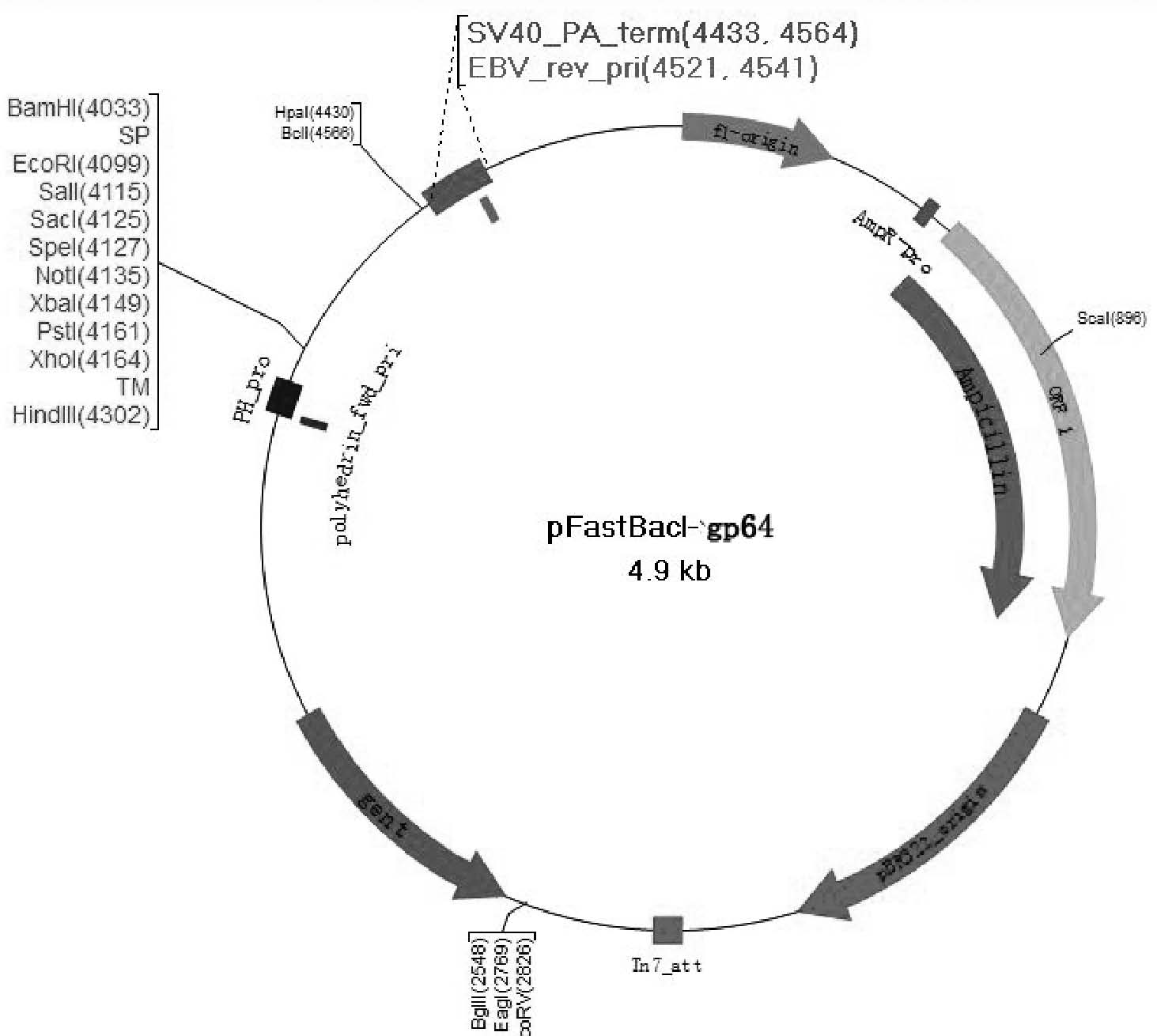
InactiveCN104711288AHigh homologyIncrease productionGenetic material ingredientsAntiparasitic agentsShuttle vectorMalaria
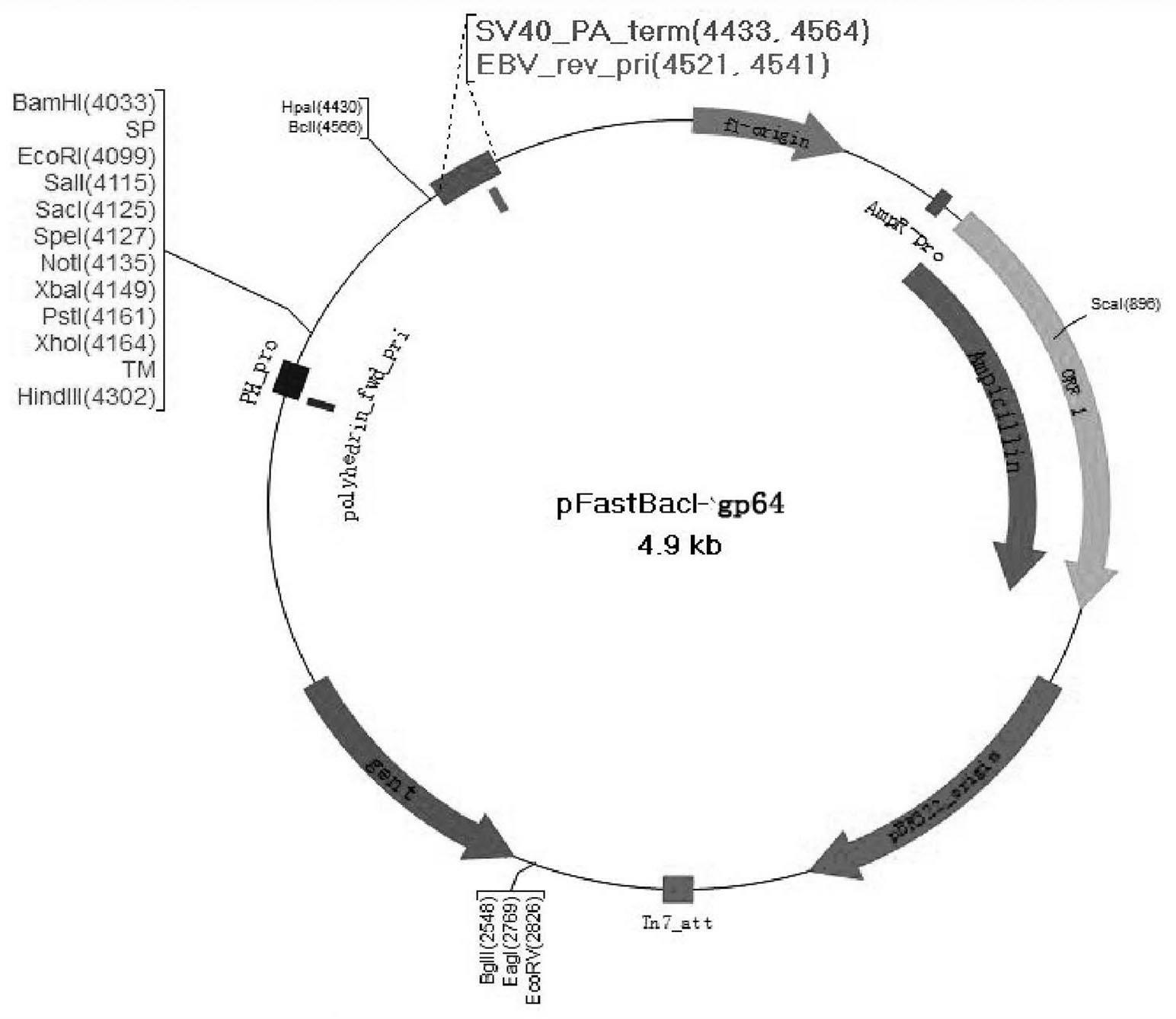
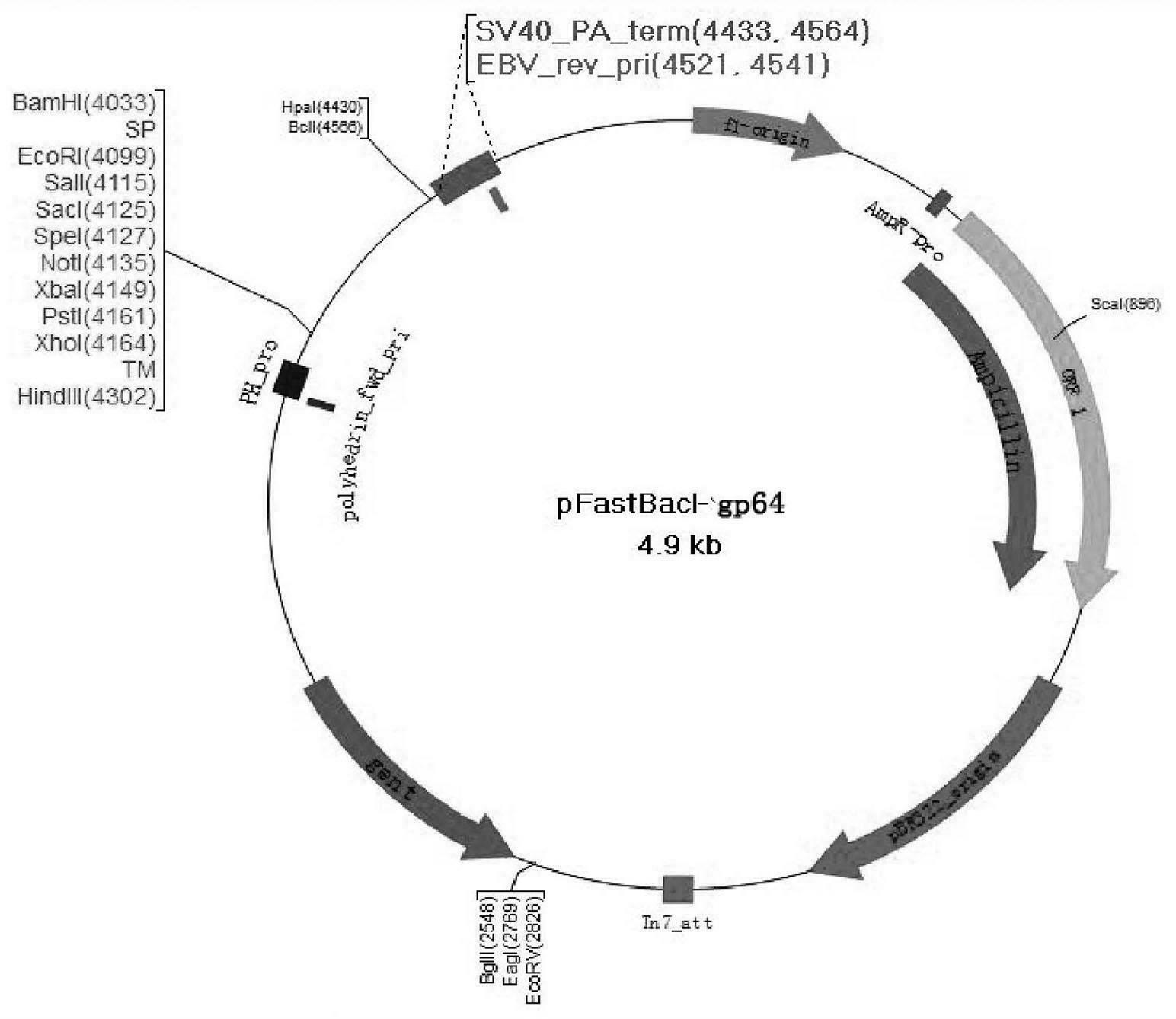
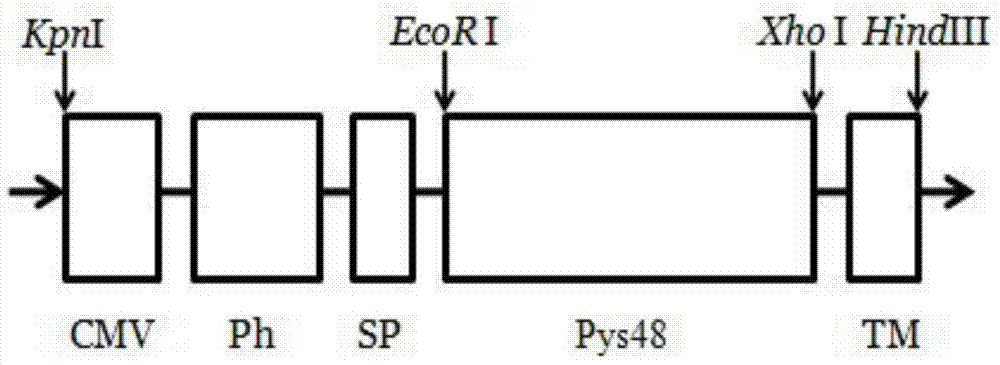
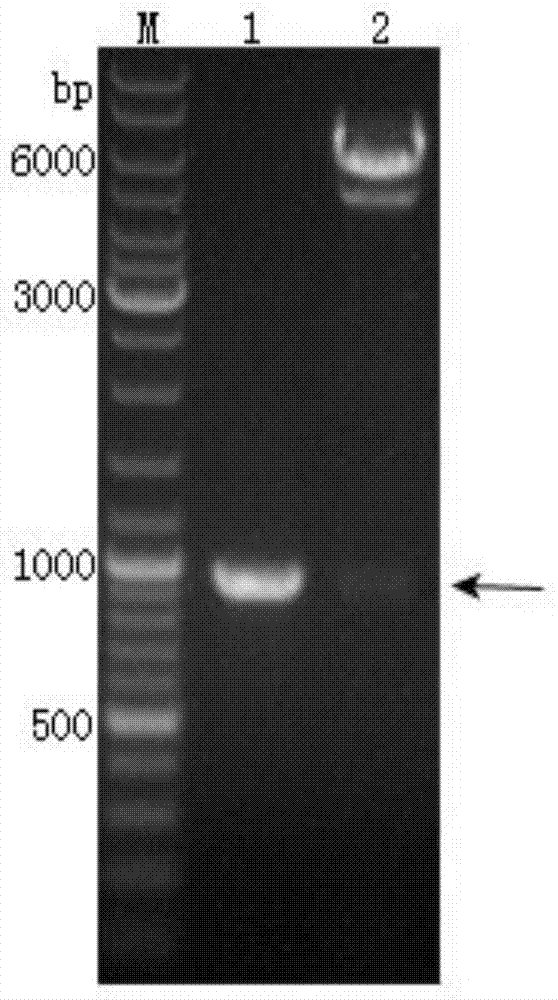
The invention provides a recombinant vector, a recombinant baculovirus prepared by from the vector and application of the virus in preparation of malaria vaccines. The recombinant vector is constructed by inserting a section of recombinant sequence into the pFastBacDual vector. The recombinant sequence is formed by: according to CMV, Ph, SP and TM sequences, adding EcoR I enzyme site and Xho I enzyme site between SP and TM, adding KpnI enzyme site in front of CMV-F, and adding HindIII enzyme site after TM-R. The recombinant baculovirus is obtained by: inserting a Plasmodium yoelii Pys48 antigen gene into the recombinant vector, conducting homologous recombination with the genome of a shuttle vector Bacmid through transposition, then transfecting a bombyx mori cell, and performing packaging in the bombyx mori cell. Through simple separation and purification of the recombinant baculovirus, an antibody with good titer can be prepared, thus providing a reference for study of P48 malaria vaccines.
Owner:特菲(天津)生物医药科技有限公司
Process for producing antigenic substance
InactiveUS20060233789A1Wide rangeHinders its propagationBiocideSnake antigen ingredientsEmbryoMalaria
An object of the present invention is to provide a means for producing an antigenic component with the retained native antigenicity using a cell-free protein synthesis. In particular, it is an object to provide a means for producing an antigenic component without depending on codon usage, like expressing an antigenic component from a gene containing a large amount of AT. The present inventors have made a strenuous study to solve the matters described above and successfully completed the present invention by preparing an antigenic component with the retained antigenicity, in particular a malaria antigen useful for manufacturing a malaria vaccine, through a system with the use of a wheat embryo among cell-free protein synthesis means.
Owner:CELLFREE SCI
Adenoviral vector-based malaria vaccines
The invention provides adenoviral vectors comprising an adenoviral genome comprising heterologous antigen-encoding nucleic acid sequences, such as Plasmodium nucleic acid sequences, operably linked to promoters. The invention further provides a method of inducing an immune response against malaria in a mammal comprising administering the adenoviral vectors to the mammal.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Malaria vaccine
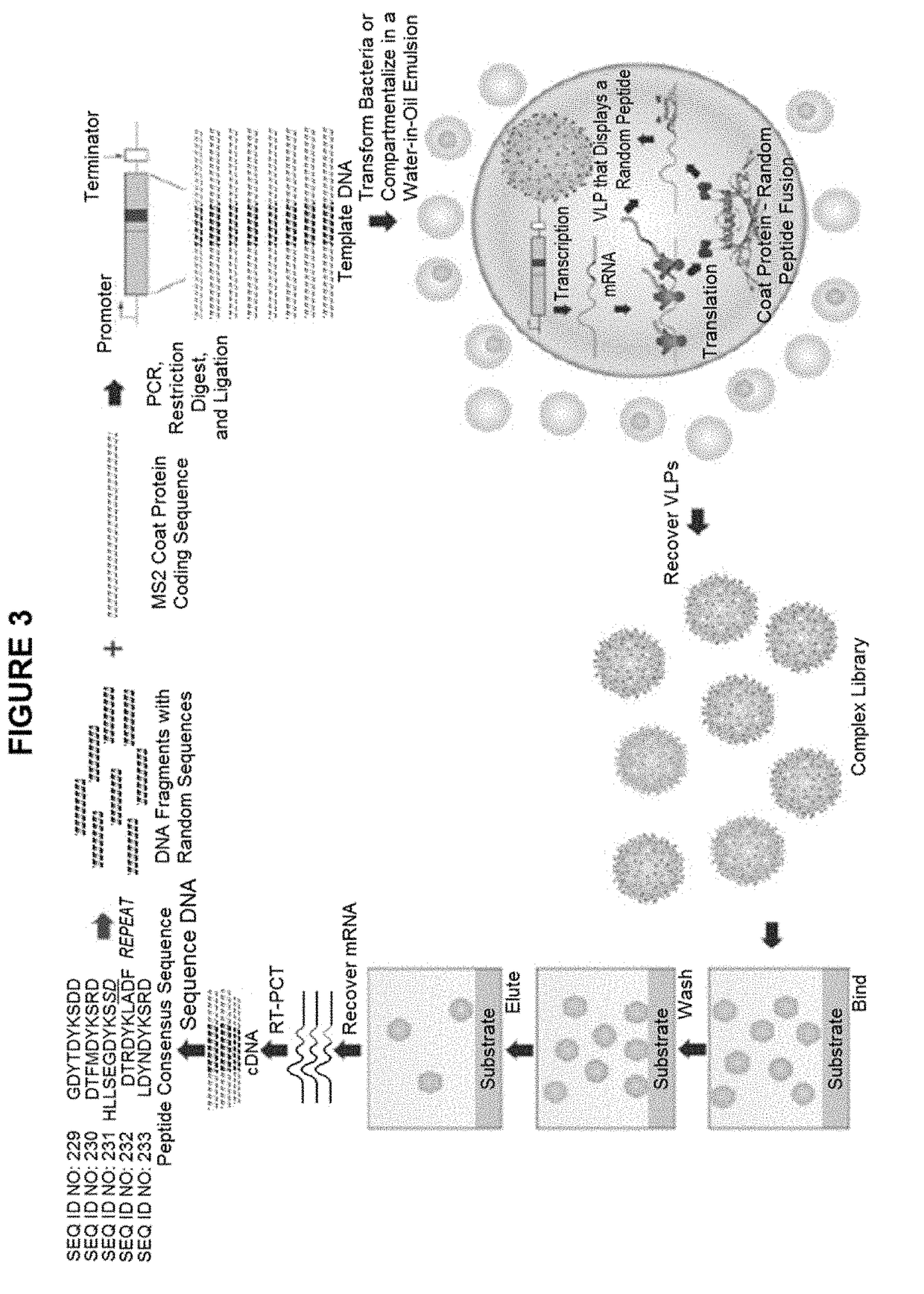
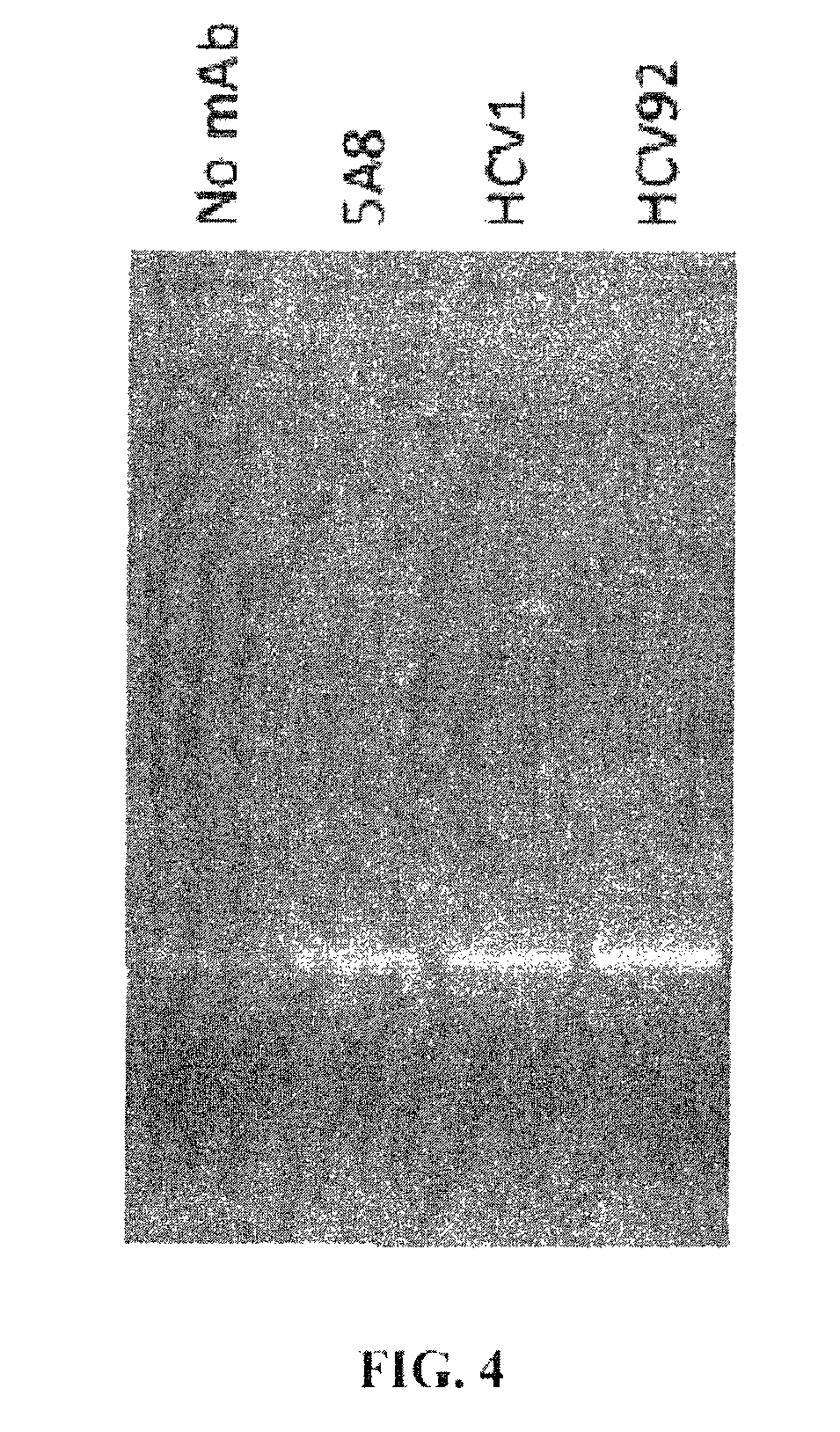
ActiveUS9943580B2Antibody mimetics/scaffoldsViral/bacteriophage medical ingredientsHeterologousBacteriophage
Embodiments are directed to malaria vaccines comprising a bacteriophage VLP displaying a heterologous peptide identified by affinity selection as an anti-malaria mimotope.
Owner:STC UNM
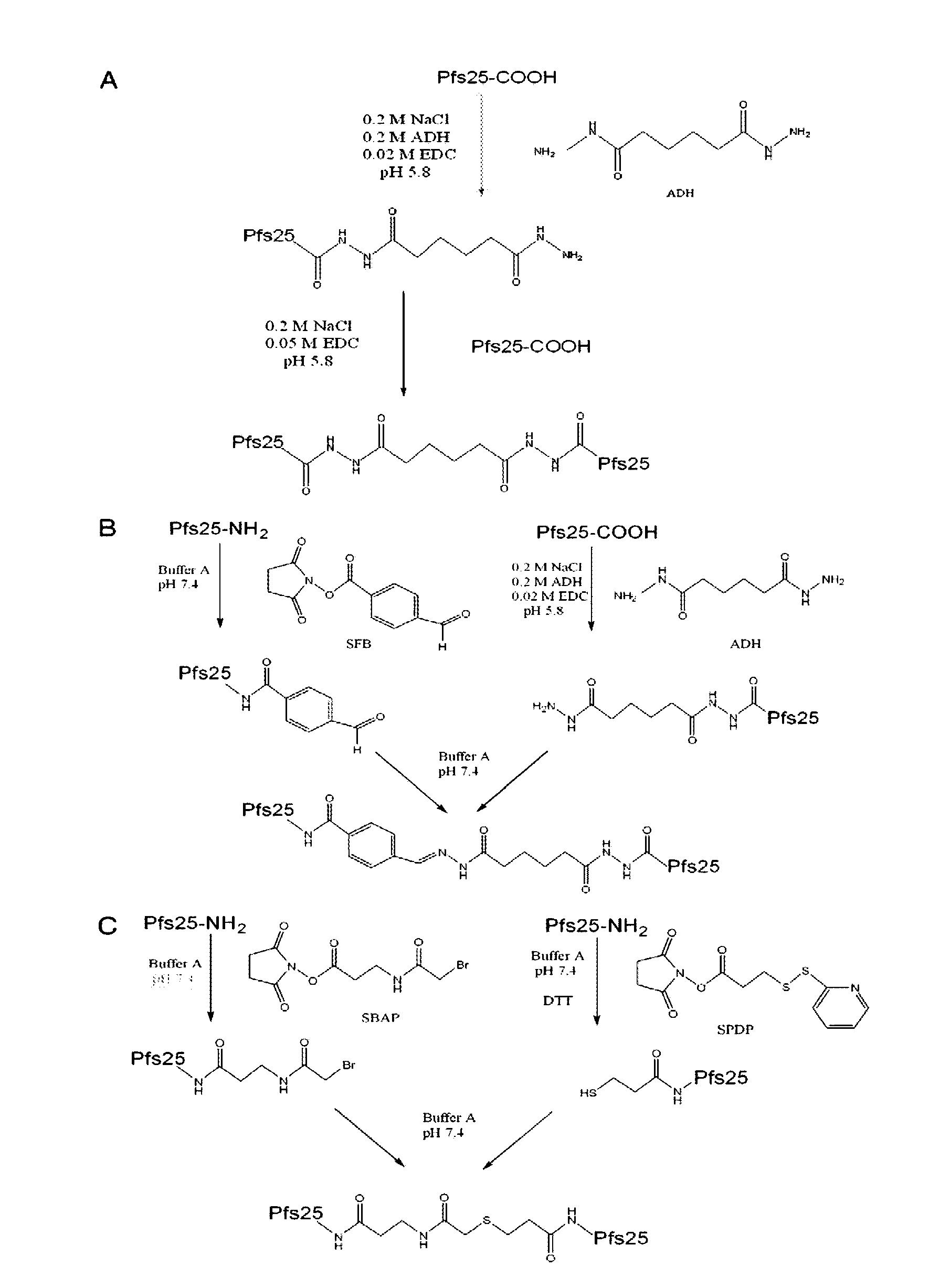
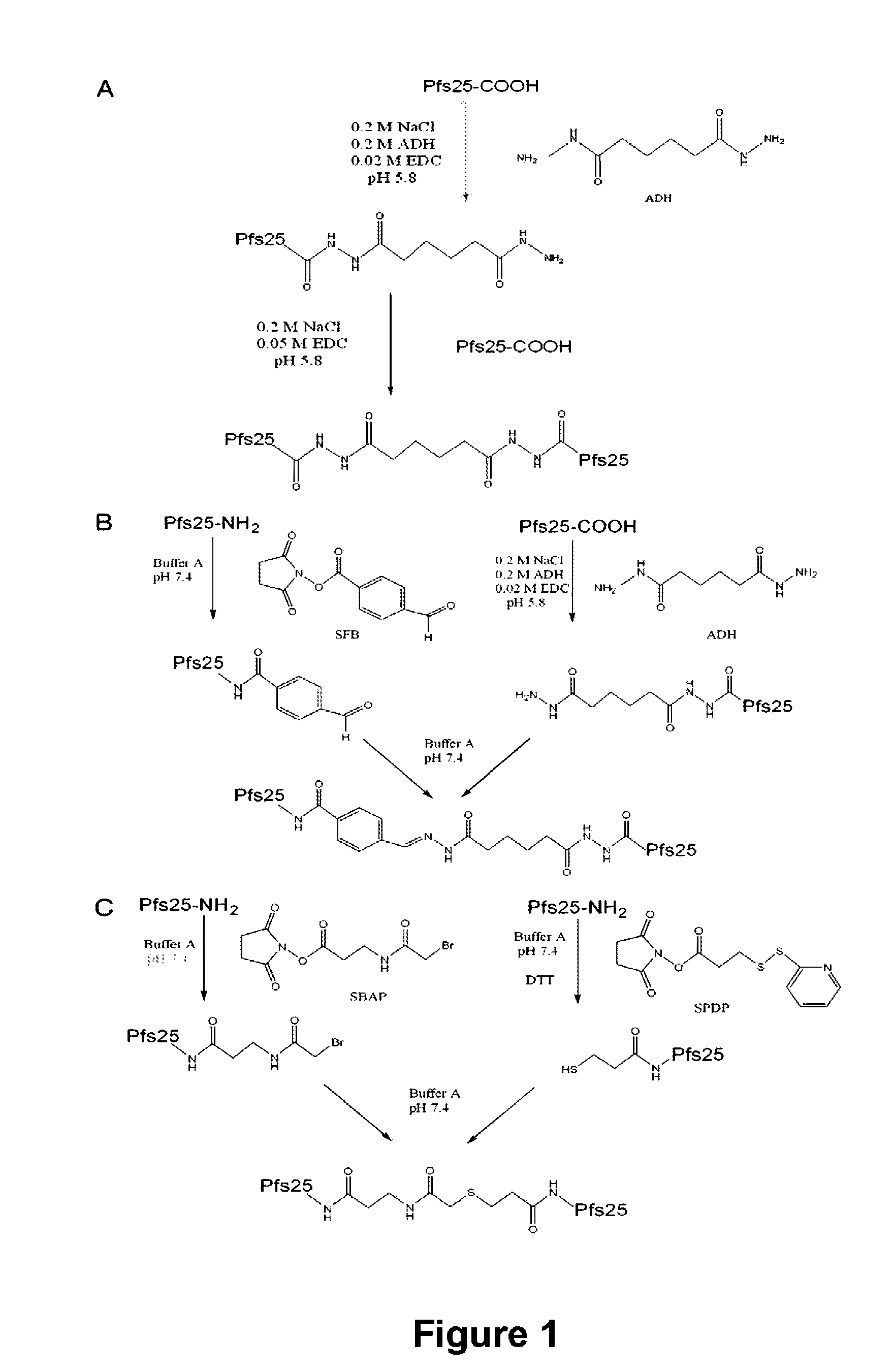
Conjugates of plasmodium falciparum surface proteins as malaria vaccines
InactiveUS20100183678A1Effective vaccineImprove the level ofCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsMedicineMalarial parasites
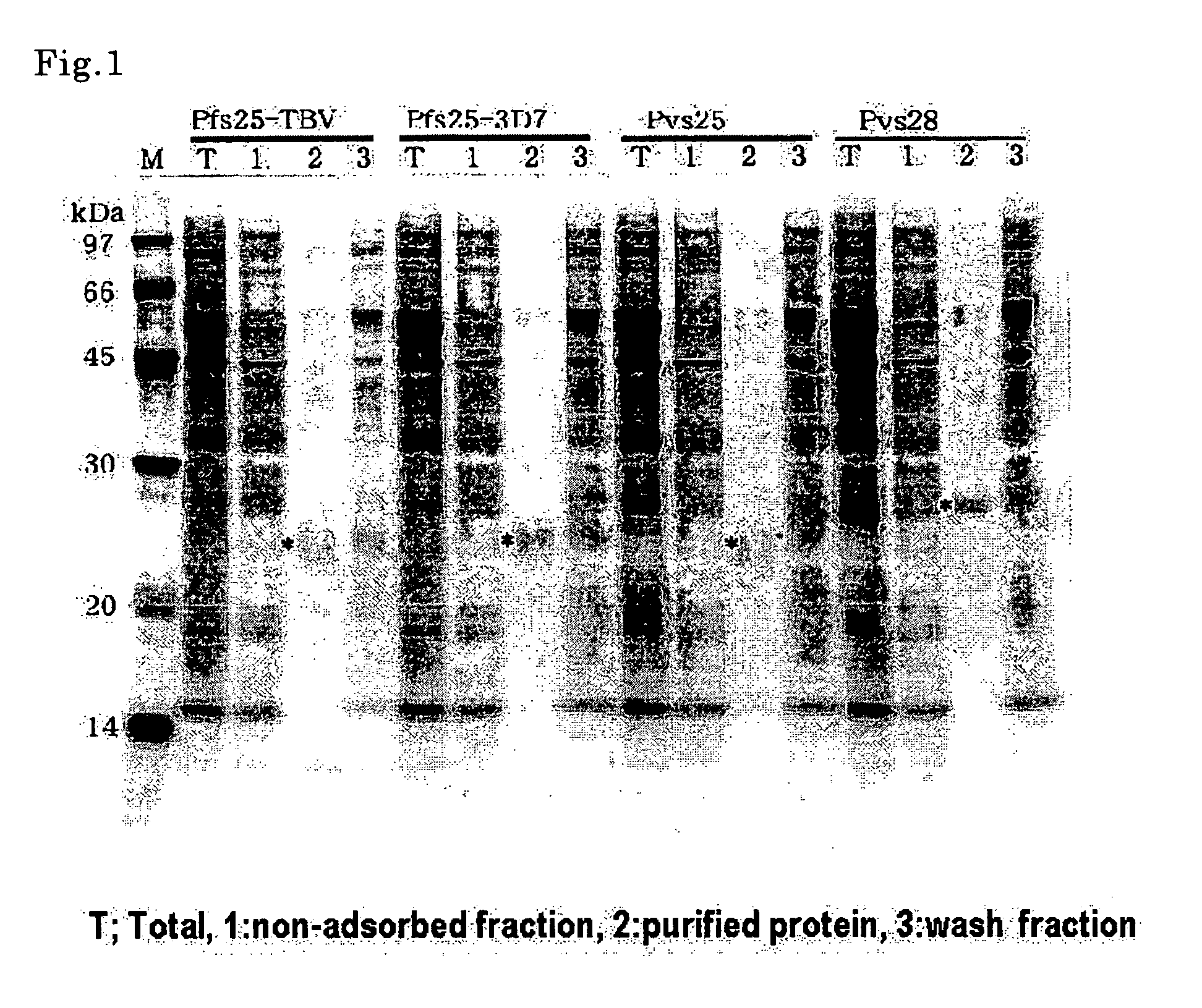
Conjugates of ookinete surface protein Pfs25 are provided that are efficacious as vaccines against Plasmodium falciparum, the most severe form of malaria. Conjugates of ookinete surface protein Pvs25 for use as a vaccine against Plasmodium vivax are also provided. Methods for preparing the conjugates, which comprise the ookinete surface protein bound onto itself or onto another protein by a linking group, are also provided.
Owner:UNITED STATES OF AMERICA
Malaria vaccines based on apicomplexan ferlins, ferlin-like proteins and other C2-domain containing proteins
The present invention relates to peptides comprising at least one antigenic determinant or epitope of an apicomplexan Ferlin, Ferlin-like protein and / or another C2-domain containing protein for use as malaria vaccines. It further relates to compositions comprising said peptides and to the use of such compositions as malaria vaccines.
Owner:MALVA GMBH
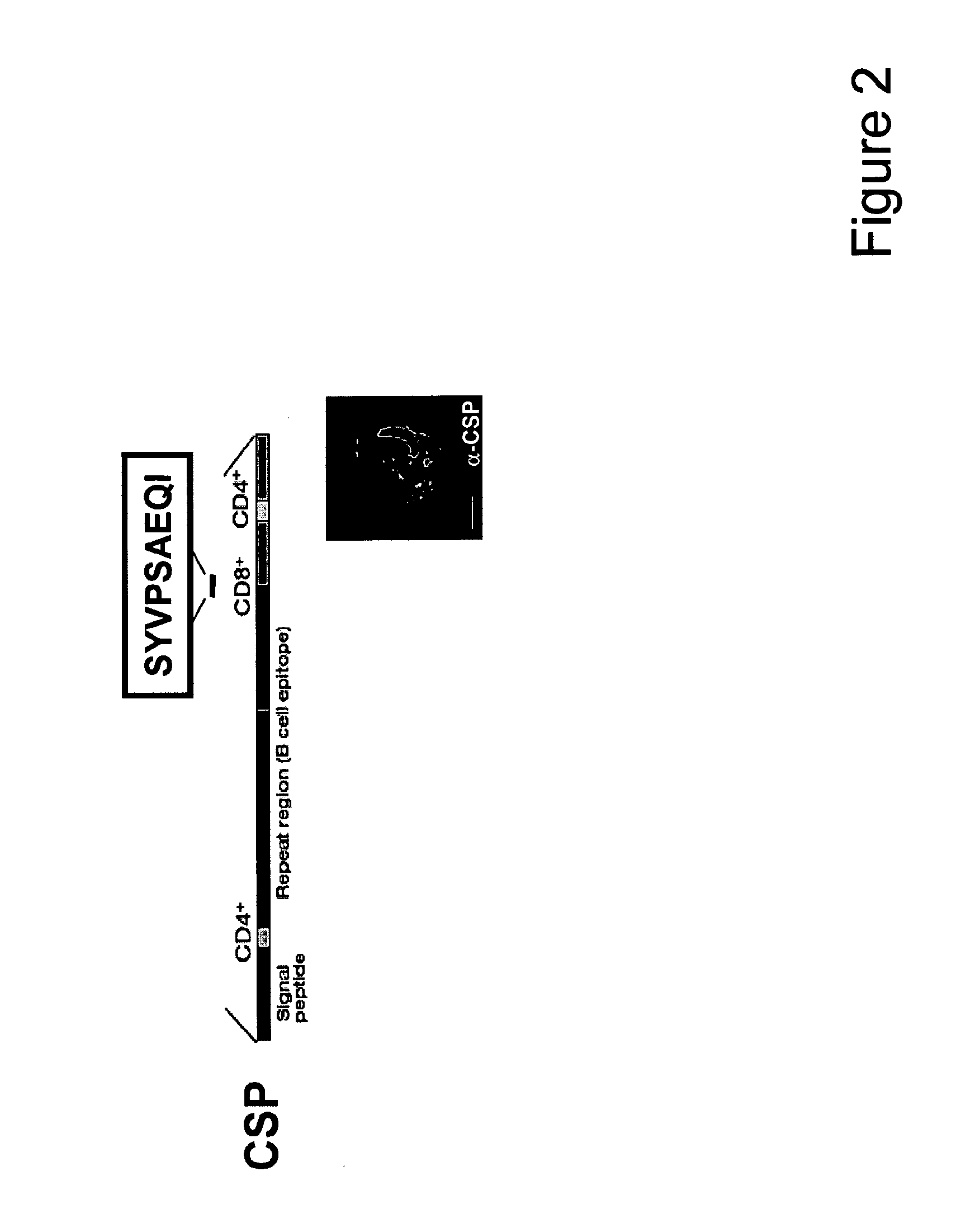
Plasmodium falciparum HLA class I restricted T-cell epitopes
InactiveUS20110117120A1Protozoa antigen ingredientsBiological material analysisCD8Malarial parasites
The invention relates immunogenic polypeptides and epitopes from Plasmodium falciparum protein AMA1. The epitopes contain HLA class I binding motifs and stimulate an anti-malaria CD8+ T-cell response. The polypeptides can be incorporated into immunogenic formulations against malaria. Additionally, the antigens are useful for facilitating evaluation of immunogenicity of candidate malaria vaccines.
Owner:SEDEGAH MARTHA +1
Malaria vaccine
InactiveUS20020160017A1Peptide/protein ingredientsGenetic material ingredientsPlasmodium merozoiteMalaria
A non-naturally occurring variant of a C-terminal fragment of a Plasmodium merozoite surface protein-1 (MSP-1) wherein said variant has (i) a reduced affinity, compared with a naturally occurring Plasmodium MSP-119, for at least one first antibody capable of blocking the binding of a second antibody, which second antibody inhibits the proteolytic cleavage of Plasmodium MSP-142 and (ii) substantially the same affinity for at least one third antibody compared with said naturally occurring Plasmodium MSP-119. which third antibody inhibits the proteolytic cleavage of Plasmodium MSP-142 is provided for use in an anti-malarial vaccine.
Owner:MEDICAL RESEARCH COUNCIL
Malaria vaccine
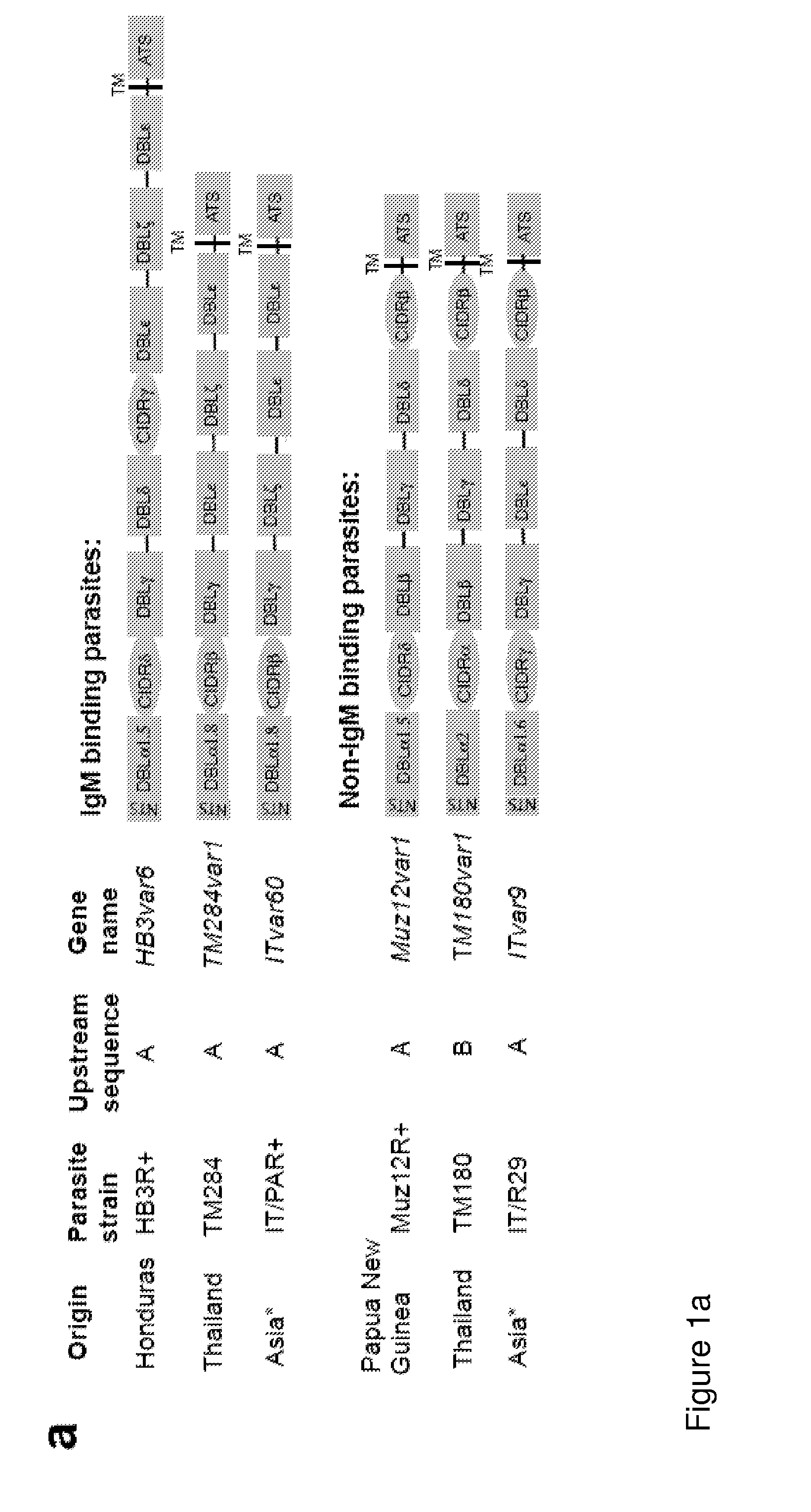
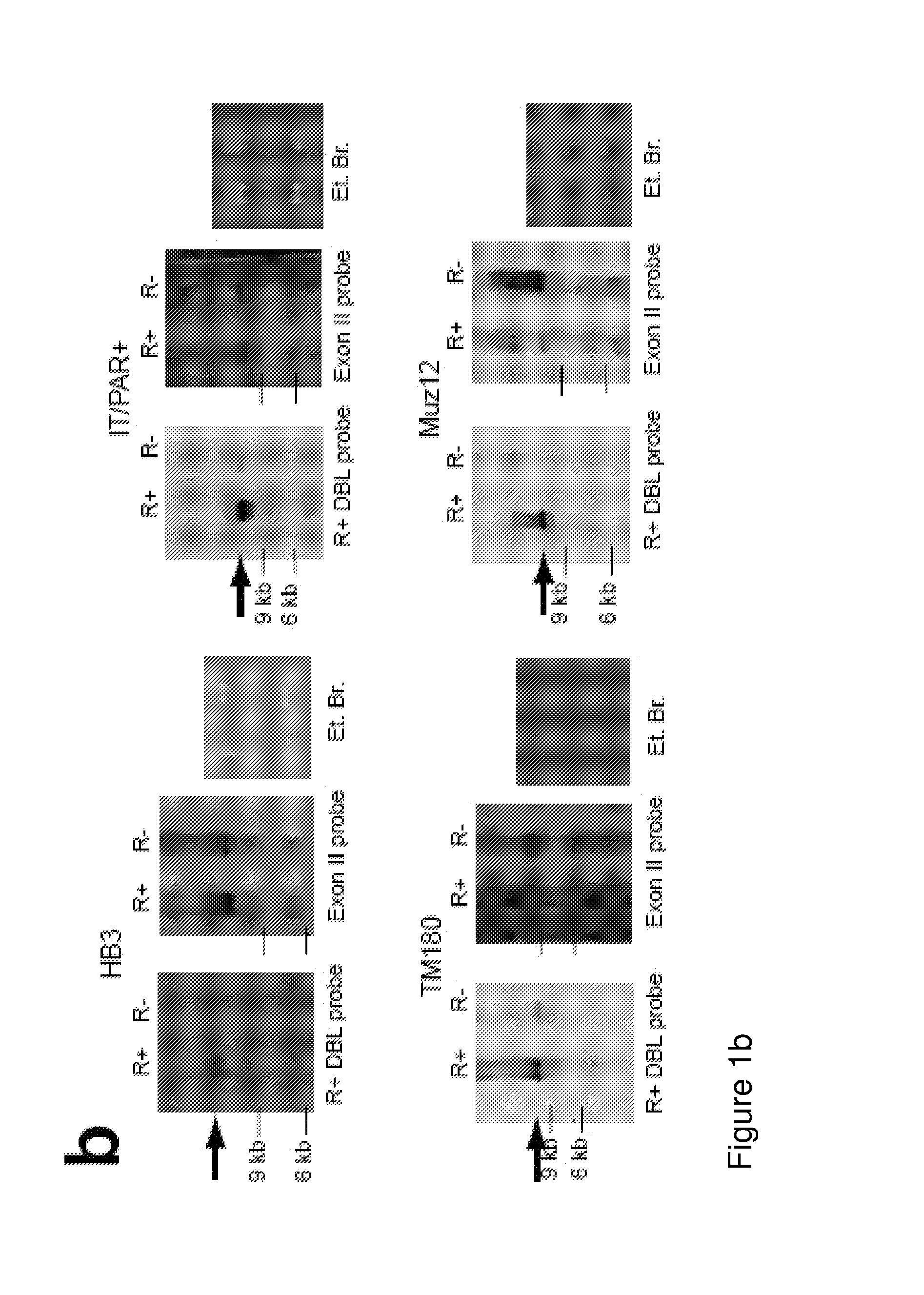
ActiveUS20140322240A1Enhance antibody responseAlleviating and reducing and eliminating symptomAntibody ingredientsImmunoglobulinsEpitopeErythrocyte membrane
The present invention provides an antigenically restricted subset of the highly variant PfEMP1 rosetting antigen which possess epitopes which may be exploited to raise immune responses effective against many diverse strains and isolates of the malaria parasite, Plasmodium falciparum. In this regard, the invention provides one or more P. falciparum Erythrocyte Membrane Protein-1 (PfEMP1) antigen(s) or a fragment or fragments thereof, for use in raising immune responses in humans.
Owner:THE UNIV COURT OF THE UNIV OF EDINBURGH
Chloroplast-derived human vaccine antigens against malaria
Disclosed is a method of making a malaria vaccine, the method comprising stably transforming a plant by inserting into its plastid genome a nucleic acid sequence encoding and operable to constitutively express a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both; harvesting the stably transformed plant in whole or in part; purifying the expressed malaria antigenic polypeptide from the harvested plant; and packaging the purified antigenic polypeptide under sterile conditions in an amount for a predetermined dosage. Also disclosed is an oral vaccine effective in raising malaria antibodies in a susceptible host, the vaccine comprising leaf material from an edible plant containing plastids stably transformed to constitutively express a fusion polypeptide consisting essentially of cholera toxin B subunit and a malaria antigenic polypeptide selected from AMA-1, MSP-1 or both.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
Malaria vaccine
ActiveUS20060024324A1Improve purification effectSimple filterPeptide/protein ingredientsProtozoaStaphylococcus lactisHybrid protein
A fusion protein, from P. falciparum Glutamate-rich protein(GLURP) genetically coupled to P. falciparum Merozoite surface protein 3 (MSP3) was produced in Lactococcus lactis as a secreted recombinant GLURP-MSP3 hybrid protein and experiments showed that the GLURP-part of the hybrid increased the overall antibody response. Immunizations with the hybrid protein consistently generated a stronger antibody response against the individual GLURP and MSP3 domains than a mixture of the two recombinant molecules injected at one site or the individual recombinant molecules injected simultaneously at two different sites. The difference was most pronounced for the MSP3-specific antibody response suggesting that T cell epitopes located in the GLURP RO-region provide help for B-cell epitopes in the MSP3 region. Moverover, when the animals were injected with a mixture of GLURP and MSP3, individual mice tended to mount a predominant antibody response against either molecule: in some animals GLURP was immunodominant whereas in other animals MSP3 was the dominant immunogen. Additionally, the hybrid was also more antigenic than the individual recombinant proteins since the ELISA-titer of naturally occurring IgG antibodies, in clinically
Owner:STATENS SERUM INST
Malaria vaccine
InactiveUS7078043B2Improve efficiencyMinimize disruptionPeptide/protein ingredientsGenetic material ingredientsMalariaMerozoite Surface Protein 1
Owner:MEDICAL RESEARCH COUNCIL
Vaccines for malaria
InactiveUS9364525B2Antibody mimetics/scaffoldsAntiinfectivesPolyclonal antibodiesLipoprotein particle
The present invention relates to a novel lipoprotein particle, methods for preparing and purifying the same, its use in medicine, particularly in the prevention of malarial infections, compositions / vaccines containing the particle or antibodies against the protein particle such as monoclonal or polyclonal antibodies and use of the same, particularly in therapy. In particular it relates to an immunogenic protein particle comprising the following monomers:a. a fusion protein comprising sequences derived from a CS protein of P. vivax and the S antigen of Hepatitis B (CSV-S), andb. a fusion protein comprising sequences derived from CS protein of P. falciparum and S antigen of Hepatitis B (RTS), andc. optionally the S antigen derived from Hepatitis B.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com